Dural Sinus Arteriovenous Malformation in the Fetus. Case Report and Discussion of the Literature
Abstract
:1. Introduction
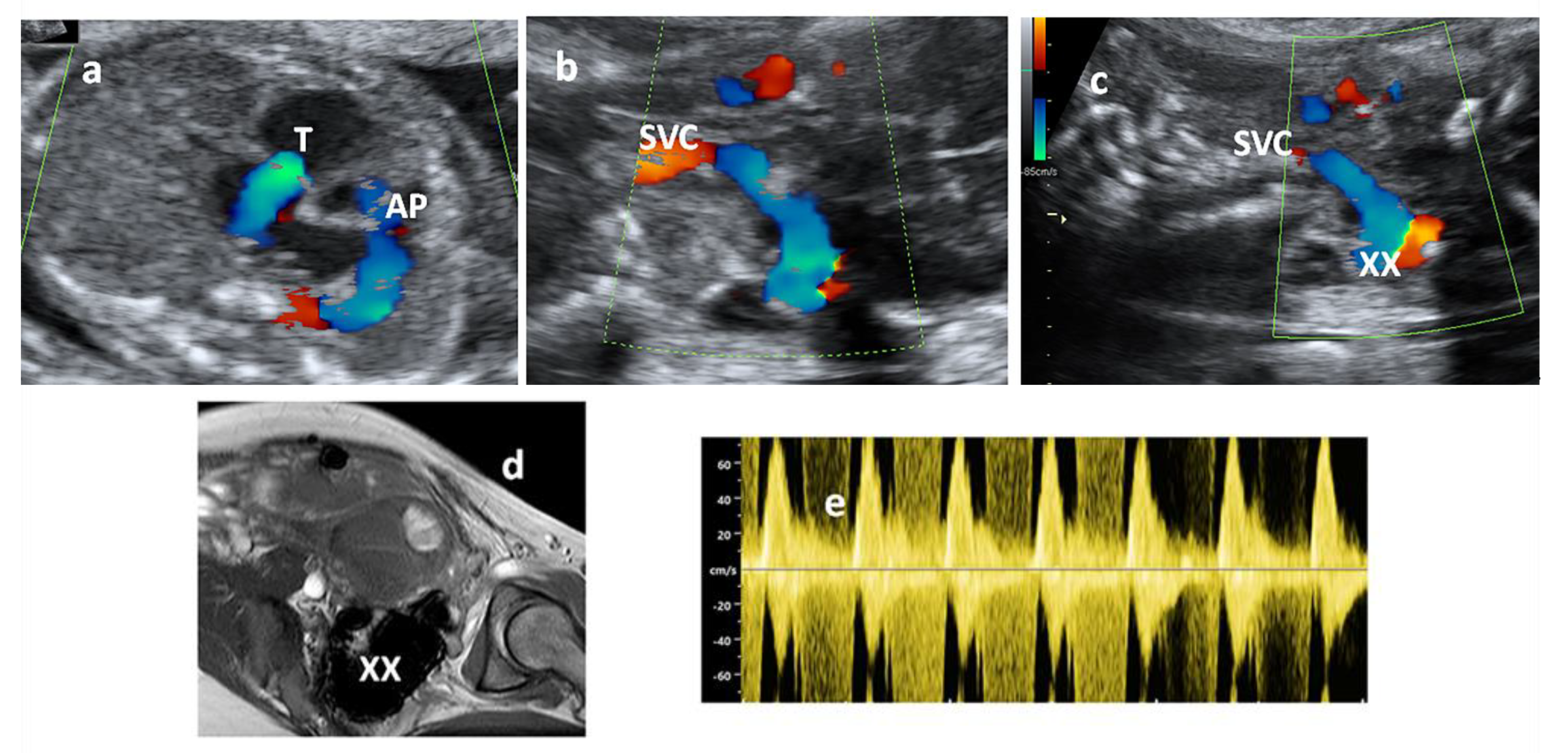
2. Case Report
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasjaunias, P. Vascular Siseases in Neonated, Infants and Children: Interventional Neuroradiology Management, 1st ed.; Spring: Berlin/Heidelberg, Germany; New York, NY, USA, 1997. [Google Scholar]
- Borden, J.A.; Wu, J.K.; Shucart, W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malfomations and implications for treatment. J. Neurosurg. 1995, 82, 166–179. [Google Scholar] [CrossRef]
- Barbosa, M.; Mahadevan, J.; Weon, Y.C.; Yoshida, Y.; Ozanne, A.; Rodesch, G.; Alvarez, H.; Lasjaunias, P. Dural sinus malformations (DSM) with giant lakes, in neonates and infants: Review of 30 consecutive cases. Interv. Neuroradiol. 2003, 9, 407–424. [Google Scholar] [CrossRef]
- Cognard, C.; Gobin, Y.P.; Pierot, L.; Bailly, A.L.; Houdart, E.; Casasco, A.; Chiras, J.; Merland, J.J. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995, 194, 671–680. [Google Scholar] [CrossRef]
- Roccatagliata, L.; Bracard, S.; Holmin, S.; Söderman, M.; Rodesch, G. Pediatric intracranial arteriovenous shunts: A global overview. Child’s Nerv. Syst. 2013, 29, 907–919. [Google Scholar] [CrossRef]
- Miller, C.; Guillaume, D. Management of midline dural sinus malformations and review of the literature. Child’s Nerv. Syst. 2016, 32, 1449–1461. [Google Scholar] [CrossRef]
- Comstock, C.H.; Kirk, J.S. Arterovenous malformations: Locations and evolution in the fetal brain. J. Ultrasound Med. 1991, 10, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Ishiguro, T.; Kitano, S.; Sakamoto, H.; Nakamura, H. Serial Antenatal Sonographic Observation of Cerebral Dural Sinus Malformation. Am. J. Neuroradiol. 2004, 25, 1446–1448. [Google Scholar]
- Rossi, A.; De Biasio, P.; Scarso, E.; Gandolfo, C.; Pavanello, M.; Morana, G.; Venturini, P.L.; Tortori-Donati, P. Prenatal MR imaging of dural sinus malformation: A case report. Prenat. Diagn. 2005, 26, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Hu, D.; Xiao, P.; Yang, W.; Chen, X. Dural Sinus Malformation Imaging in the Fetus: Based on 4 Cases and Literature Review. J. Stroke Cerebrovasc. Dis. 2018, 27, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, D.L.; Winer, N.; Gallot, D.; Lopes, K.; Perrotin, F.; Fluncker, S.; Geissler, F.; Beaufrere, A.M.; Vendittelli, F.; Couture, C.; et al. Prenatal diagnosis of thrombosis of the dural sinuses: Report of six cases, review of the literature and suggested management. Ultrasound Obstet. Gynecol. 2008, 32, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Legendre, G.; Picone, O.; Levaillant, J.M.; Delavaucoupet, J.; Ozanne, A.; Frydman, R.; Senat, M.V. Prenatal diagnosis of a spontaneous dural sinus thrombosis. Prenat. Diagn. 2009, 29, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Merzoug, V.; Flunker, S.; Drissi, C.; Eurin, D.; Grangé, G.; Garel, C.; Richter, B.; Geissler, F.; Couture, A.; Adamsbaum, C. Dural sinus malformation (DSM) in fetuses. Diagnostic value of prenatal MRI and follow-up. Eur. Radiol. 2008, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.V.; Hardin, V.; Davis, M.; Chang, E.; Rumboldt, Z. Thrombosed Fetal Dural Sinus Malformation Diagnosed with Magnetic Resonance Imaging. Obstet. Gynecol. 2008, 111, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.R.; Embleton, N.; Bradburn, M.; Connolly, D.J.A.; Mandefield, L.; Mooney, C.; Griffiths, P.D. Accuracy of in-utero MRI to detect fetal brain abnormalities and prognosticate developmental outcome: Postnatal follow-up of the MERIDIAN cohort. Lancet Child Adolesc. Heal. 2020, 4, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, D.; Chen, J.; Pearl, M.; Huang, J.; Gemmete, J.; Kathuria, S. Intracranial Dural Arteriovenous Fistulas: Classification, Imaging Findings, and Treatment. Am. J. Neuroradiol. 2012, 33, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.M.; Chen, M.M.; McDonald, M.; McGehrin, K.; Steinberg, J.; Handwerker, J.; LaBuzetta, J.N. Cranial dural arteriovenous fistula. A Treatable Mimic. Stroke 2018, 49, e332–e334. [Google Scholar] [CrossRef]
- Iampreechakul, P.; Wangtanaphat, K.; Lertbutsayanukul, P.; Wattanasen, Y.; Siriwimonmas, S. Spontaneous Closure of a Cavernous Sinus Dural Arteriovenous Fistula with Spinal Perimedullary Drainage (Cognard V) during Attempted Transvenous Embolization. Asian J. Neurosurg. 2019, 14, 1268–1274. [Google Scholar] [CrossRef]
- Jagadeesan, B.D.; Grande, A.; Guillaume, D.J.; Nascene, D.; Tummala, R.P. The role of percutaneous embolization techniques in the management of dural sinus malformations with atypical angioarchitecture in neonates: Report of 2 cases. J. Neurosurg. Pediatr. 2015, 16, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.; Storey, A.; Olson, H.E.; Soul, J.; Estroff, J.A.; Trenor, C.C.; Cooper, B.K.; Smith, E.R.; Orbach, D.B. Imaging features and prognostic factors in fetal and postnatal torcular dural sinus malformations, part I: Review of experience at Boston Children’s Hospital. J. Neurointerv. Surg. 2018, 10, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Storey, A.; Olson, H.E.; Soul, J.; Estroff, J.A.; Trenor, C.C.; Cooper, B.K.; Smith, E.R.; Orbach, D.B. Imaging features and prognostic factors in fetal and postnatal torcular dural sinus malformations, part II: Synthesis of the literature and patient management. J. NeuroInterv. Surg. 2017, 10, 471–475. [Google Scholar] [CrossRef]
- Requejo, F.; Tcherbbis, V.; Gonzalez, M.L.; Argañaraz, R.; Marelli, J.M.; Mantese, B. Dural sinus malformation with giant pouch (DSMGP): Symptoms and treatment. Child’s Nerv. Syst. 2020, 36, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, M.E.; Tworetzky, W.; Morash, D.; Friedman, K.G. Cardiac Findings in the Fetus with Cerebral Arteriovenous Malformation Are Associated with Adverse Outcome. Fetal Diagn. Ther. 2016, 41, 108–114. [Google Scholar] [CrossRef]
- Jhaveri, S.; Berenstein, A.; Srivastava, S.; Shigematsu, T.; Geiger, M.K. High Output Cardiovascular Physiology and Outcomes in Fetal Diagnosis of Vein of Galen Malformation. Pediatr. Cardiol. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-G.; Zhang, Y.-Y.; Nie, F.; Peng, M.-J.; Li, Y.-Z.; Li, P.-L. Diagnosis of foetal vein of galen aneurysmal malformation by ultrasound combined with magnetic resonance imaging: A case series. BMC Med. Imaging 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; De Carolis, M.P.; Tempera, A.; Pedicelli, A.; Rollo, M.; Luca, E.; De Luca, D.; Conti, G.; Piastra, M.; Morena, T.C. Outcome of Neonates with Vein of Galen Malformation Presenting with Severe Heart Failure: A Case Series. Am. J. Perinatol. 2018, 36, 169–175. [Google Scholar] [CrossRef]
- Paladini, D.; Deloison, B.; Rossi, A.; Chalouhi, G.E.; Gandolfo, C.; Sonigo, P.; Buratti, S.; Millischer, A.E.; Tuo, G.; Ville, Y.; et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: Retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet. Gynecol. 2017, 50, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fesslova, V.; Colli, A.M.; Boito, S.; Fabietti, I.; Triulzi, F.; Persico, N. Dural Sinus Arteriovenous Malformation in the Fetus. Case Report and Discussion of the Literature. Diagnostics 2021, 11, 1651. https://doi.org/10.3390/diagnostics11091651
Fesslova V, Colli AM, Boito S, Fabietti I, Triulzi F, Persico N. Dural Sinus Arteriovenous Malformation in the Fetus. Case Report and Discussion of the Literature. Diagnostics. 2021; 11(9):1651. https://doi.org/10.3390/diagnostics11091651
Chicago/Turabian StyleFesslova, Vlasta, Anna Maria Colli, Simona Boito, Isabella Fabietti, Fabio Triulzi, and Nicola Persico. 2021. "Dural Sinus Arteriovenous Malformation in the Fetus. Case Report and Discussion of the Literature" Diagnostics 11, no. 9: 1651. https://doi.org/10.3390/diagnostics11091651
APA StyleFesslova, V., Colli, A. M., Boito, S., Fabietti, I., Triulzi, F., & Persico, N. (2021). Dural Sinus Arteriovenous Malformation in the Fetus. Case Report and Discussion of the Literature. Diagnostics, 11(9), 1651. https://doi.org/10.3390/diagnostics11091651





