Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Information Sources
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction and Collection
2.5. Risk-of-Bias Assessment and Quality Assessment of Studies
3. Results
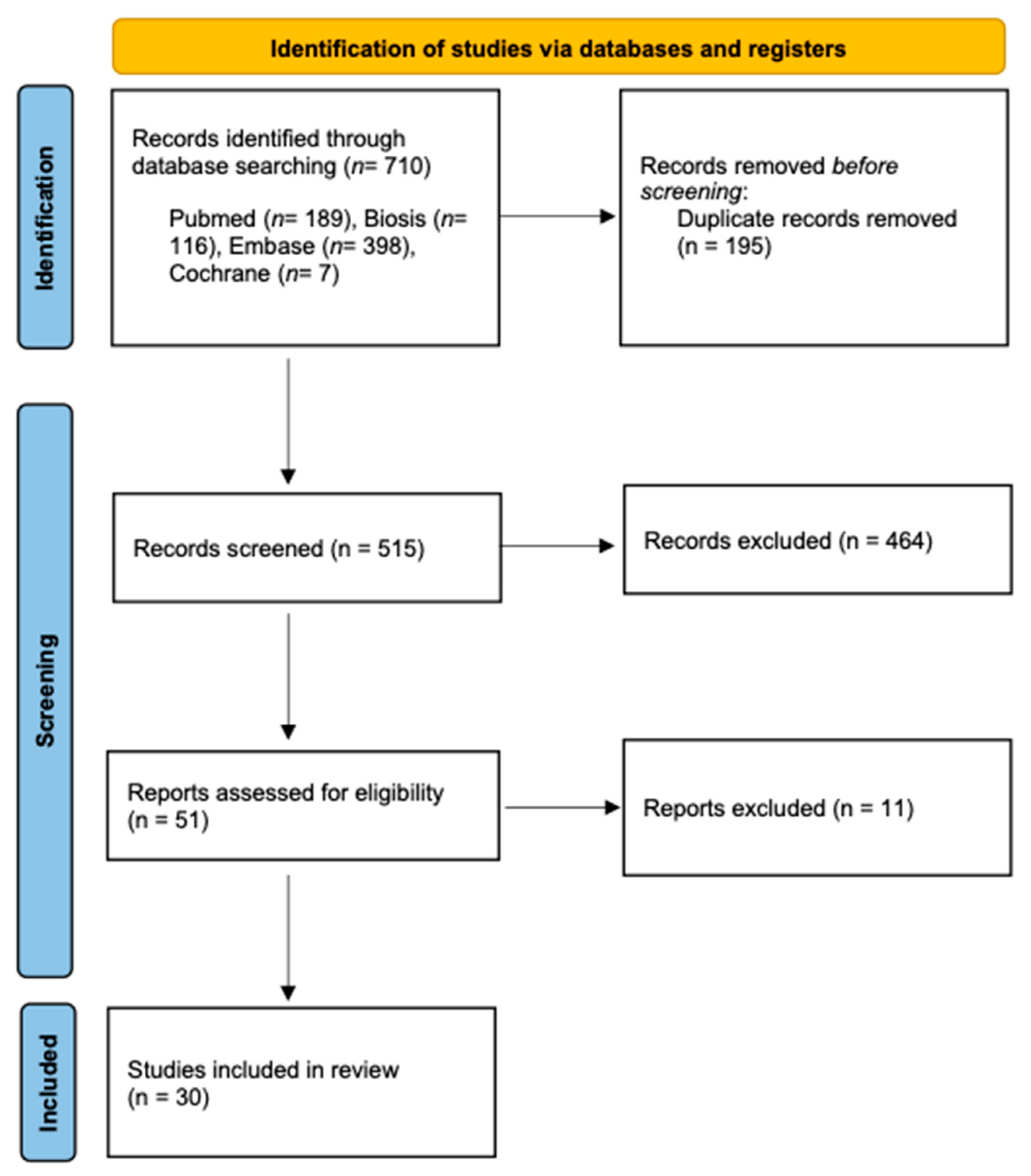
3.1. Study Selection
3.2. Study Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3DAC PROPELLER | Three-dimensional anisotropy contrast periodically rotated overlapping parallel lines with enhanced reconstruction |
| CISS | Constructive interference in steady state |
| DESS-WE | Double-echo steady-state with water excitation sequence |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion weighted imaging |
| EPI | Echo planar imaging |
| FFE | Fast field echo |
| FIESTA | Fast Imaging Employing Steady-state Acquisition |
| GE | Gradient echo |
| MP-RAGE | Magnetization-prepared rapid gradient-echo |
| PD | Proton density |
| PETRA | Phase encode time reduction acquisition |
| PSIF | Reversed fast imaging with steady-state precession |
| SE | Spin-echo |
| SPACE | Sampling perfection with application optimized contrasts using different flip angle evolution |
| SPAIR | Spectral adiabatic inversion recovery |
| SPGR | Spoiled gradient recalled |
| SPIR | Spectral Presaturation with Inversion Recovery |
| STIR | Short tau inversion recovery |
| T1 | T1 weighted image |
| T2 | T2 weighted image |
| T2* | T2 relaxation |
| TFE | Turbo field echo |
| TIRM | Turbo inversion recovery magnitude |
| TSE | Turbo spin echo |
| VIBE | Volumetric interpolated breath-hold examination |
References
- Libersa, P.; Savignat, M.; Tonnel, A. Neurosensory disturbances of the inferior alveolar nerve: A retrospective study of complaints in a 10-year period. J. Oral Maxillofac. Surg. 2007, 65, 1486–1489. [Google Scholar] [CrossRef]
- Jerjes, W.; Swinson, B.; Moles, D.R.; El-Maaytah, M.; Banu, B.; Upile, T.; Kumar, M.; Al Khawalde, M.; Vourvachis, M.; Hadi, H.; et al. Permanent sensory nerve impairment following third molar surgery: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, e1–e7. [Google Scholar] [CrossRef]
- Qi, W.; Lei, J.; Liu, Y.N.; Li, J.N.; Pan, J.; Yu, G.Y. Evaluating the risk of post-extraction inferior alveolar nerve injury through the relative position of the lower third molar root and inferior alveolar canal. Int. J. Oral Maxillofac. Surg. 2019, 48, 1577–1583. [Google Scholar] [CrossRef]
- Sigron, G.R.; Pourmand, P.P.; Mache, B.; Stadlinger, B.; Locher, M.C. The most common complications after wisdom-tooth removal: Part 1: A retrospective study of 1,199 cases in the mandible. Swiss Dent. J. 2014, 124, 1042–1056. [Google Scholar]
- Pippi, R.; Spota, A.; Santoro, M. Prevention of Lingual Nerve Injury in Third Molar Surgery: Literature Review. J. Oral Maxillofac. Surg. 2017, 75, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.P. Observations on the recovery of sensation following inferior alveolar nerve injuries. Br. J. Oral Maxillofac. Surg. 1988, 26, 177–189. [Google Scholar] [CrossRef]
- Lam, N.P.; Donoff, R.B.; Kaban, L.B.; Dodson, T.B. Patient satisfaction after trigeminal nerve repair. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Dental MRI: A road beyond CBCT. Eur. Radiol. 2020, 30, 6389–6391. [Google Scholar] [CrossRef] [PubMed]
- Tsapaki, V. Radiation protection in dental radiology—Recent advances and future directions. Phys. Med. 2017, 44, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Stratis, A.; Zhang, G.; Jacobs, R.; Bogaerts, R.; Bosmans, H. The growing concern of radiation dose in paediatric dental and maxillofacial CBCT: An easy guide for daily practice. Eur. Radiol. 2019, 29, 7009–7018. [Google Scholar] [CrossRef] [PubMed]
- Sodickson, A.; Baeyens, P.F.; Andriole, K.P.; Prevedello, L.M.; Nawfel, R.D.; Hanson, R.; Khorasani, R. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009, 251, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gahleitner, A.; Solar, P.; Nasel, C.; Homolka, P.; Youssefzadeh, S.; Ertl, L.; Schick, S. [Magnetic resonance tomography in dental radiology (dental MRI)]. Radiologe 1999, 39, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Olt, S.; Jakob, P.M. Contrast-enhanced dental MRI for visualization of the teeth and jaw. Magn. Reson. Med. 2004, 52, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Kim, S.; Lund, N.L. Magnetic resonance imaging of human teeth. J. Endod. 1992, 18, 237–244. [Google Scholar] [CrossRef]
- Idiyatullin, D.; Corum, C.; Moeller, S.; Prasad, H.S.; Garwood, M.; Nixdorf, D.R. Dental magnetic resonance imaging: Making the invisible visible. J. Endod. 2011, 37, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Al-Haj Husain, A.; Stadlinger, B.; Winklhofer, S.; Müller, M.; Piccirelli, M.; Valdec, S. Mandibular Third Molar Surgery: Intraosseous Localization of the Inferior Alveolar Nerve Using 3D Double-Echo Steady-State MRI (3D-DESS). Diagnostics 2021, 11, 1245. [Google Scholar] [CrossRef]
- Burian, E.; Probst, F.A.; Weidlich, D.; Cornelius, C.P.; Maier, L.; Robl, T.; Zimmer, C.; Karampinos, D.C.; Ritschl, L.M.; Probst, M. MRI of the inferior alveolar nerve and lingual nerve-anatomical variation and morphometric benchmark values of nerve diameters in healthy subjects. Clin. Oral Investig. 2020, 24, 2625–2634. [Google Scholar] [CrossRef]
- Fujii, H.; Fujita, A.; Yang, A.; Kanazawa, H.; Buch, K.; Sakai, O.; Sugimoto, H. Visualization of the Peripheral Branches of the Mandibular Division of the Trigeminal Nerve on 3D Double-Echo Steady-State with Water Excitation Sequence. AJNR Am. J. Neuroradiol. 2015, 36, 1333–1337. [Google Scholar] [CrossRef] [Green Version]
- Geethanath, S.; Vaughan, J.T. Accessible magnetic resonance imaging: A review. J. Magn. Reson. Imaging 2019, 49, e65–e77. [Google Scholar] [CrossRef]
- Gray, C.F.; Redpath, T.W.; Smith, F.W.; Staff, R.T. Advanced imaging: Magnetic resonance imaging in implant dentistry. Clin. Oral Implant. Res. 2003, 14, 18–27. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; Group, Q. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M.; Kolokythas, A. Inferior alveolar and lingual nerve imaging. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2011, 19, 35–46. [Google Scholar] [CrossRef]
- Politis, C.; Ramírez, X.B.; Sun, Y.; Lambrichts, I.; Heath, N.; Agbaje, J.O. Visibility of mandibular canal on panoramic radiograph after bilateral sagittal split osteotomy (BSSO). Surg. Radiol. Anat. 2013, 35, 233–240. [Google Scholar] [CrossRef]
- Petersen, L.B.; Olsen, K.R.; Matzen, L.H.; Vaeth, M.; Wenzel, A. Economic and health implications of routine CBCT examination before surgical removal of the mandibular third molar in the Danish population. Dentomaxillofac. Radiol. 2015, 44, 20140406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasel, C.; Gahleitner, A.; Breitenseher, M.; Czerny, C.; Solar, P.; Imhof, H. Dental MR tomography of the mandible. J. Comput. Assist. Tomogr. 1998, 22, 498–502. [Google Scholar] [CrossRef]
- Nasel, C.; Gahleitner, A.; Breitenseher, M.; Czerny, C.; Glaser, C.; Solar, P.; Imhof, H. Localization of the mandibular neurovascular bundle using dental magnetic resonance imaging. Dentomaxillofac. Radiol. 1998, 27, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Salvolini, E.; De Florio, L.; Regnicolo, L.; Salvolini, U. Magnetic Resonance applications in dental implantology: Technical notes and preliminary results. Radiol. Med. 2002, 103, 526–529. [Google Scholar]
- Cassetta, M.; Pranno, N.; Barchetti, F.; Sorrentino, V.; Lo Mele, L. 3.0 Tesla MRI in the early evaluation of inferior alveolar nerve neurological complications after mandibular third molar extraction: A prospective study. Dentomaxillofac. Radiol. 2014, 43, 20140152. [Google Scholar] [CrossRef] [Green Version]
- Cassetta, M.; Pranno, N.; Pompa, V.; Barchetti, F.; Pompa, G. High resolution 3-T MR imaging in the evaluation of the trigeminal nerve course. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 257–264. [Google Scholar]
- Mazza, D.; Di Girolamo, M.; Cecchetti, F.; Baggi, L. Appearance of normal MRI anatomy of the lingual nerve using steady-state free precession sequences at 3-T. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 1), 19–26. [Google Scholar]
- Kotaki, S.; Sakamoto, J.; Kretapirom, K.; Supak, N.; Sumi, Y.; Kurabayashi, T. Diffusion tensor imaging of the inferior alveolar nerve using 3T MRI: A study for quantitative evaluation and fibre tracking. Dentomaxillofac. Radiol. 2016, 45, 20160200. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Kaneda, T.; Fujita, Y.; Kato, M.; Sakayanagi, M.; Minami, M. Diffusion tensor tractography for the inferior alveolar nerve (V3): Initial experiment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 270–274. [Google Scholar] [CrossRef]
- Demirturk Kocasarac, H.; Geha, H.; Gaalaas, L.R.; Nixdorf, D.R. MRI for Dental Applications. Dent. Clin. N. Am. 2018, 62, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, D.; Gambarini, G.; Capuani, S.; Testarelli, L. Nuclear Magnetic Resonance Imaging in Endodontics: A Review. J. Endod. 2018, 44, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, U.; Eisenbeiss, A.K.; Scheifele, C.; Nelson, K.; Bock, M.; Hennig, J.; von Elverfeldt, D.; Herdt, O.; Flügge, T.; Hövener, J.B. Dental MRI using wireless intraoral coils. Sci. Rep. 2016, 6, 23301. [Google Scholar] [CrossRef] [Green Version]
- Prager, M.; Heiland, S.; Gareis, D.; Hilgenfeld, T.; Bendszus, M.; Gaudino, C. Dental MRI using a dedicated RF-coil at 3 Tesla. J. Craniomaxillofac. Surg. 2015, 43, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Zuniga, J.R.; Panchal, N.; Cheng, J.; Chhabra, A. Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: Experience in a tertiary care centre. Eur. Radiol. 2016, 26, 3392–3400. [Google Scholar] [CrossRef]
- Dessouky, R.; Xi, Y.; Zuniga, J.; Chhabra, A. Role of MR Neurography for the Diagnosis of Peripheral Trigeminal Nerve Injuries in Patients with Prior Molar Tooth Extraction. AJNR Am. J. Neuroradiol. 2018, 39, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Kress, B.; Gottschalk, A.; Anders, L.; Stippich, C.; Palm, F.; Bähren, W.; Sartor, K. High-resolution dental magnetic resonance imaging of inferior alveolar nerve responses to the extraction of third molars. Eur. Radiol. 2004, 14, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Gerber, S.; Solbach, T.; Anders, L.; Bähren, W.; Kress, B. Quantitative analysis of contrast enhanced MRI of the inferior alveolar nerve in inflammatory changes of the mandible. Rofo 2003, 175, 1344–1348. [Google Scholar] [CrossRef]
- Kress, B.; Gottschalk, A.; Stippich, C.; Palm, F.; Bähren, W.; Sartor, K. MR imaging of traumatic lesions of the inferior alveolar nerve in patients with fractures of the mandible. AJNR Am. J. Neuroradiol. 2003, 24, 1635–1638. [Google Scholar] [PubMed]
- Terumitsu, M.; Matsuzawa, H.; Seo, K.; Watanabe, M.; Kurata, S.; Suda, A.; Nakada, T. High-contrast high-resolution imaging of posttraumatic mandibular nerve by 3DAC-PROPELLER magnetic resonance imaging: Correlation with the severity of sensory disturbance. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burian, E.; Sollmann, N.; Ritschl, L.M.; Palla, B.; Maier, L.; Zimmer, C.; Probst, F.; Fichter, A.; Miloro, M.; Probst, M. High resolution MRI for quantitative assessment of inferior alveolar nerve impairment in course of mandible fractures: An imaging feasibility study. Sci. Rep. 2020, 10, 11566. [Google Scholar] [CrossRef]
- Kress, B.; Gottschalk, A.; Anders, L.; Stippich, C.; Palm, F.; Bähren, W.; Sartor, K. Topography of the inferior alveolar nerve in relation to cystic processes of the mandible in dental MRI. Rofo 2003, 175, 67–69. [Google Scholar] [CrossRef]
- Deng, W.; Chen, S.L.; Zhang, Z.W.; Huang, D.Y.; Zhang, X.; Li, X. High-resolution magnetic resonance imaging of the inferior alveolar nerve using 3-dimensional magnetization-prepared rapid gradient-echo sequence at 3.0T. J. Oral Maxillofac. Surg. 2008, 66, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, F.; Liu, D.; Zheng, C.; Kong, X.; Shu, S.; Li, D.; Wang, L. Visualization of the morphology and pathology of the peripheral branches of the cranial nerves using three-dimensional high-resolution high-contrast magnetic resonance neurography. Eur. J. Radiol. 2020, 132, 109137. [Google Scholar] [CrossRef]
| Focused Question (PICO) | Does Direct Visualization of the IAN and LN Using MRI Provide Accurate and Feasible Preoperative Diagnostical Information in Healthy Subjects and Patients Undergoing Oral and Maxillofacial Surgery? | |
|---|---|---|
| Search strategy | Population | Human studies (patients and/or healthy subjects), aged older than 12 years undergoing oral and maxillofacial surgical interventions near the IAN and LN. #1—((inferior alveolar nerve [MeSH]) OR (lingual nerve [MeSH]) OR (mandibular nerve [MeSH]) OR (trigeminal nerve [MeSH])) |
| Intervention | Magnetic resonance imaging. #2—((magnetic resonance imaging [MeSH]) OR (MRI) OR (nuclear magnetic resonance imaging [MeSH]) OR (NMR) OR (diffusion tensor imaging [MeSH]) OR (DTI) OR (ultra-short echo-time [MeSH]) OR (UTE) OR (maxillofacial imaging)) #3—((visualization) OR (neurography)) | |
| Comparison | Not applicable. Conventional preoperative radiological assessment. #4—((computed tomography [MeSH]) OR (cone-beam computed tomography [MeSH]) #5—(panoramic radiography [MeSH]) | |
| Outcome | Feasibility and accuracy of preoperative radiological assessment of the IAN and LN using MRI. #6—((accuracy) OR (feasibility) OR (signal-to-noise-ratio [MeSH])) | |
| Search combination(s) | (#1) AND (#2 or #3) OR (#6) | |
| Study Number | Author, Year, Country | Study | Sample Size | Age | Study Objectives | Nerves | MRI Sequences | Field Strengths | Type of MRI Coil | Outcome (Feasibility/Accuracy) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Miloro et al., 1997, USA | Assessment of the lingual nerve in the third molar region using magnetic resonance imaging | 10 | 21–35 years | Determination of the exact in situ location of the LN in the third molar region using high-resolution magnetic resonance imaging. | LN | Phase encode time reduction acquisition (PETRA) sequence | 1.5 T | surface coil | LN visualization in the molar region using the HR-MRI sequence. Assessment of the nerve diameter, the different shapes of the nerve, and the mean vertical and horizontal distances to the lingual crest and the lingual plate of the mandible. |
| 2 | Nasel et al., 1998, Austria | Dental MR tomography of the mandible | 5 | 28–55 years | Presentation of a new imaging technique (dental MR tomography). | IAN | T1- and T2 sequences were performed with gradient echo (GE) and spin echo (SE) techniques -3D T1 GE using a turbo field echo (TFE) -3D T2* TFE | 1.0 T | Standard neck-quad coil | Visualization of anatomic features on T1 GE images, neurovascular bundle on T2 images, and nerve exit at the mental foramen. |
| 3 | Nasel et al., 1998, Austria | Localization of the mandibular neurovascular bundle using dental magnetic resonance imaging | 11 | 32–57 years | Reliability of visualizing the neurovascular bundle of the mandible using 3D MPR-MRI for the jaws. | IAN | GE sequence with a spectral fat suppression pre-impulse | 1.0 T | Standard neck-quad coil | Visualization of the mandibular canal and neurovascular bundle. |
| 4 | Salvolini et al., 2002, Italy | Magnetic resonance applications in dental implantology: technical notes and preliminary results | 10 | N/A | Evaluation of the potential use of magnetic resonance imaging in dental implantology, including the identification of the mandibular nerve. | IAN | T2 TSE, T1 SE and T2 GE | 1.0 T | Brain coil | Identification of the intraosseous course of the IAN within the mandibular canal. |
| 5 | Kress et al., 2003, Germany | Topography of the inferior alveolar nerve in relation to cystic processes of the mandible in dental MRI | 13 | 15–71 years | Determination whether preoperative MRI could depict the continuous course of the neurovascular bundle and its positional relationship to cystic processes of the mandible. | IAN | T2 TSE-axial, Proton density (PD) FFE-sagittal, PD FFE-coronal | 1.5 T | surface coil | High-resolution visualization in minimal cystic processes and identifying the neurovascular bundle in the sagittal and coronal sequences. |
| 6 | Kress et al., 2003, Germany | High-resolution MR technique allowing visualization of the course of the inferior alveolar nerve along cystic processes | N/A | N/A | Visualization of the entire course of the IAN in the presence of cystic lesions using high-resolution MRI. | IAN | T2 TSE-axial, T1 TSE sequence, a pd GE—sagittal and coronal | N/A | Head coil | Successful display of the IAN throughout its course. |
| 7 | Kress et al., 2003, Germany | MR imaging of traumatic lesions of the inferior alveolar nerve in patients with fractures of the mandible | 23 | 18–64 years | Visualization of the neurovascular bundle in patients with mandibular fracture and investigation of post-traumatic changes in IAN signal intensities. | IAN | Object-oriented sagittal view T1 sequences and pd sequences | 1.5 T | Surface coil | Feasibility of displaying the disruption of the neurovascular bundle and intraoperative confirmation of these findings. |
| 8 | Gottschalk et al., 2003, Germany | Quantitative analysis of contrast enhanced MRI of the inferior alveolar nerve in inflammatory changes of the mandible | 30 | 18–52 years | Quantitative analysis of the IAN’s signal intensity using contrast-enhanced MRI in inflammatory lesions of the mandible. | IAN | T1- fast-field-echo (FFE) GE-proset-sequence | 1.5 T | Surface coil | Assessing the MR signal intensity of the neurovascular bundle and mapping of physiological and pathophysiological states of the IAN. |
| 9 | Kress et al., 2004, Germany | High-resolution dental magnetic resonance imaging of inferior alveolar nerve responses to the extraction of third molars | 71 | 18–62 years | Assessment of signal intensity measurements in the neurovascular bundle after third molar extraction treatment using a contrast agent. | IAN | T2 TSE-axial, T1 FFE proset fat saturation-sagittal, contrast agent no delay | N/A | Surface coil | Accurate visualization of the neurovascular bundle, with postoperatively increased signal intensity measurements at the defined regions of interest. |
| 10 | Mori et al., 2008, Japan | Diffusion tensor tractography for the inferior alveolar nerve (V3): initial experiment | 10 | 21–35 years | Evaluation of diffusion tensor tractography (DTT) in the mandible to visualize the IAN. | IAN | Single-shot echo planar imaging (EPI) sequence with a SENSE factor of 2 | 1.5 T | SENSE Flex S coil | The automatic fiber tracking method made the IAN visualization possible, providing more information overlapping with T1 images. |
| 11 | Deng et al., 2008, China | High-resolution magnetic resonance imaging of the inferior alveolar nerve using 3-dimensional magnetization-prepared rapid gradient-echo sequence at 3.0 T | 18 | 18–42 years | Visualization of the anatomy of the IAN in healthy subjects and patients with mandibular diseases. | IAN | 3D magnetization-prepared rapid gradient-echo (3D MP-RAGE) | 3.0 T | Standard neck and CP spine array coil | High-resolution images with a high signal-to-noise ratio and excellent contrast of the IAN in the intact mandible and in patients with mandibular trauma could be obtained using the 3D-MPRAGE MR sequence. |
| 12 | Terumitsu et al., 2008, Japan | Morphologic evaluation of the inferior alveolar nerve in patients with sensory disorders by high-resolution 3D volume rendering magnetic resonance neurography on a 3.0-T system | 16 | N/A | Evaluation of the morphological appearance of the IAN in patients who reported persistent sensory dysfunction due to unilateral IAN injury. | IAN | T1 fast spoiled gradient-recalled echo (fast SPGR) sequence with fat suppression (Chemical shift selective pulse (CHESS)) | 3.0 T | 8-channel neurovascular phased-array coil | High-resolution 3-dimensional volume rendering magnetic resonance neurography (3DVR-MRN) visualized the course of the IAN and its morphological changes. |
| 13 | Krasny et al., 2012, Germany | Anatomic variations of neural canal structures of the mandible observed by 3-Tesla magnetic resonance imaging | 64 | N/A | Evaluation of anatomical variations of the mandibular canal, the neurovascular bundle, mental canal, incisive canal, and the nutrient canals in patients having nonspecific head or neck pain. | IAN | modified 3D high-resolution T2 sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) sequence | 3.0 T | head and neck coil | Precise visualization of the IAN’s anatomical variations in the fully dentate mandible of the volunteers, providing the detection of entire structures and structural abnormalities accurately. |
| 14 | Terumitsu et al., 2013, Japan | Evaluating fine structure of the injured trigeminal nerve and tissue using 3D anisotropy contrast imaging on a 3.0-T system | 20 | N/A | Distinguishment of the course of the nerve fibers from the connective tissue mass in patients with injured IAN or LN. | IAN + LN | Three-dimensional anisotropy contrast periodically rotated overlapping parallel lines with enhanced reconstruction (3DAC PROPELLER) diffusion-weighted imaging (DWI) | 3.0 T | N/A | Successful delineation of the lesion from the surrounding connective tissues, providing textural details indicating possible severity of sensory dysfunction. |
| 15 | Cassetta et al., 2014, Italy | High-resolution 3-T MR imaging in the evaluation of the trigeminal nerve course | 78 | 17–71 years | Qualitative evaluation of the course of four segments of the trigeminal nerve, including the extracranial segments of the mandibular nerve. | IAN + LN | 3D T2 fast imaging employing steady-state acquisition sequence (3D FIESTA); T1 fast spoiled gradient recalled echo sequence (3D SPGR) | 3.0 T | 8-channel neurovascular phased-array coil | Accurate visualization of the trigeminal nerve and its branches, especially the mandibular nerve, with high inter- and intra-rater variability. |
| 16 | Cassetta et al., 2014, Italy | 3.0 Tesla MRI in the early evaluation of inferior alveolar nerve neurological complications after mandibular third molar extraction: a prospective study. | 196 | 19–32 years | Investigation of the use of 3.0 Tesla MRI to predict possible sensitivity disturbances qualitatively and quantitatively in the IAN after third molar extraction treatment. | IAN | 3D FIESTA; 3D SPGR | 3.0 T | 8-channel neurovascular phased-array coil | No differences in the course of the IAN were observed preoperatively and postoperatively with good inter- and intra-rater variability. |
| 17 | Assaf et al., 2014, Germany | Evaluation of four different optimized magnetic-resonance-imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T | 12 | 25–63 years | Evaluation of four optimized noncontrast MRI sequences on the visibility of osseous and dental maxillary and mandibular structures on healthy subjects. | IAN + LN | T1; fat saturated T1; fat saturated T2 and constructive interference steady state (CISS). | 3.0 T | 20-channel head-and-neck coil | The evaluated sequences provided excellent 2D and 3D visualization of osseous and dental structures, allowing its use in the differentiation of the mandibular canal, the IAN, the mental foramen, and the LN. |
| 18 | Fujii et al., 2015, Germany | Visualization of the peripheral branches of the mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence | 86 | 17–88 years | Detectability of the six extracranial branches of the mandibular nerve. | IAN + LN | 3D double-echo steady-state with water excitation sequence (3D-DESS-WE) | 3.0 T | 20-channel head-neck coil | Successful visualization of the LN and IAN based on a 5-point scale. |
| 19 | Manoliu et al., 2016, Switzerland | MR neurographic orthopantomogram: ultrashort Echo-Time Imaging of Mandibular Bone and Teeth Complemented with High-Resolution Morphological and Functional MR Neurography | 10 | 20–50 years | Investigation of a new technique for MR neurographic orthopantomograms that uses ultrashort echo time (UTE) imaging to visualize bones and teeth. | IAN | 3D PETRA single-echo sequence; 3D DWI reversed fast imaging with steady-state precession (3D-PSIF); 3D T2 SPACE sequence with short tau inversion recovery (STIR) fat suppression; accelerated diffusion-tensor-imaging (DTI) prototype sequence (2D SMS-accelerated RESOLVE) | 3.0 T | 64-channel phased array coil | The image quality was excellent for all sequences using the suggested MR neurographic orthopantomogram. All sequences performed excellently in the anatomical delineation of the mandibular canal and IAN with high diagnostic confidence. In particular, the 3D PETRA and 3D PSIF provided a complete representation of the mandibular canal in all volunteers. |
| 20 | Cox et al., 2016, USA | Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: experience in a tertiary care centre | 17 | 14–69 years | Applicability of magnetic resonance neurography in the assessment of peripheral trigeminal neuropathies in patients with suspected trigeminal neuropathies (IAN and LN). | IAN + LN | CISS 3D sequence axial, T1 and T2 spectral adiabatic inversion recovery (SPAIR) sequences axial, DTI axial, 3D STIR SPACE coronal, 3D DW PSIF coronal | 1.5 T | Multichannel head coil | Excellent image quality could be achieved in the treatment of peripheral trigeminal neuropathy, by additionally showing moderate to excellent correlation with intraoperative results. |
| 21 | Kotaki et al., 2016, Japan | Diffusion tensor imaging of the inferior alveolar nerve using 3T MRI: a study for quantitative evaluation and fibre tracking | 46 | 20–36 years | Qualitative and quantitative visualization and fibre tracking of the IAN in healthy participants using diffusion tensor imaging. | IAN | DTI: SE-based single-shot EPI with fat suppression by STIR sequence, 3D T1 with MP-RAGE | 3.0 T | 16 channel neck and head coil | DTI is a feasible technique for quantitative analysis and imaging of the IAN, providing quantitative data of the diffusivity and anisotropy of microscopic water movement within nerve fibers while simultaneously obtaining three-dimensional visualization of the nerve fibers. |
| 22 | Probst et al., 2017, Germany | Magnetic resonance imaging of the inferior alveolar nerve with special regard to metal artifact reduction | 7 | N/A | Investigation of the capacities and limitations of IAN MRI in patients with IAN disruption with respect to metal artifacts. | IAN | WARP sequences, T2 TSE coronal, T2 TSE axial, T2 TSE Spectral Presaturation with Inversion Recovery (SPIR) parasagittal, T1 TSE parasagittal, T2 TSE parasagittal, 3D CISS triplanar reconstructions, 3D T1 volumetric interpolated breath-hold examination (VIBE) triplanar reconstructions | 1.5 T and 3.0 T | 12-channel head coil with an additional four-channel surface coil | Assessment of better image quality at 3.0 T. Achievement of average to excellent image quality of the IAN and its structural changes by using the WARP sequence taking into account the severity of metallic artifacts. The findings correlated with clinical symptoms. |
| 23 | Terumitsu et al., 2017, Japan | High-contrast high-resolution imaging of post-traumaticmandibular nerve by 3DAC-PROPELLER magneticresonance imaging: correlation with the severity of sensory disturbance | 19 | 27–62 years | Assessment of high-resolution magnetic resonance imaging of the IAN and LN after trauma-related injury and the correlation between the morphological classification and the degree of severity of the sensory disorder and morphologic characteristics of the surrounding connective tissue. | IAN + LN | 3DAC PROPELLER DWI pulse sequence | 3.0 T | Neurovascular coil or a custom 3-inch surface coil | Successful visualization of the internal structures within the lesion, delineation of the IAN and LN after traumatic injury and display of the morphologic features in relation with the severity of the patient’s sensory disturbance. |
| 24 | Dessouky et al., 2018, USA | Role of MR neurography for the diagnosis of peripheral trigeminal nerve injuries in patients with prior molar tooth extraction | 42 | N/A | Evaluation of the diagnostic accuracy of MR neurography for the assessment of damaged peripheral trigeminal nerves in patients with previous third molar extraction and its correlation with the intraoperative findings. | IAN + LN | 3D PSIF, T1, T2 fat suppressed, DWI | 1.5 T and 3.0 T | Multichannel head coil | Reliability and accuracy for the diagnosis of post-traumatic nerve injury after molar tooth extractions. Neuropathy could be determined by various qualitative and quantitative criteria. |
| 25 | Burian et al., 2019, Germany | MRI of the inferior alveolar nerve and lingual nerve—anatomical variation and morphometric benchmark values of nerve diameters in healthy subjects | 30 | 21–32 years | Examination of the reliability of various black bone MRI sequences for direct visualization of the IAN and LN in healthy volunteers. | IAN + LN | 3D STIR 3D DESS, 3D T1 FFE | 3.0 T | 16-channel Head Neck Spine Coil | Excellent feasibility of direct visualization of the complete course of the IAN and the proximal course of the LN. |
| 26 | Mazza et al., 2020, Italy | Appearance of normal MRI anatomy of the lingual nerve using steady-state free precession sequences at 3-T | 24 | 18–84 years | Application of a steady-state free precession (SSFP) MRI sequence to investigate the direct visualization of the LN. | LN | FIESTA | 3.0 T | Head and neck multiarray coil | Visualization of the LN throughout its course from the mandibular nerve to the mylohyoid muscle in excellent image quality. |
| 27 | Burian et al., 2020, Germany | High resolution MRi for quantitative assessment of inferior alveolar nerve impairment in course of mandible fractures: an imaging feasibility study | 30 | 16–57 years | Visualization of the IAN course in patients with unilateral mandibular fracture and healthy patients using different “black bone” MRI sequences. | IAN | 3D STIR, 3D DESS, 3D T1 FFE | 3.0 T | 16-channel head-neck-spine coil | Black bone MRI sequences generally allowed good visibility, with the 3D T1 FFE sequence and the 3D STIR sequence providing good visualization of the fracture and the IAN. |
| 28 | Wu et al., 2020, China | Visualization of the morphology and pathology of the peripheral branches of the cranial nerves using three-dimensional high-resolution high-contrast magnetic resonance neurography | 35 | 18–69 years | Visualization of the peripheral cranial nerves, including the IAN and LN in healthy and pathological volunteers. | IAN | T1 TSE axial, T2 TSE axial, T2 turbo inversion recovery magnitude (T2-TIRM) coronal, conventional (noncontrast enhanced) 3D SPACE STIR (cMRN), contrast enhanced T1-SPAIR and contrast enhanced 3D SPACE STIR (HRHCMRN) axial. | 3.0 T | head and neck coil | Very good visualization of the morphology of the IAN and LN and the pathologies affecting them. |
| 29 | Beck et al., 2021, Austria | Is MRI a viable alternative to CT/CBCT to identify the course of the inferior alveolar nerve in relation to the roots of the third molars? | 53 | N/A | Evaluation of the spatial relationship between the IAN and the mandibular third molar (MTM) using MRI or CT/CBCT images. | IAN | PD T2 TSE FS axial, PD TSE FS coronal | 3.0 T | 64 channel head-and-neck coil | Observation of good inter- and intra-rater concordance in evaluating the spatial relationship between IAN and MTM. In addition, MRI images provided benefits in the identification of accessory IAN in comparison to CT/CBCT. |
| 30 | Al-Haj Husain et al., 2021, Switzerland | Mandibular third molar surgery: intraosseous localization of the inferior alveolar nerve using 3D double-echo steady-state MRI (3D-DESS) | 19 | 18–63 years | Evaluation of the IAN’s intraosseous position within the mandibular canal’s osseous boundaries in patients undergoing third molar extraction treatment using preoperative cone-beam computed tomography and magnetic resonance imaging. Assessment of a conversion factor between the two imaging modalities. | IAN | 3D-DESS-WE | 3.0 T | 64 channel head-and-neck coil | Accurate simultaneous visualization of the nerve tissues within osseous boundaries. A conversion factor from IAC in CBCT and MRI to IAN in MRI was determined. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Haj Husain, A.; Solomons, M.; Stadlinger, B.; Pejicic, R.; Winklhofer, S.; Piccirelli, M.; Valdec, S. Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review. Diagnostics 2021, 11, 1657. https://doi.org/10.3390/diagnostics11091657
Al-Haj Husain A, Solomons M, Stadlinger B, Pejicic R, Winklhofer S, Piccirelli M, Valdec S. Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review. Diagnostics. 2021; 11(9):1657. https://doi.org/10.3390/diagnostics11091657
Chicago/Turabian StyleAl-Haj Husain, Adib, Mark Solomons, Bernd Stadlinger, Rada Pejicic, Sebastian Winklhofer, Marco Piccirelli, and Silvio Valdec. 2021. "Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review" Diagnostics 11, no. 9: 1657. https://doi.org/10.3390/diagnostics11091657
APA StyleAl-Haj Husain, A., Solomons, M., Stadlinger, B., Pejicic, R., Winklhofer, S., Piccirelli, M., & Valdec, S. (2021). Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review. Diagnostics, 11(9), 1657. https://doi.org/10.3390/diagnostics11091657






