Clinically Silent Small Vessel Disease of the Brain in Patients with Obstructive Sleep Apnea Hypopnea Syndrome
Abstract
:1. Introduction
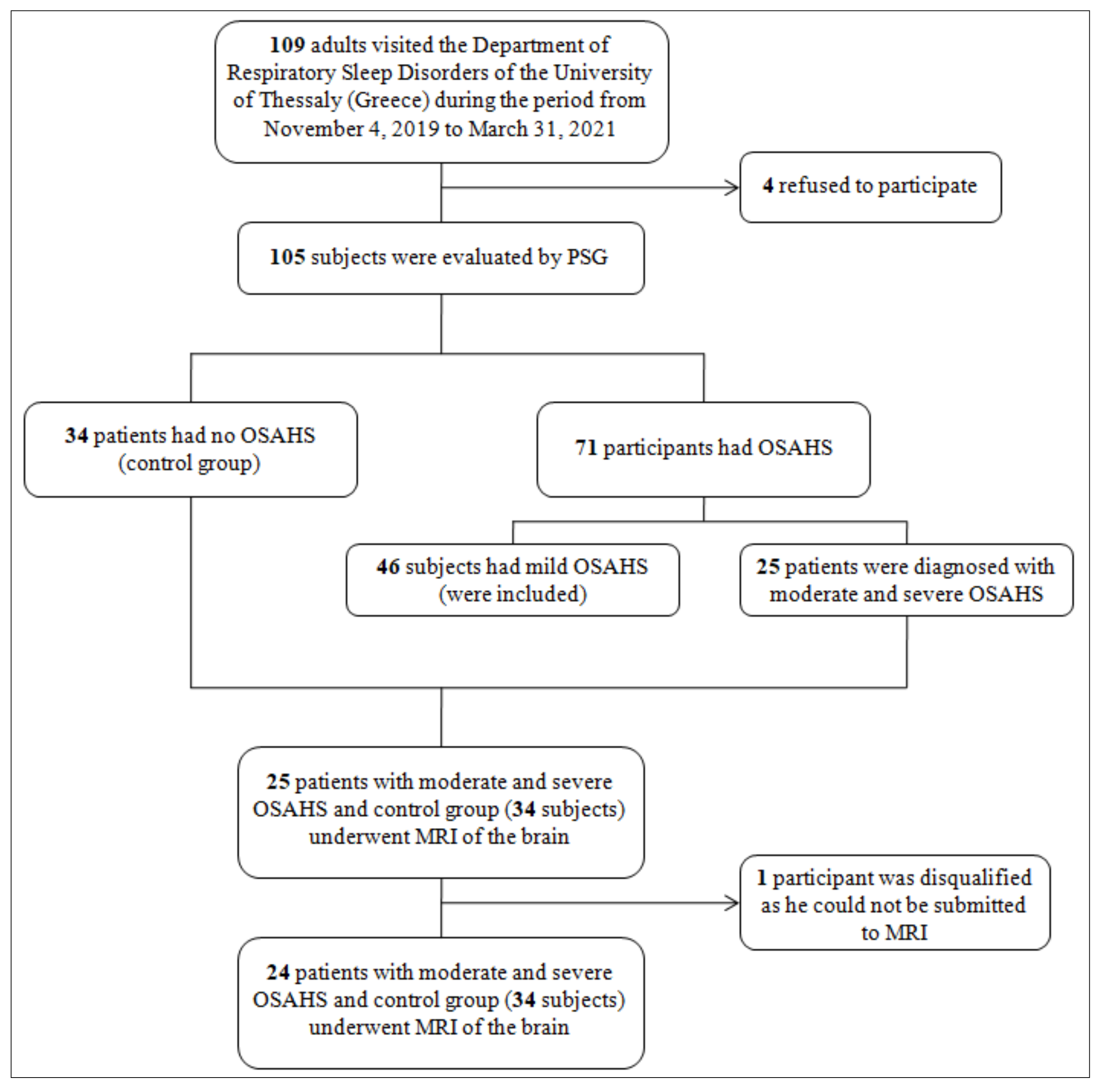
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethics
2.3. Polysomnography
2.4. MRI Brain Protocol and Assessment
2.5. Statistical Analysis
3. Results
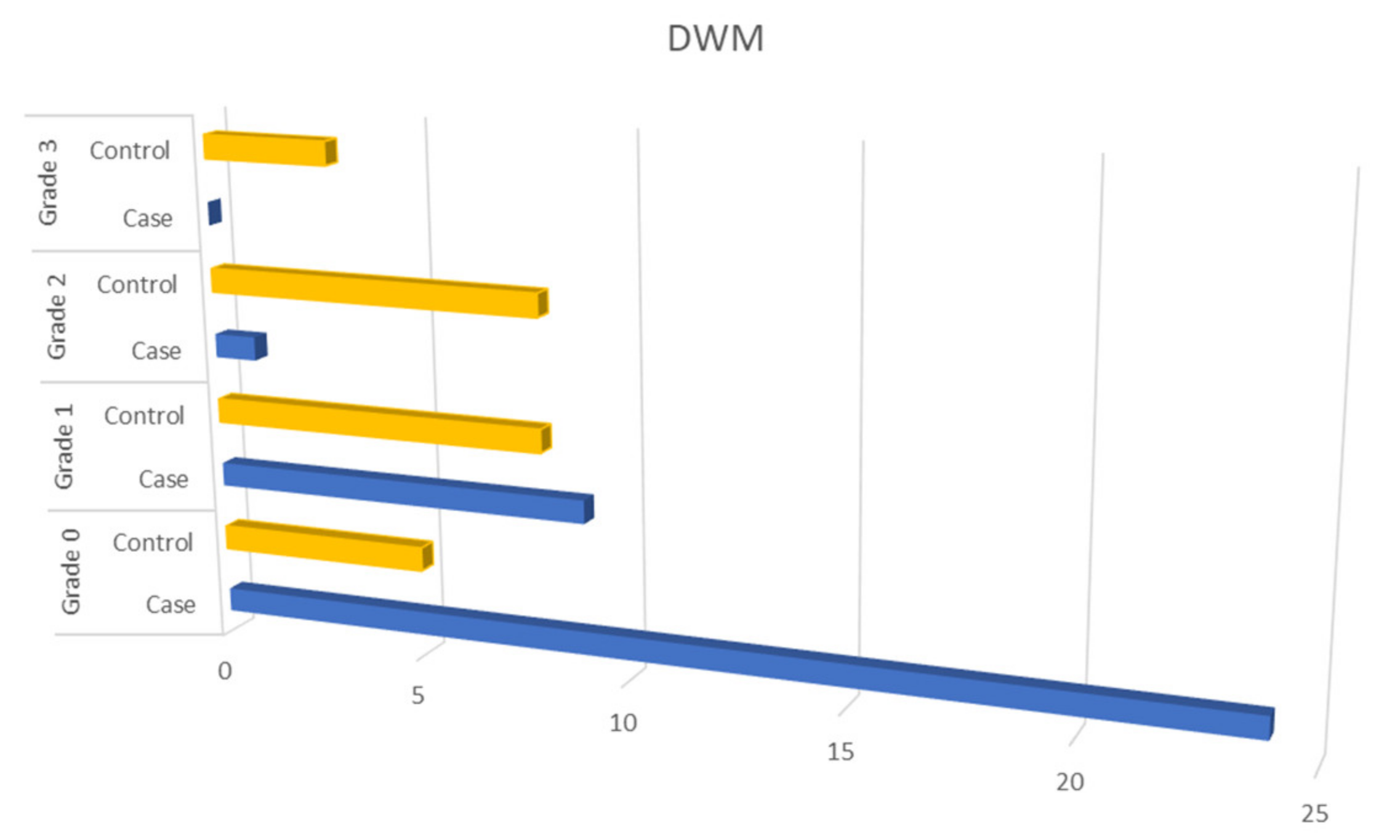
MRI Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar]
- Lévy, P.; Tamisier, R.; Minville, C.; Launois, S.; Pépin, J.L. Sleep apnoea syndrome in 2011: Current concepts and future directions. Eur. Respir. Rev. 2011, 20, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Durgan, D.J.; Bryan, R.M., Jr. Cerebrovascular consequences of obstructive sleep apnea. J. Am. Heart Assoc. 2012, 1, e000091. [Google Scholar] [CrossRef] [Green Version]
- Kepplinger, J.; Barlinn, K.; Boehme, A.K.; Gerber, J.; Puetz, V.; Pallesen, L.P.; Schrempf, W.; Dzialowski, I.; Albright, K.C.; Alexandrov, A.V.; et al. Association of sleep apnea with clinically silent microvascular brain tissue changes in acute cerebral ischemia. J. Neurol. 2014, 261, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Haula, T.M.; Puustinen, J.; Takala, M.; Holm, A. Wake-up strokes are linked to obstructive sleep apnea and worse early functional outcome. Brain Behav. 2021, 11, e2284. [Google Scholar] [CrossRef]
- Riglietti, A.; Fanfulla, F.; Pagani, M.; Lucini, D.; Malacarne, M.; Manconi, M.; Ferretti, G.; Esposito, F.; Cereda, C.W.; Pons, M. Obstructive and Central Sleep Apnea in First Ever Ischemic Stroke are Associated with Different Time Course and Autonomic Activation. Nat. Sci. Sleep 2021, 13, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet. Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO) The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004, 27 (Suppl. S1), 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, S.M.; Chaudhary, U.J.; Brown, M.M.; Yousry, T.A.; Kallis, C.; Jäger, H.R.; Werring, D.J. The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 2009, 73, 1759–1766. [Google Scholar] [CrossRef]
- Smith, E.E.; Saposnik, G.; Biessels, G.J.; Doubal, F.N.; Fornage, M.; Gorelick, P.B.; Greenberg, S.M.; Higashida, R.T.; Kasner, S.E.; Seshadri, S. American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; and Council on Hypertension. Prevention of Stroke in Patients with Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e44–e71. [Google Scholar]
- ZanonZotin, M.C.; Sveikata, L.; Viswanathan, A.; Yilmaz, P. Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management. Curr. Opin Neurol. 2021, 34, 246–257. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [PubMed]
- Chokesuwattanaskul, A.; Lertjitbanjong, P.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Mao, M.A.; Cheungpasitporn, W.; Chokesuwattanaskul, R. Impact of obstructive sleep apnea on silent cerebral small vessel disease: A systematic review and meta-analysis. Sleep Med. 2020, 68, 80–88. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Yuan, R.; Liu, M.; Hao, Z. Association of obstructive sleep apnea and cerebral small vessel disease: A systematic review and meta-analysis. Sleep 2020, 43, zsz264. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Park, J.H.; Choi, K.H.; Chang, Y.; Moon, J.; Kim, J.H.; Choi, Y.; Kim, Y.J.; Lee, H.W. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017, 30, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Norman, J.E.; Srinivasan, V.J.; Rutledge, J.C. Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am. J. Neurodegener Dis. 2016, 5, 171–177. [Google Scholar]
- Del Brutto, O.H.; Mera, R.M.; Zambrano, M.; Castillo, P.R. Relationship between obstructive sleep apnea and neuroimaging signatures of cerebral small vessel disease in community-dwelling older adults. The Atahualpa Project. Sleep Med. 2017, 37, 10–12. [Google Scholar] [CrossRef]
- van Dijk, E.J.; Prins, N.D.; Vermeer, S.E.; Vrooman, H.A.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. C-reactive protein and cerebral small-vessel disease: The Rotterdam Scan Study. Circulation 2005, 112, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozal, D.; Serpero, L.D.; Kheirandish-Gozal, L.; Capdevila, O.S.; Khalyfa, A.; Tauman, R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep 2010, 33, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8. [Google Scholar] [CrossRef]
- Lombardi, C.; Pengo, M.F.; Parati, G. Systemic hypertension in obstructive sleep apnea. J. Thorac. Dis. 2018, 10 (Suppl. S34), 4231–4243. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J. Am. Heart Assoc. 2015, 4, e002454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennum, P.; Børgesen, S.E. Intracranial pressure and obstructive sleep apnea. Chest 1989, 95, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, J.; Hajak, G.; Sander, D.; Schulz-Varszegi, M.; Rüther, E.; Conrad, B. Assessment of intracranial hemodynamics in sleep apnea syndrome. Stroke 1992, 23, 1427–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Sleep Parameters and Characteristics | OSAHS Patients (n = 24) |
|---|---|
| Age (years) | 58 ± 11.9 |
| Gender (Males) | 15 (62.5%) |
| BMI (Kg/m2) | 31.4 ± 6.2 |
| ESS | 9.9 ± 4.3 |
| Sleep hours | 7.3 ± 1.2 |
| S1 (%) | 4.9 ± 2.6 |
| S2 (%) | 63.3 ± 11.6 |
| S3–4 (%) | 10.1 ± 8.5 |
| REM (%) | 17.5 ± 28.4 |
| AI (events/h) | 16.3 ± 17.3 |
| HI (events/h) | 24.1 ± 13.4 |
| AHI (events/h) | 40.4 ± 21.1 |
| ODI (events/h) | 41.2 ± 23.2 |
| MinSpO2 (%) | 76.5 ± 12.8 |
| T < 90% (min) | 36.8 ± 65.6 |
| Mean heart rate (beats/min) | 68.1 ± 9.7 |
| Vascular Diseases | OSAHS Patients (n = 24) N (%) | Controls (n = 34) N (%) |
|---|---|---|
| Hypertension | 12 (50%) | 7 (20.6%) |
| Coronary artery disease | 4 (16.7%) | 1 (2.9%) |
| Atrial fibrillation | 2 (8.3%) | 1 (2.9%) |
| Diabetes | 4 (16.7%) | 0 (0%) |
| Pulmonary Embolism | 1 (4.2%) | 0 (0%) |
| Dyslipidemia | 14 (58.3%) | 3 (8.8%) |
| Stroke | 3 (12.5%) | 0 (0%) |
| Peripheral arterial disease | 1 (4.2%) | 0 (0%) |
| Independent Variable | OSAHS | Sig | aOR 95% CI | Adjusting (Univariate Analysis p < 0.1) | |
|---|---|---|---|---|---|
| Dependent variable | DWM | Grade:0 | ref | Age, gender, diabetes, dyslipidemia, hypertension, coronary artery disease, atrial fibrillation. | |
| Grade:1 | 0.033 | 8.66 (1.19–63.08) | |||
| Grade:2 | 0.862 | - | |||
| Grade:3 | 0.999 | - | |||
| PVWM | Grade:0 | ref | Age, gender, dyslipidemia, hypertension, coronary artery disease, atrial fibrillation, valvular heart disease. | ||
| Grade:1 | 0.002 | 104.98 (5.15–2141) | |||
| Grade:2 | 0.006 | 329.75 (5.32–204.38) | |||
| B-WMH | Yes/No | 0.053 | 15.07 (0.97–234.65) | Age, gender, dyslipidemia, hypertension, coronary artery disease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raptis, D.G.; Sinani, O.; Rapti, G.G.; Papanikolaou, A.; Dadouli, K.; Ntellas, P.; Kapsalaki, E.Z.; Malli, F.; Gourgoulianis, K.I.; Xiromerisiou, G. Clinically Silent Small Vessel Disease of the Brain in Patients with Obstructive Sleep Apnea Hypopnea Syndrome. Diagnostics 2021, 11, 1673. https://doi.org/10.3390/diagnostics11091673
Raptis DG, Sinani O, Rapti GG, Papanikolaou A, Dadouli K, Ntellas P, Kapsalaki EZ, Malli F, Gourgoulianis KI, Xiromerisiou G. Clinically Silent Small Vessel Disease of the Brain in Patients with Obstructive Sleep Apnea Hypopnea Syndrome. Diagnostics. 2021; 11(9):1673. https://doi.org/10.3390/diagnostics11091673
Chicago/Turabian StyleRaptis, Dimitrios G., Olga Sinani, Georgia G. Rapti, Aikaterini Papanikolaou, Katerina Dadouli, Panagiotis Ntellas, Eftychia Z. Kapsalaki, Foteini Malli, Konstantinos I. Gourgoulianis, and Georgia Xiromerisiou. 2021. "Clinically Silent Small Vessel Disease of the Brain in Patients with Obstructive Sleep Apnea Hypopnea Syndrome" Diagnostics 11, no. 9: 1673. https://doi.org/10.3390/diagnostics11091673
APA StyleRaptis, D. G., Sinani, O., Rapti, G. G., Papanikolaou, A., Dadouli, K., Ntellas, P., Kapsalaki, E. Z., Malli, F., Gourgoulianis, K. I., & Xiromerisiou, G. (2021). Clinically Silent Small Vessel Disease of the Brain in Patients with Obstructive Sleep Apnea Hypopnea Syndrome. Diagnostics, 11(9), 1673. https://doi.org/10.3390/diagnostics11091673








