Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HPV Genotyping
2.3. RNA Isolation from Plasma and RT-qPCR
2.4. Statistical Analyzes
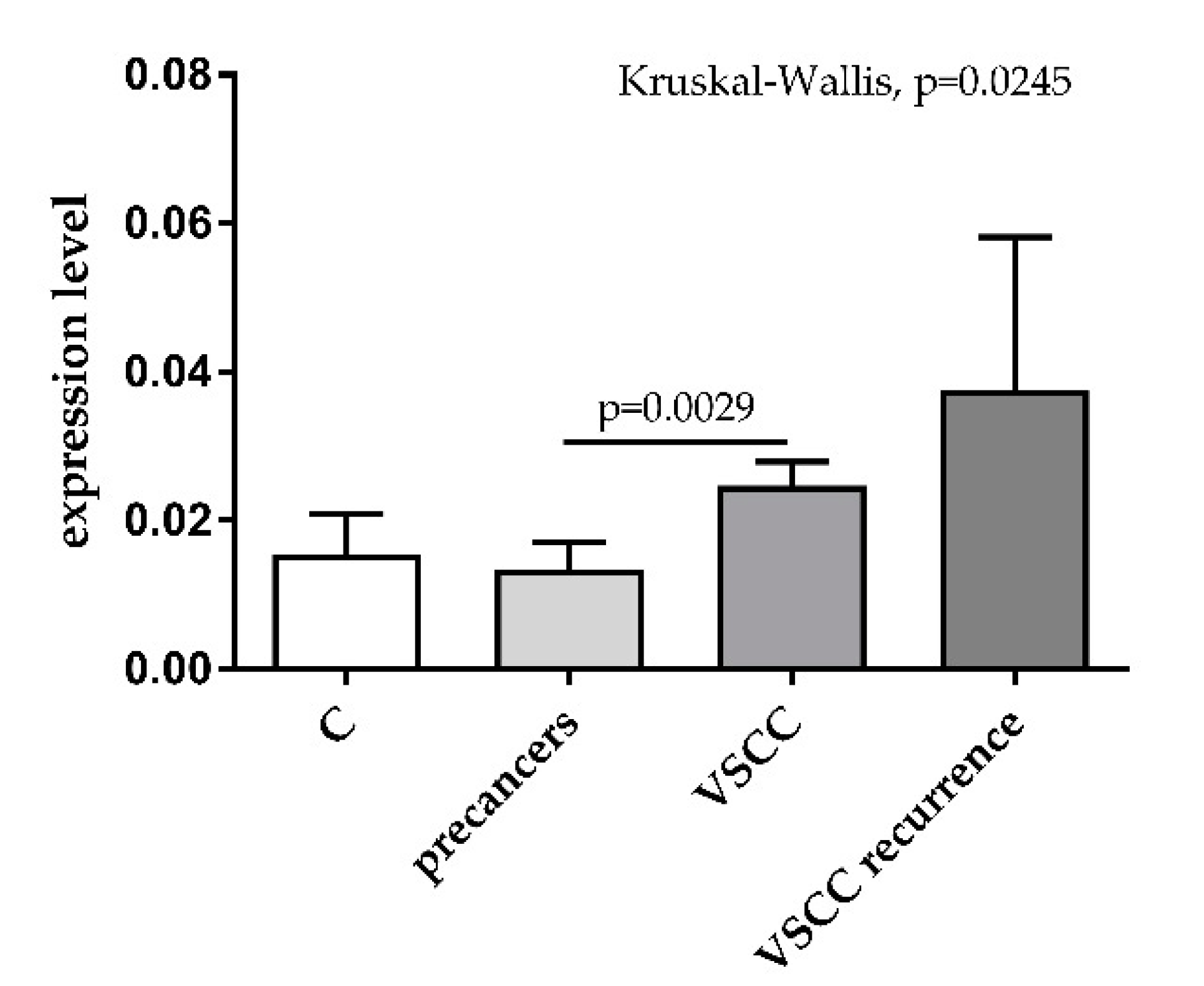
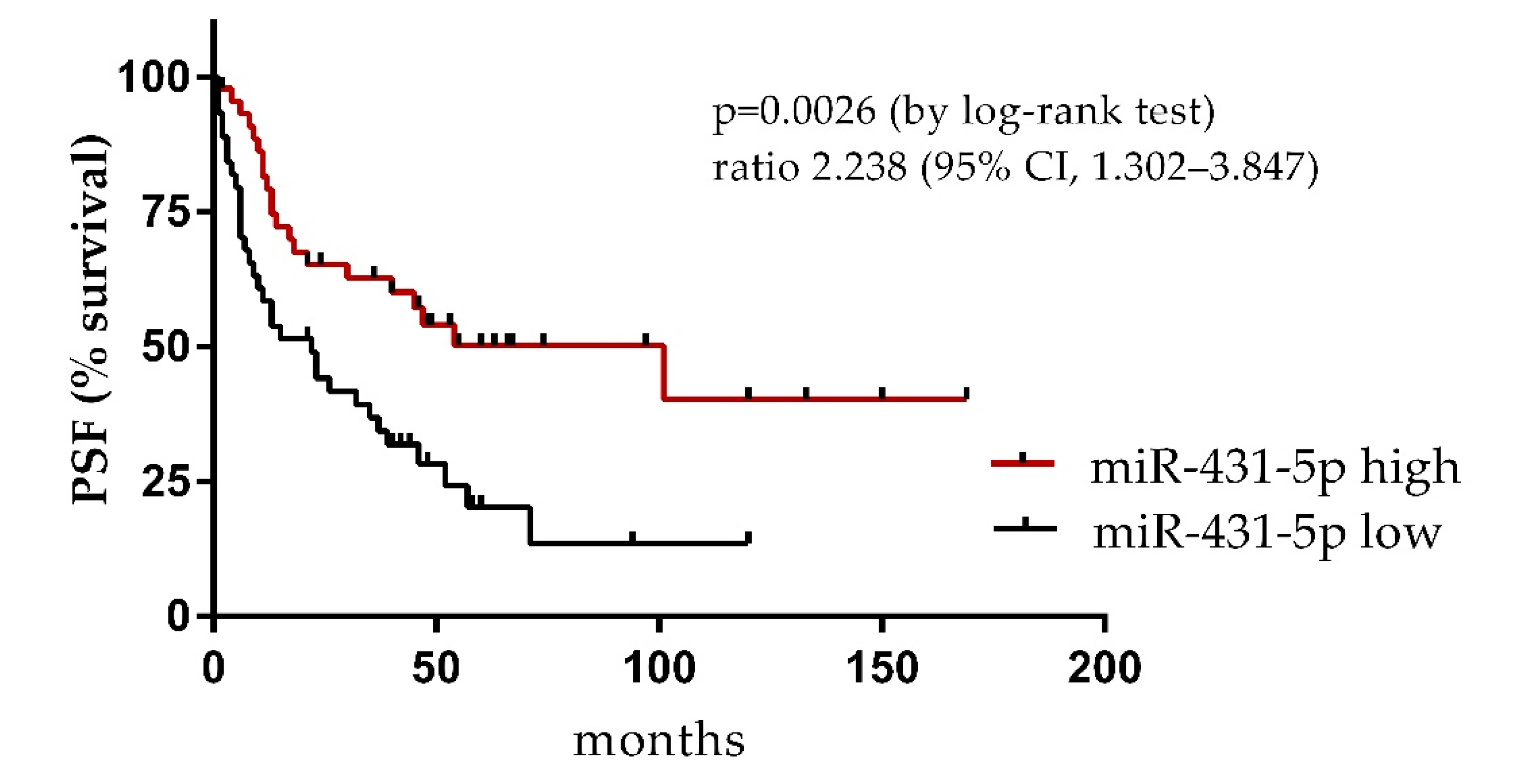
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojciechowska, U.; Didkowska, J.; Michałek, I.; Olasek, P.; Ciuba, A. Cancer in Poland in 2018; Maria Sklodowska-Curie National Research Institute of Oncology: Warsaw, Poland, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Vulvar Cancer. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 1 June 2021).
- Cohen, P.A.; Anderson, L.; Eva, L.; Scurry, J. Clinical and molecular classification of vulvar squamous pre-cancers. Int. J. Gynecol. Cancer 2019, 29, 821–828. [Google Scholar] [CrossRef]
- Van de Nieuwenhof, H.P.; Massuger, L.F.; van der Avoort, I.A.; Bekkers, R.L.; Casparie, M.; Abma, W.; van Kempen, L.C.L.T.; de Hullu, J.A. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur. J. Cancer 2009, 45, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Akerman, G.; Dussour, C.; Haddad, B.; Paniel, B.J.; Rouzier, R. Epidemiology of vulvar intra-epithelial neoplasias. Gynecol. Obstet. Fertil. 2007, 35, 1251–1256. [Google Scholar] [CrossRef]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Van Seters, M.; van Beurden, M.; de Craen, A.J. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol. Oncol. 2005, 97, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Chafe, W.; Richards, A.; Morgan, L.; Wilkinson, E. Unrecognized invasive carcinoma in vulvar intraepithelial neoplasia (VIN). Gynecol. Oncol. 1988, 31, 154–165. [Google Scholar] [CrossRef]
- Brinton, L.A.; Thistle, J.E.; Liao, L.M.; Trabert, B. Epidemiology of vulvar neoplasia in the NIH-AARP Study. Gynecol. Oncol. 2017, 145, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, S.K.D.; Rao, G.; Dey, A.; Mukherjee, P.; Wren, J.D.; Bhattacharya, R. Small Non-Coding-RNA in Gynecological Malignancies. Cancers 2021, 13, 1085. [Google Scholar] [CrossRef]
- Liolios, T.; Kastora, S.L.; Colombo, G. MicroRNAs in Female Malignancies. Cancer Inform. 2019, 18, 1176935119828746. [Google Scholar] [CrossRef] [Green Version]
- De Melo Maia, B.; Lavorato-Rocha, A.M.; Rodrigues, L.S.; Coutinho-Camillo, C.M.; Baiocchi, G.; Stiepcich, M.M.; Rocha, R.M. microRNA portraits in human vulvar carcinoma. Cancer Prev. Res. 2013, 6, 1231–1241. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wu, X. miRNA expression profile of vulvar squamous cell carcinoma and identification of the oncogenic role of miR-590-5p. Oncol. Rep. 2016, 35, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valihrach, L.; Androvic, P.; Kubista, M. Circulating miRNA analysis for cancer diagnostics and therapy. Mol. Asp. Med. 2020, 72, 100825. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Arai, M.; Jiang, X.; Sugaya, S.; Kanda, T.; Fujii, K.; Yokosuka, O. Downregulation of microRNA-431 by human interferon-beta inhibits viability of medulloblastoma and glioblastoma cells via upregulation of SOCS6. Int. J. Oncol. 2014, 44, 1685–1690. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Ren, F.; Rong, M.; Dang, Y.; Luo, Y.; Luo, D.; Chen, G. Correlation between down-expression of miR-431 and clinicopathological significance in HCC tissues. Clin. Transl. Oncol. 2015, 17, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Rapa, I.; Votta, A.; Felice, B.; Righi, L.; Giorcelli, J.; Scarpa, A.; Volante, M. Identification of MicroRNAs Differentially Expressed in Lung Carcinoid Subtypes and Progression. Neuroendocrinology 2015, 101, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Osaki, M.; Onuma, K.; Yumioka, T.; Iwamoto, H.; Sejima, T.; Okada, F. Identification of MicroRNAs Involved in Resistance to Sunitinib in Renal Cell Carcinoma Cells. Anticancer Res. 2017, 37, 2985–2992. [Google Scholar] [PubMed]
- Zieba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piascik, A.; Kowalewska, M. Somatic mutation profiling of vulvar cancer: Exploring therapeutic targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, K.; Misiek, M.; Kowalik, A.; Bakula-Zalewska, E.; Kopczynski, J.; Zielinska, A.; Kowalewska, M. Normalizers for microRNA quantification in plasma of patients with vulvar intraepithelial neoplasia lesions and vulvar carcinoma. Tumour Biol. 2017, 39, 1010428317717140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, M.N.; McMurray, H.R.; Land, H.; Almudevar, A. On non-detects in qPCR data. Bioinformatics 2014, 30, 2310–2316. [Google Scholar] [CrossRef]
- Duica, F.; Condrat, C.E.; Danila, C.A.; Boboc, A.E.; Radu, M.R.; Xiao, J.; Predescu, D.V. MiRNAs: A Powerful Tool in Deciphering Gynecological Malignancies. Front. Oncol. 2020, 10, 591181. [Google Scholar] [CrossRef]
- De Melo Maia, B.; Ling, H.; Monroig, P.; Ciccone, M.; Soares, F.A.; Calin, G.A.; Rocha, R.M. Design of a miRNA sponge for the miR-17 miRNA family as a therapeutic strategy against vulvar carcinoma. Mol. Cell. Probes 2015, 29, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Movahedi, M.; Rejali, M.; Maleki, F.; Moetamani-Ahmadi, M.; Seifi, S.; Avan, A. The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell. Physiol. 2019, 234, 1289–1294. [Google Scholar] [CrossRef]
- Hulstaert, E.; Morlion, A.; Levanon, K.; Vandesompele, J.; Mestdagh, P. Candidate RNA biomarkers in biofluids for early diagnosis of ovarian cancer: A systematic review. Gynecol. Oncol. 2021, 160, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Yuan, X.; Xu, N.; Zhao, S.; Hou, L.; Zhang, N. miR-431-5p regulates cell proliferation and apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis by targeting XIAP. Arthritis Res. Ther. 2020, 22, 231. [Google Scholar] [CrossRef]
- Jiang, Q.; Cheng, L.; Ma, D.; Zhao, Y. FBXL19-AS1 exerts oncogenic function by sponging miR-431-5p to regulate RAF1 expression in lung cancer. Biosci. Rep. 2019, 39, BSR20181804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zeng, C.; Hu, S.; Wang, L.; Liu, J. ATG3, a Target of miR-431-5p, Promotes Proliferation and Invasion of Colon Cancer via Promoting Autophagy. Cancer Manag. Res. 2019, 11, 10275–10285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.T.; Li, X.X.; Zeng, L.W. Circ_0001742 promotes tongue squamous cell carcinoma progression via miR-431-5p/ATF3 axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10300–10312. [Google Scholar] [PubMed]
- Kanaan, Z.; Roberts, H.; Eichenberger, M.R.; Billeter, A.; Ocheretner, G.; Pan, J.; Galandiuk, S. A plasma microRNA panel for detection of colorectal adenomas: A step toward more precise screening for colorectal cancer. Ann. Surg. 2013, 258, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Quan, L.; Liu, A. Identification of key microRNAs associated with diffuse large B-cell lymphoma by analyzing serum microRNA expressions. Gene 2018, 642, 205–211. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, Z.; Yuan, Q.; Lu, X. Downregulation of MiR-431 expression associated with lymph node metastasis and promotes cell invasion in papillary thyroid carcinoma. Cancer Biomark. 2018, 22, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Schubert, M.; Garbrecht, N.; Weigel, M.T.; Jonat, W.; Mundhenke, C.; Günther, V. Vulvar cancer: Epidemiology, clinical presentation, and management options. Int. J. Womens Health 2015, 7, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, F.; Forte, S.; Ardighieri, L.; Bonetti, E.; Fernando, B.; Sartori, E.; Odicino, F. Multivariate analysis of prognostic factors in primary squamous cell vulvar cancer: The role of perineural invasion in recurrence and survival. Eur. J. Surg. Oncol. 2019, 45, 2115–2119. [Google Scholar] [CrossRef] [PubMed]

| Patients Enrolled (n) | Median Age (Years) (Range) | FIGO | G | hrHPV Status | |
|---|---|---|---|---|---|
| HSIL | 21 | 57.1 (26.3–83.4) | |||
| dVIN | 8 | 50.4 (18.8–79.5) | |||
| Primary VSCC | 107 | 72.4 (37.3–94.2) | I n = 56 II n = 3 III n = 30 IV n = 3 N/A n = 6 | G1 n = 32 G2 n = 37 G3 n = 13 N/A n = 16 | negative n = 26 positive n = 44 N/A n = 28 |
| Recurrent VSCC | 9 | 74.8 (85.8–72.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujko, M.; Zalewski, K.; Szczyrek, M.; Kowalik, A.; Boresowicz, J.; Długosz, A.; Goryca, K.; Góźdź, S.; Kowalewska, M. Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions. Diagnostics 2021, 11, 1706. https://doi.org/10.3390/diagnostics11091706
Bujko M, Zalewski K, Szczyrek M, Kowalik A, Boresowicz J, Długosz A, Goryca K, Góźdź S, Kowalewska M. Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions. Diagnostics. 2021; 11(9):1706. https://doi.org/10.3390/diagnostics11091706
Chicago/Turabian StyleBujko, Mateusz, Kamil Zalewski, Martyna Szczyrek, Artur Kowalik, Joanna Boresowicz, Angelika Długosz, Krzysztof Goryca, Stanisław Góźdź, and Magdalena Kowalewska. 2021. "Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions" Diagnostics 11, no. 9: 1706. https://doi.org/10.3390/diagnostics11091706
APA StyleBujko, M., Zalewski, K., Szczyrek, M., Kowalik, A., Boresowicz, J., Długosz, A., Goryca, K., Góźdź, S., & Kowalewska, M. (2021). Circulating Hsa-miR-431-5p as Potential Biomarker for Squamous Cell Vulvar Carcinoma and Its Premalignant Lesions. Diagnostics, 11(9), 1706. https://doi.org/10.3390/diagnostics11091706






