Sequential Organ Failure Assessment Outperforms Quantitative Chest CT Imaging Parameters for Mortality Prediction in COVID-19 ARDS
Abstract
:1. Introduction
2. Materials and Methods
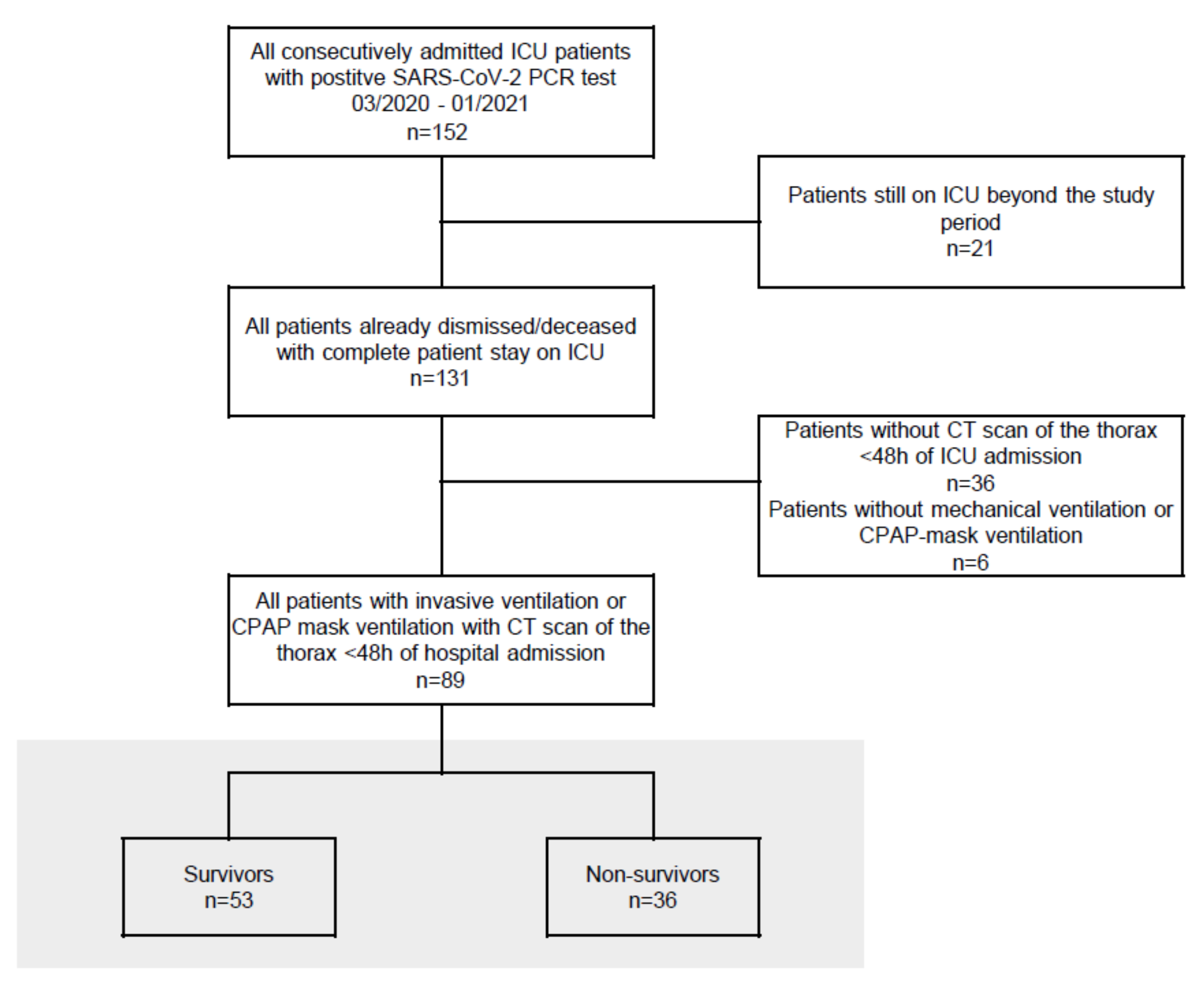
2.1. Patient Data
2.2. Image Acquisition
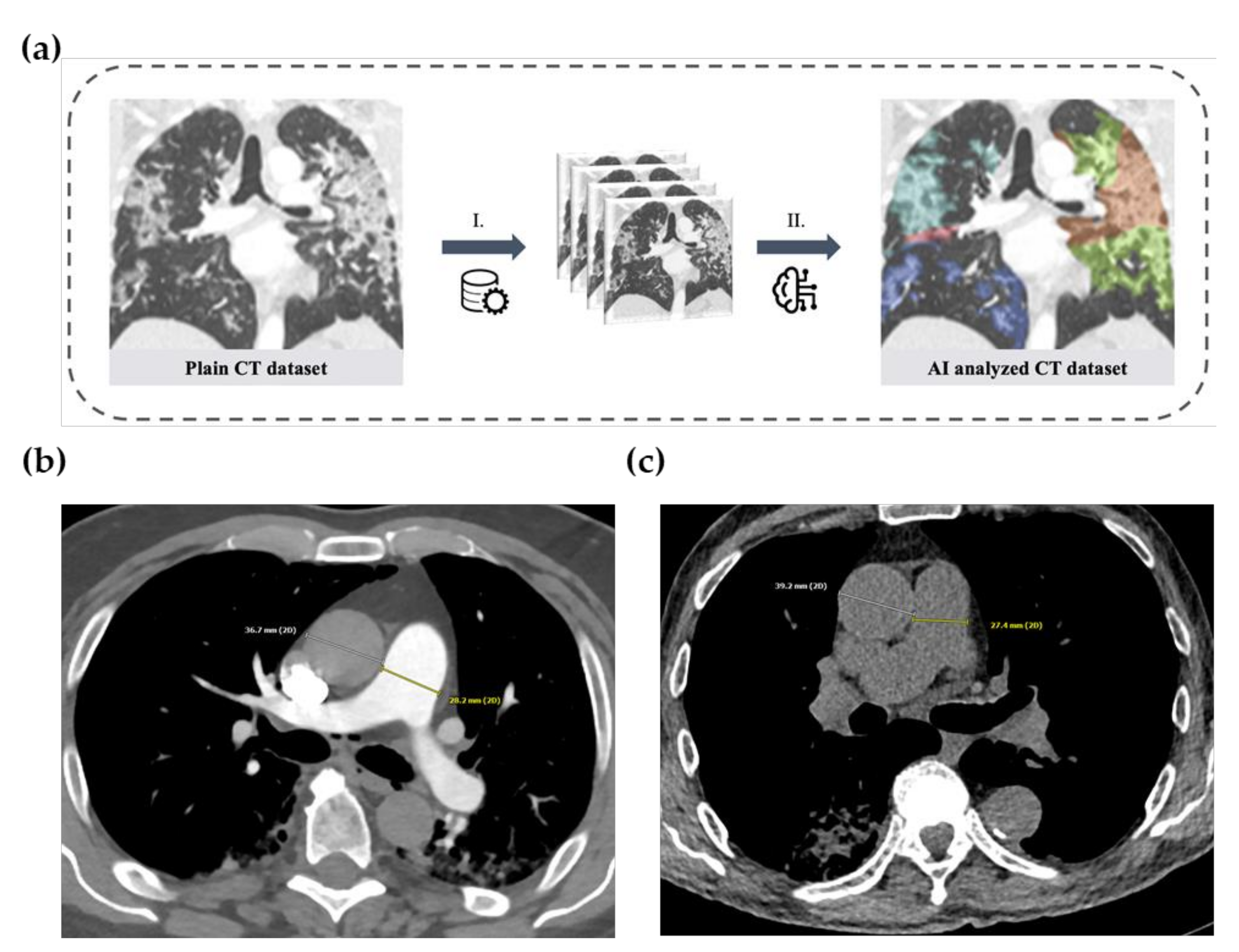
2.3. Artificial-Intelligence-Based Quantification of Lung Involvement
2.4. Prediction Parameters for the Regression Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics and Demographic Data
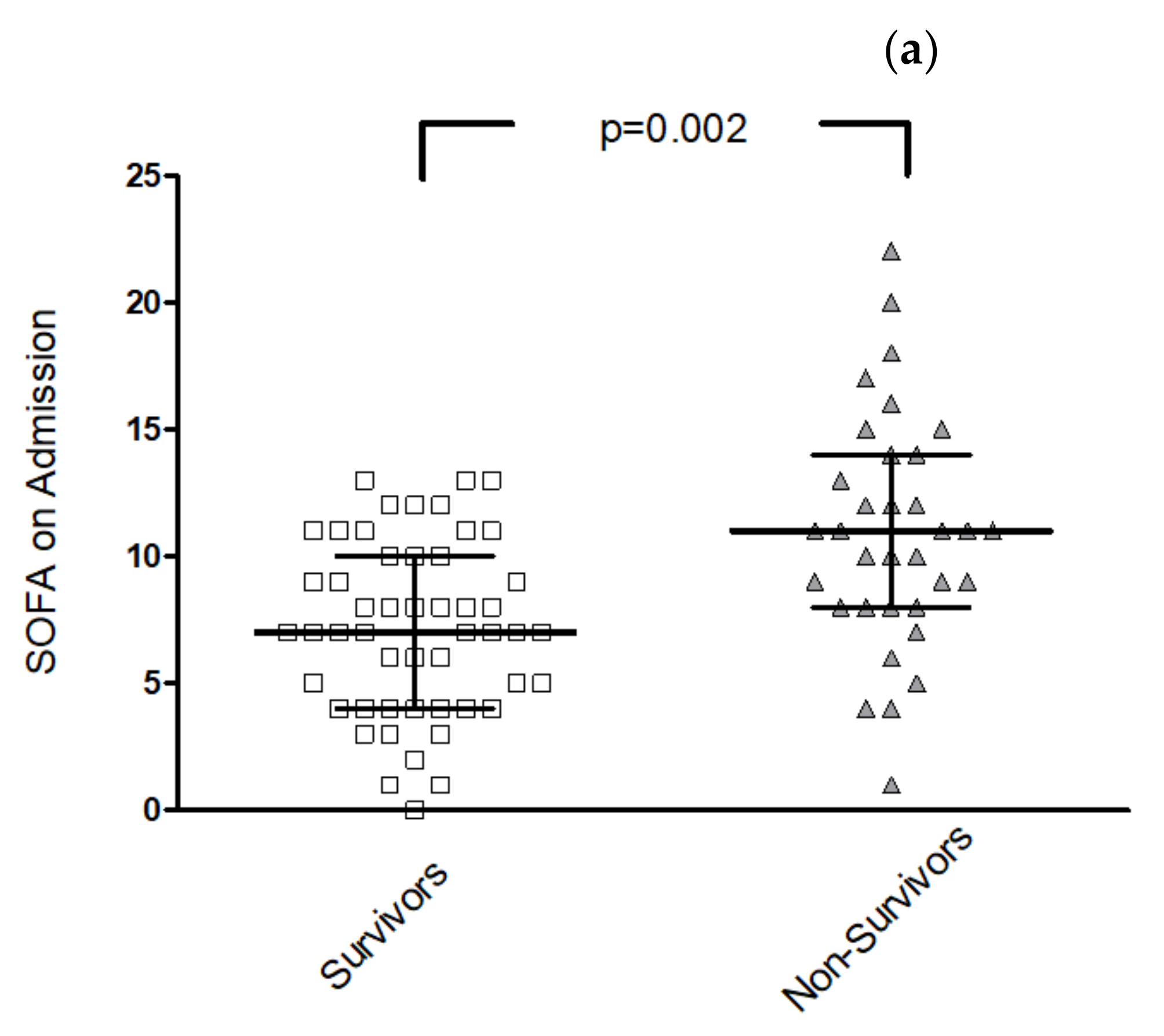
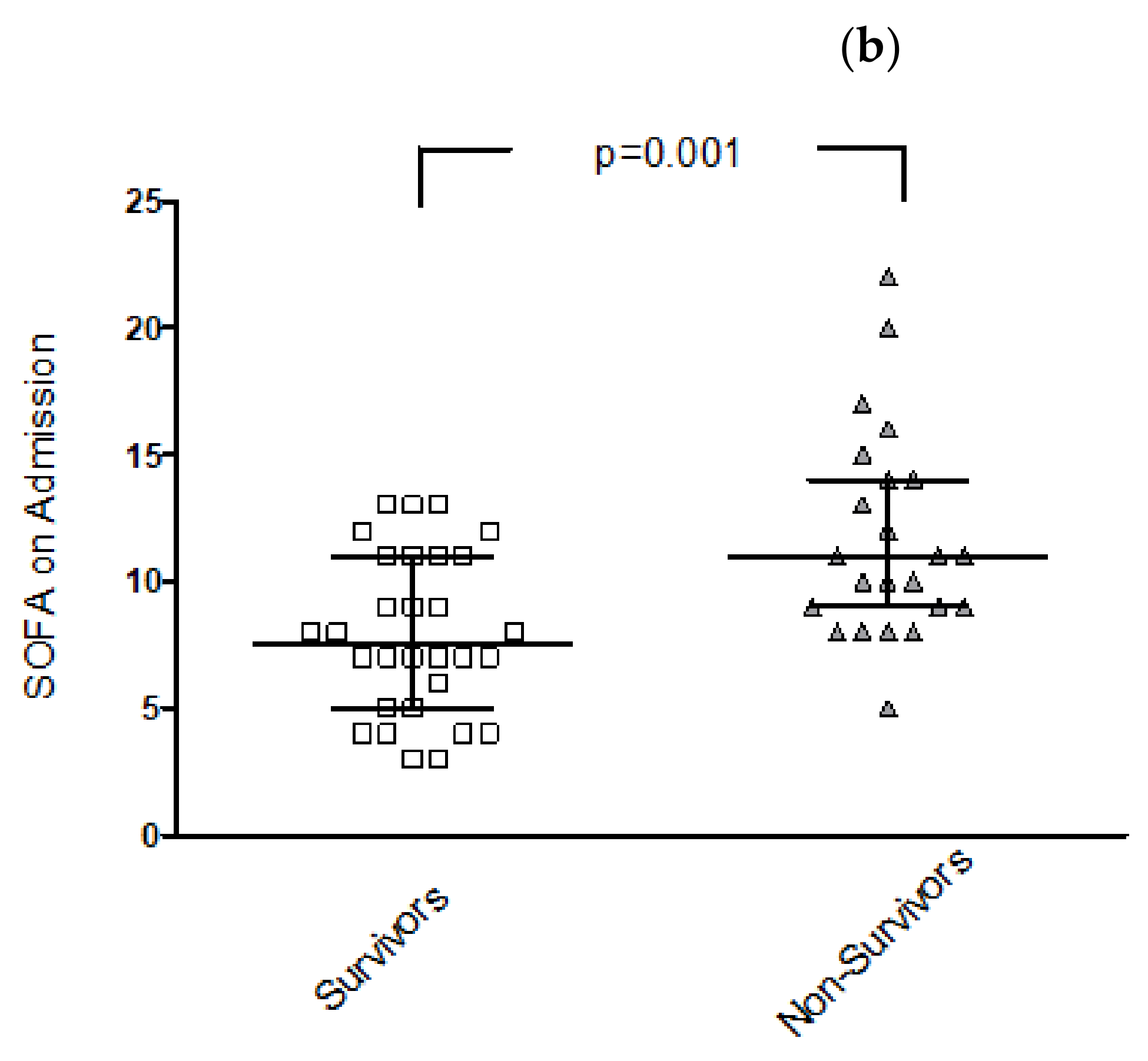
3.2. Differences in Clinical and Imaging Parameters for Survivors vs. Non-Survivors
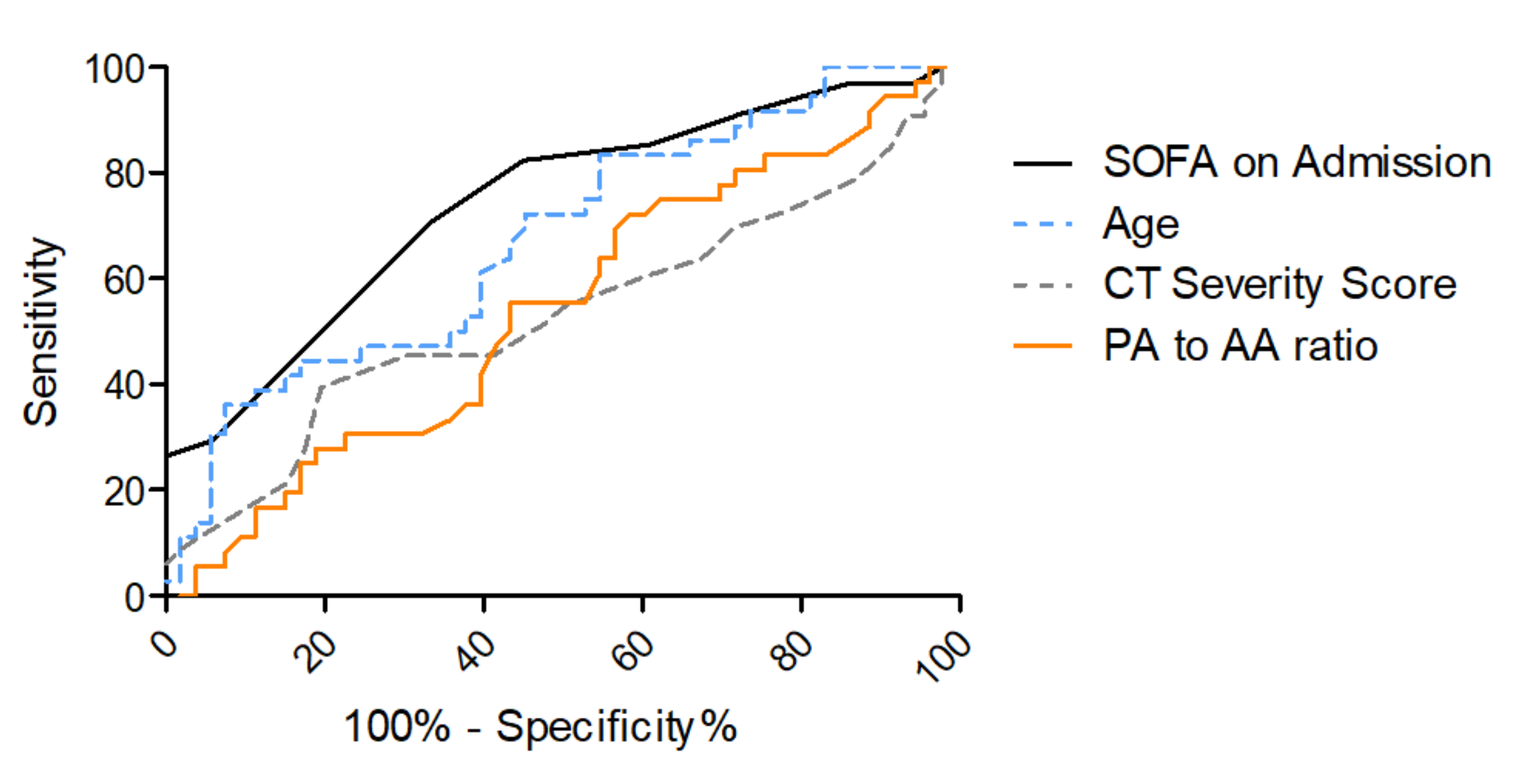
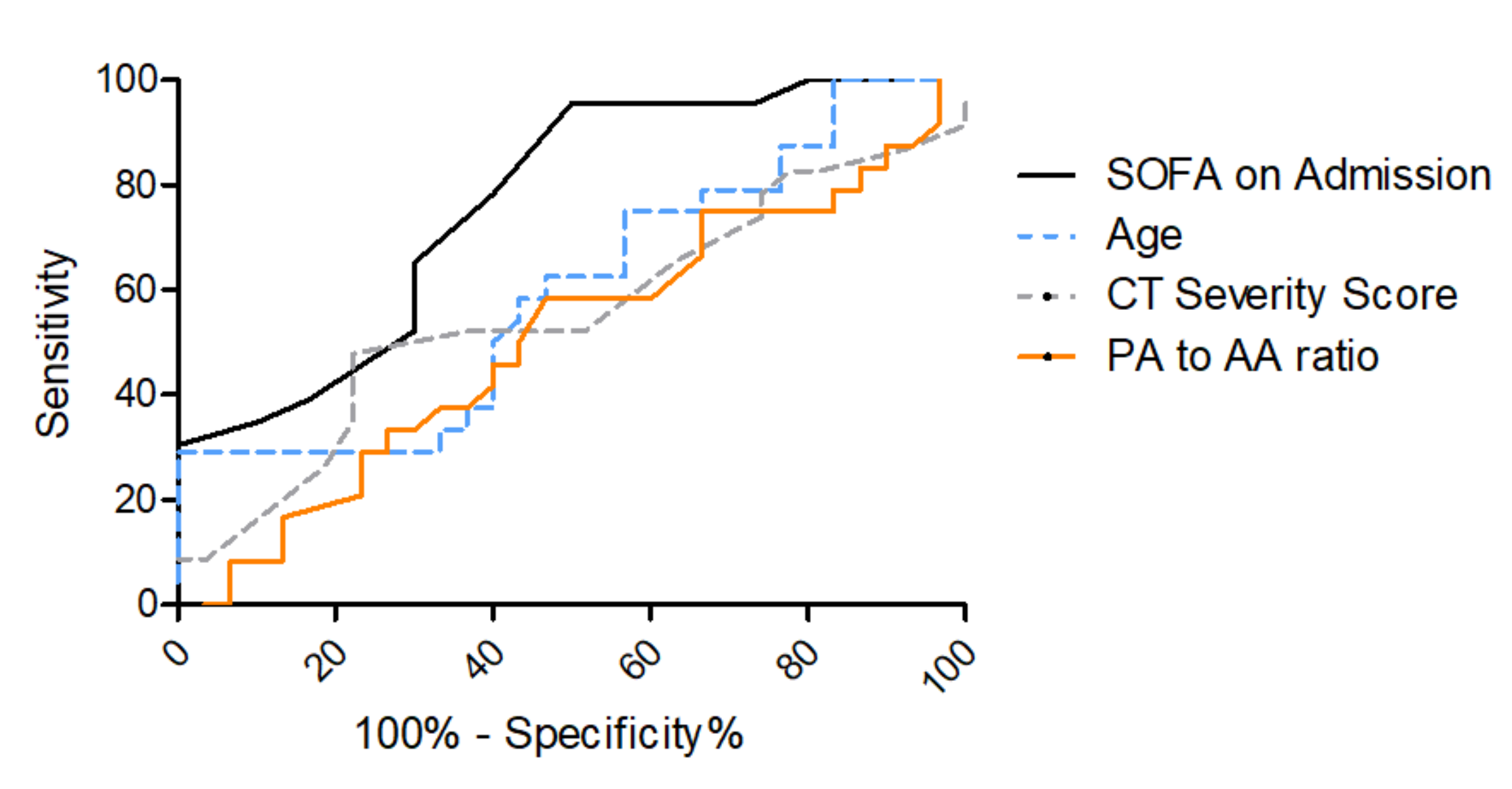
3.3. Risk Stratification for In-Hospital Mortality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The Socio-Economic Implications of the Coronavirus Pandemic (COVID-19): A Review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829. [Google Scholar] [CrossRef]
- Contreras, S.; Priesemann, V. Risking Further COVID-19 Waves despite Vaccination. Lancet Infect. Dis. 2021, 21, 745–746. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, M.; Dong, X.; Zhang, J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.; Fu, W.; Li, W.; et al. Risk Factors for Severe and Critically Ill COVID-19 Patients: A Review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in Patients with COVID-19: A Systematic Review and Meta-Analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case Characteristics, Resource Use, and Outcomes of 10,021 Patients with COVID-19 Admitted to 920 German Hospitals: An Observational Study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Abate, S.M.; Ahmed Ali, S.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit Admission and Outcomes among Patients with Coronavirus: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef] [PubMed]
- Immovilli, P.; Morelli, N.; Antonucci, E.; Radaelli, G.; Barbera, M.; Guidetti, D. COVID-19 Mortality and ICU Admission: The Italian Experience. Crit. Care 2020, 24, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected with COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality among Patients with COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Song, J.; Deane, A.M.; Plummer, M.P. Global Impact of Coronavirus Disease 2019 Infection Requiring Admission to the ICU. Chest 2021, 159, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from Intensive Care in Patients with COVID-19: A Systematic Review and Meta-analysis of Observational Studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Lai, C.-C.; Yeh, Y.-P.; Chang-Chuan, C.; Chen, H.-H. Progression from Pneumonia to ARDS as a Predictor for Fatal COVID-19. J. Infect. Public Health 2021, 14, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.A.; Zaidi, S.T.R. Mortality in COVID-19 Patients with Acute Respiratory Distress Syndrome and Corticosteroids Use: A Systematic Review and Meta-Analysis. Expert Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.-J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, Q.; Yao, S.; Luo, L.; Zhou, W.; Mao, X.; Li, J.; Duan, J.; Yan, Z.; Yang, M.; et al. Early Prediction of Disease Progression in COVID-19 Pneumonia Patients with Chest CT and Clinical Characteristics. Nat. Commun. 2020, 11, 4968. [Google Scholar] [CrossRef]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative Chest CT Analysis in COVID-19 to Predict the Need for Oxygenation Support and Intubation. Eurt. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.H.; Wani, A.H.; Shah, N.N.; Yaseen, M.; Jehangir, M. Chest CT Features of Coronavirus Disease-19 (COVID-19) Pneumonia: Which Findings on Initial CT Can Predict an Adverse Short-Term Outcome? BJR Open 2020, 2, 20200016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N.; Fang, C.; Shan, F.; Mei, X.; Shi, J.; et al. CT Quantification of Pneumonia Lesions in Early Days Predicts Progression to Severe Illness in a Cohort of COVID-19 Patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investig. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gresser, E.; Rueckel, J.; Puhr-Westerheide, D.; Schwarze, V.; Fink, N.; Kunz, W.G.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; Ingrisch, M.; et al. Prognostic Value of Admission Chest CT Findings for Invasive Ventilation Therapy in COVID-19 Pneumonia. Diagnostics 2020, 10, 1108. [Google Scholar] [CrossRef]
- Gresser, E.; Reich, J.; Sabel, B.O.; Kunz, W.G.; Fabritius, M.P.; Rübenthaler, J.; Ingrisch, M.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; et al. Risk Stratification for ECMO Requirement in COVID-19 ICU Patients Using Quantitative Imaging Features in CT Scans on Admission. Diagnostics 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Caruso Neves, C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef]
- Edwards, P.D.; Bull, R.K.; Coulden, R. CT Measurement of Main Pulmonary Artery Diameter. BJR 1998, 71, 1018–1020. [Google Scholar] [CrossRef]
- Chan, A.L.; Juarez, M.M.; Shelton, D.K.; MacDonald, T.; Li, C.-S.; Lin, T.-C.; Albertson, T.E. Novel Computed Tomographic Chest Metrics to Detect Pulmonary Hypertension. BMC Med. Imaging 2011, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Smits, H.; Knoops, A.J.G.; Korst, M.B.J.M.; Samson, T.; Scholten, E.T.; Schalekamp, S.; Schaefer-Prokop, C.M.; Philipsen, R.H.H.M.; Meijers, A.; et al. COVID-19 on Chest Radiographs: A Multireader Evaluation of an Artificial Intelligence System. Radiology 2020, 296, E166–E172. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Raschke, R.A.; Agarwal, S.; Rangan, P.; Heise, C.W.; Curry, S.C. Discriminant Accuracy of the SOFA Score for Determining the Probable Mortality of Patients with COVID-19 Pneumonia Requiring Mechanical Ventilation. JAMA 2021, 325, 1469–1470. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.C.; Dewaswala, N.; Tuarez, F.R.; Pino, J.; Chait, R.; Chen, K.; Reddy, R.; Abdallah, A.; AL Abbasi, B.; Torres, P.; et al. Validation of sofa score in critically ill patients with COVID-19. Chest 2020, 158, A613. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical Characteristics and Day-90 Outcomes of 4244 Critically Ill Adults with COVID-19: A Prospective Cohort Study. Intensive Care Med. 2021, 47, 60–73. [CrossRef]
- The PROGRESS Study Group; Ahnert, P.; Creutz, P.; Horn, K.; Schwarzenberger, F.; Kiehntopf, M.; Hossain, H.; Bauer, M.; Brunkhorst, F.M.; Reinhart, K.; et al. Sequential Organ Failure Assessment Score Is an Excellent Operationalization of Disease Severity of Adult Patients with Hospitalized Community Acquired Pneumonia—Results from the Prospective Observational PROGRESS Study. Crit. Care 2019, 23, 110. [Google Scholar] [CrossRef] [Green Version]
| COVID-19 ICU-Patients (n = 89) | |||
|---|---|---|---|
| Patient Data | |||
| Age | 65 | (53–73) | |
| Male Sex | 70 | (78.7%) | |
| Body Mass Index | 27 | (25–33) | |
| SOFA Score on Admission * | 8 | (6–11) | |
| Lactate on Admission | 1.3 | (1.0–1.8) | |
| Oxygenation Index on Admission ** | 168 | (110–226) | |
| Comorbidities | |||
| Diabetes | 31 | (34.4%) | |
| Hypertension | 55 | (61.1%) | |
| Heart Disease | 31 | (34.4%) | |
| Pulmonary Disease | 16 | (17.8%) | |
| Chronic Kidney Disease | 9 | (10.1%) | |
| Active Malignancy | 9 | (10.1%) | |
| Immunosuppression | 7 | (7.8%) | |
| ARDS Type on Admission *** | |||
| Mild | 24 | (29.6%) | |
| Moderate | 39 | (48.1%) | |
| Severe | 15 | (18.5%) | |
| No ARDS on Admission | 3 | (3.7%) | |
| CT Features on Admission | |||
| CT-Severity Score **** | 15 | (11–20) | |
| CT-Percentage of Lung Involvement **** | 36 | (20–57) | |
| Pulmonary artery to ascending aorta ratio | 0.86 | (0.78–0.94) | |
| In-Hospital-Mortality | |||
|---|---|---|---|
| Independent Variables | Odds Ratio | CI | p Value |
| Age | 1.067 | 1.004–1.134 | 0.036 * |
| Sex | 0.231 | 0.048–1.118 | 0.069 |
| BMI | 1.044 | 0.936–1.164 | 0.444 |
| SOFA on Admission | 1.409 | 1.171–1.696 | <0.001 * |
| CT Severity Score on Admission | 1.046 | 0.941–1.163 | 0.402 |
| PA-to-AA Ratio | 0.086 | 0.001–12.934 | 0.337 |
| N = 53 Survivors, N = 36 Non-Survivors | ||||||
|---|---|---|---|---|---|---|
| Survivors (n = 51) vs. Non-Survivors (n = 34) | AUC (95% CI) | Y-Index | Discriminative Value | Sensitivity | Specificity | |
| SOFA Score on Admission | 0.74 | 0.63–0.85 | 0.37 | 7.5 | 0.82 | 0.55 |
| Survivors (n = 53) vs. Non-Survivors (n = 36) | ||||||
| Age | 0.68 | 0.56–0.79 | 0.26 | 57.7 | 0.83 | 0.45 |
| N = 30 Survivors, N = 23 Non-Survivors | ||||||
|---|---|---|---|---|---|---|
| Survivors (n = 30) vs. Non-Survivors (n = 23) | AUC (95% CI) | Y-Index | Discriminative Value | Sensitivity | Specificity | |
| SOFA Score on Admission | 0.77 | 0.64–0.89 | 0.46 | 7.5 | 0.96 | 0.50 |
| Survivors (n = 30) vs. Non-Survivors (n = 24) | ||||||
| Age | 0.60 | 0.44–0.75 | 0.29 | 57.4 | 0.29 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puhr-Westerheide, D.; Reich, J.; Sabel, B.O.; Kunz, W.G.; Fabritius, M.P.; Reidler, P.; Rübenthaler, J.; Ingrisch, M.; Wassilowsky, D.; Irlbeck, M.; et al. Sequential Organ Failure Assessment Outperforms Quantitative Chest CT Imaging Parameters for Mortality Prediction in COVID-19 ARDS. Diagnostics 2022, 12, 10. https://doi.org/10.3390/diagnostics12010010
Puhr-Westerheide D, Reich J, Sabel BO, Kunz WG, Fabritius MP, Reidler P, Rübenthaler J, Ingrisch M, Wassilowsky D, Irlbeck M, et al. Sequential Organ Failure Assessment Outperforms Quantitative Chest CT Imaging Parameters for Mortality Prediction in COVID-19 ARDS. Diagnostics. 2022; 12(1):10. https://doi.org/10.3390/diagnostics12010010
Chicago/Turabian StylePuhr-Westerheide, Daniel, Jakob Reich, Bastian O. Sabel, Wolfgang G. Kunz, Matthias P. Fabritius, Paul Reidler, Johannes Rübenthaler, Michael Ingrisch, Dietmar Wassilowsky, Michael Irlbeck, and et al. 2022. "Sequential Organ Failure Assessment Outperforms Quantitative Chest CT Imaging Parameters for Mortality Prediction in COVID-19 ARDS" Diagnostics 12, no. 1: 10. https://doi.org/10.3390/diagnostics12010010
APA StylePuhr-Westerheide, D., Reich, J., Sabel, B. O., Kunz, W. G., Fabritius, M. P., Reidler, P., Rübenthaler, J., Ingrisch, M., Wassilowsky, D., Irlbeck, M., Ricke, J., & Gresser, E. (2022). Sequential Organ Failure Assessment Outperforms Quantitative Chest CT Imaging Parameters for Mortality Prediction in COVID-19 ARDS. Diagnostics, 12(1), 10. https://doi.org/10.3390/diagnostics12010010







