Metabolic Tumour Volume as a Predictor of Survival for Sinonasal Tract Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Parameters
2.2. F18-FDG Uptake Parameters
2.3. Statistical Analysis
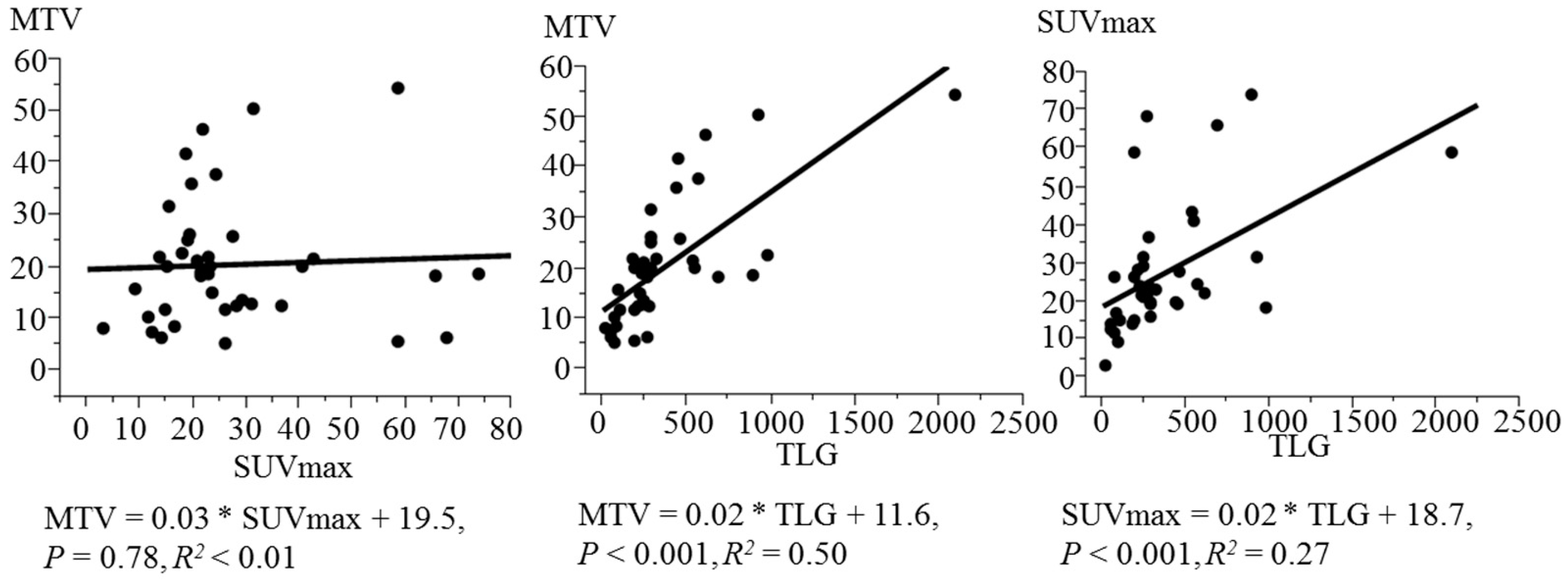
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bracigliano, A.; Tatangelo, F.; Perri, F.; Di Lorenzo, G.; Tafuto, R.; Ottaiano, A.; Clemente, O.; Barretta, M.; Losito, N.; Santorsola, M.; et al. Malignant Sinonasal Tumors: Update on Histological and Clinical Management. Curr. Oncol. 2021, 28, 2420–2438. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, P.; Merlotti, A.; Olmetto, E.; Bianchi, A.; Desideri, I.; Bacigalupo, A.; Franco, P.; Franzese, C.; Orlandi, E.; Livi, L.; et al. What is the prognostic impact of FDG PET in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2122–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creff, G.; Devillers, A.; Depeursinge, A.; Palard-Novello, X.; Acosta, O.; Jegoux, F.; Castelli, J. Evaluation of the prognostic value of FDG PET/CT parameters for patients with surgically treated head and neck Cancer: A systematic review. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Ziai, P.; Hayeri, M.R.; Salei, A.; Salavati, A.; Houshmand, S.; Alavi, A.; Teytelboym, O.M. Role of Optimal Quantification of FDG PET Imaging in the Clinical Practice of Radiology. Radiographics 2016, 36, 481–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Nishio, M.; Nakanishi, H.; Hanai, N.; Hirakawa, H.; Kodaira, T.; Tamaki, T.; Hasegawa, Y. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol. Lett. 2016, 12, 1493–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujima, N.; Hirata, K.; Shiga, T.; Yasuda, K.; Onimaru, R.; Tsuchiya, K.; Kano, S.; Mizumachi, T.; Homma, A.; Kudo, K.; et al. Semi-quantitative analysis of pre-treatment morphological and intratumoral characteristics using 18F-fluorodeoxyglucose positron-emission tomography as predictors of treatment outcome in nasal and paranasal squamous cell carcinoma. Quant. Imaging Med. Surg. 2018, 8, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Fujiwara, M.; Kitajima, K.; Tanooka, M.; Terada, T.; Noguchi, K.; Ishikura, R.; Kamikonya, N.; Yamakado, K. Clinical T staging is superior to fluorodeoxyglucose positron emission tomography for predicting local outcomes after intra-arterial infusion chemoradiotherapy for maxillary sinus squamous cell carcinoma. Nagoya J. Med Sci. 2018, 80, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Pak, K.; Yi, K.-I.; Kim, I.J.; Roh, H.-J.; Cho, K.-S. Prognostic value of tumoral heterogeneity and volumetric parameters as measured by F18-FDG PET/CT in sinonasal cancer. Eur. Arch. Oto-Rhino-Laryngol. 2016, 274, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hanai, N.; Nishikawa, D.; Fukuda, Y.; Koide, Y.; Kodaira, T.; Tachibana, H.; Tomita, N.; Makita, C.; Hasegawa, Y. The Charlson comorbidity index is a prognostic factor in sinonasal tract squamous cell carcinoma. Jpn. J. Clin. Oncol. 2016, 46, 646–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Kimura, K.; Kodaira, T.; Tachibana, H.; Tomita, N.; Koide, Y.; Tanaka, H.; Nishikawa, D.; Koide, Y.; Beppu, S.; et al. Gross tumor volume in sinonasal tract cancer as a predictor of local recurrence after chemoradiotherapy. Jpn. J. Clin. Oncol. 2018, 48, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Batsakis, J.G.; Rice, D.H.; Solomon, A.R. The pathology of head and neck tumors: Squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg. 1980, 2, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Gencturk, M.; Caicedo-Granados, E.; Li, F.; Cayci, Z. Prediction of survival with combining quantitative18F-FDG PET/CT and DW-MRI parameters in sinonasal malignancies. Head Neck 2019, 41, 3080–3089. [Google Scholar] [CrossRef] [PubMed]
- Seol, J.-H.; Kim, J.; Lee, J.-D.; Lee, J.-G.; Yoon, J.-H.; Kim, C.-H. The Feasibility of 18F-fluorodeoxyglucose-positron Emission Tomography Uptake as a Prognostic Factor for Paranasal Sinus Malignancy. Am. J. Rhinol. Allergy 2013, 27, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.; Cheon, G.J.; Nam, H.-Y.; Kim, S.-J.; Kang, K.W.; Chung, J.-K.; Kim, E.E.; Lee, D.S. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristic | n | SUVmax | MTV | TLG | |
|---|---|---|---|---|---|
| Age | Per 1 year | 39 | 27.4 ± 16.8 | 20.4 ± 12.4 | 373.2 ± 373.0 |
| p value a | 0.90 | 0.53 | 0.41 | ||
| Sex | Man | 29 | 28.0 ± 17.4 | 21.0 ± 12.8 | 410.6 ± 420.4 |
| Woman | 10 | 25.7 ± 15.7 | 18.9 ± 11.4 | 264.4 ± 141.2 | |
| p value b | 0.82 | 0.72 | 0.42 | ||
| Maxillary sinus | 28 | 31.3 ± 17.6 | 22.0 ± 12.9 | 419.0 ± 399.1 | |
| Primary site | Nasal or ethmoid sinus | 11 | 17.7 ± 9.3 | 16.5 ± 10.5 | 256.5 ± 278.9 |
| Clinical T classification | p value b | 0.006 | 0.20 | 0.10 | |
| cT1 | 1 | 11.6 | 10.3 | 70.6 | |
| cT2 | 2 | 13.2 ± 1.2 | 6.7 ± 1.0 | 51.5 ± 2.3 | |
| cT3 | 9 | 33.4 ± 18.7 | 14.7 ± 7.5 | 259.4 ± 127.1 | |
| cT4a | 20 | 29.4 ± 18.0 | 23.5 ± 13.4 | 502.0 ± 473.2 | |
| cT4b | 7 | 20.6 ± 7.70 | 24.3 ± 12.3 | 286.6 ± 131.0 | |
| Clinical N classification | p value a | 0.92 | 0.007 | <0.001 | |
| cN0 | 29 | 26.7 ± 16.5 | 18.1 ± 10.5 | 330.1 ± 272.1 | |
| cN1 | 3 | 33.5 ± 22.0 | 39.2 ± 16.5 | 960.7 ± 990.7 | |
| cN2 | 7 | 27.9 ± 18.2 | 21.9 ± 12.6 | 300.0 ± 143.2 | |
| Clinical stage | p value a | 0.81 | 0.08 | 0.37 | |
| I | 1 | 11.6 | 10.3 | 70.6± | |
| II | 2 | 13.1 ± 1.2 | 6.7 ± 1.0 | 51.5 ± 2.3 | |
| III | 8 | 29.0 ± 14.4 | 15.8 ± 7.2 | 258.2 ± 135.8 | |
| IVA | 21 | 31.2 ± 19.5 | 22.7 ± 13.6 | 490.9 ± 464.1 | |
| IVB | 7 | 20.6 ± 7.70 | 24.3 ± 12.3 | 286.6 ± 131.0 | |
| Treatment group | p value a | 0.84 | 0.019 | 0.07 | |
| Surgery | 25 | 25.8 ± 14.1 | 19.4 ± 13.3 | 368.5 ± 432.5 | |
| Radiotherapy | 14 | 28.4 ± 18.3 | 22.3 ± 10.6 | 381.6 ± 247.5 | |
| p value b | 0.93 | 0.25 | 0.33 | ||
| Chemotherapy | Presence | 31 | 29.7 ± 17.3 | 20.2 ± 12.7 | 395.5 ± 401.7 |
| Absence | 8 | 18.6 ± 11.8 | 21.3 ± 11.9 | 286.7 ± 230.3 | |
| p value b | 0.06 | 0.61 | 0.58 | ||
| Charlson | <6 | 37 | 27.9 ± 17.1 | 19.8 ± 12.2 | 354.4 ± 368.5 |
| comorbidity index | ≥6 | 2 | 18.5 ± 0.6 | 32.1 ± 13.6 | 721.2 ± 377.6 |
| p value b | 0.28 | 0.11 | 0.09 |
| Characteristic | MTV <21.8 (n = 27) | MTV ≥21.8 (n = 12) | p Value | |
|---|---|---|---|---|
| Age | Mean ± SD | 65.1 ± 14.5 | 61.6 ± 8.0 | 0.27 a |
| Sex | Man/woman | 20/7 | 9/3 | 1.00 b |
| Primary site | Maxillary sinus/others | 19/8 | 9/3 | 1.00 b |
| Clinical T classification | cT1-4a/cT4b | 24/3 | 8/4 | 0.17 b |
| Clinical N classification | cN0/cN1-2 | 22/5 | 7/5 | 0.23 b |
| Clinical stage | cStageI–III/cStageIV | 10/17 | 1/11 | 0.12 b |
| Treatment group | Surgery/radiotherapy | 18/9 | 7/5 | 0.72 b |
| Chemotherapy | Presence/absence | 22/5 | 9/3 | 0.68 b |
| Charlson comorbidity index | <6/≥6 | 27/0 | 10/2 | 0.09 b |
| Parameter | Sinonasal Tract SCC-Specific Survival | OS | DFS | LRFS | RRFS | DMFS |
|---|---|---|---|---|---|---|
| Age (per 1 year) | ||||||
| Hazard ratio | 0.96 | 1.00 | 0.96 | 0.93 | 0.99 | 0.94 |
| 95% confidence interval | 0.91–1.00 | 0.97–1.05 | 0.92–1.01 | 0.88–0.98 | 0.91–1.10 | 0.89–0.99 |
| p value | 0.07 | 0.89 | 0.09 | 0.007 | 0.83 | 0.018 |
| Sex (man/woman) | ||||||
| Hazard ratio | 0.28 | 0.68 | 0.41 | 0.54 | 0.37 | 0.63 |
| 95% confidence interval | 0.08–1.00 | 0.24–2.13 | 0.13–1.34 | 0.14–2.32 | 0.05–3.32 | 0.16–3.18 |
| p value | 0.05 | 0.49 | 0.14 | 0.39 | 0.35 | 0.55 |
| Clinical stage (IV/I–III) | ||||||
| Hazard ratio | 1.75 | 1.16 | 1.29 | 0.74 | 1.07 | 3.18 |
| 95% confidence interval | 0.45–8.73 | 0.38–3.91 | 0.36–5.19 | 0.14–3.31 | 0.09–25.1 | 0.65–24.0 |
| p value | 0.43 | 0.80 | 0.70 | 0.69 | 0.96 | 0.16 |
| Treatment group (radiotherapy/surgery) | ||||||
| Hazard ratio | 0.64 | 1.31 | 0.94 | 1.16 | 0.73 | 0.56 |
| 95% confidence interval | 0.16–2.42 | 0.46–3.71 | 0.27–3.21 | 0.26–4.96 | 0.08–6.85 | 0.11–2.39 |
| p value | 0.52 | 0.60 | 0.93 | 0.84 | 0.77 | 0.44 |
| MTV (≥21.8/<21.8) | ||||||
| Hazard ratio | 3.69 | 2.25 | 3.38 | 5.42 | 4.74 | 1.64 |
| 95% confidence interval | 1.17–12.0 | 0.89–5.51 | 1.19–9.71 | 1.59–20.3 | 0.70–42.8 | 0.40–6.24 |
| p value | 0.026 | 0.09 | 0.023 | 0.007 | 0.11 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, H.; Tamaki, T.; Kodaira, T.; Nishio, M.; Nishikawa, D.; Beppu, S.; Terada, H.; Sawabe, M.; Hanai, N. Metabolic Tumour Volume as a Predictor of Survival for Sinonasal Tract Squamous Cell Carcinoma. Diagnostics 2022, 12, 146. https://doi.org/10.3390/diagnostics12010146
Suzuki H, Tamaki T, Kodaira T, Nishio M, Nishikawa D, Beppu S, Terada H, Sawabe M, Hanai N. Metabolic Tumour Volume as a Predictor of Survival for Sinonasal Tract Squamous Cell Carcinoma. Diagnostics. 2022; 12(1):146. https://doi.org/10.3390/diagnostics12010146
Chicago/Turabian StyleSuzuki, Hidenori, Tsuneo Tamaki, Takeshi Kodaira, Masami Nishio, Daisuke Nishikawa, Shintaro Beppu, Hoshino Terada, Michi Sawabe, and Nobuhiro Hanai. 2022. "Metabolic Tumour Volume as a Predictor of Survival for Sinonasal Tract Squamous Cell Carcinoma" Diagnostics 12, no. 1: 146. https://doi.org/10.3390/diagnostics12010146
APA StyleSuzuki, H., Tamaki, T., Kodaira, T., Nishio, M., Nishikawa, D., Beppu, S., Terada, H., Sawabe, M., & Hanai, N. (2022). Metabolic Tumour Volume as a Predictor of Survival for Sinonasal Tract Squamous Cell Carcinoma. Diagnostics, 12(1), 146. https://doi.org/10.3390/diagnostics12010146






