Application of Highly Flexible Adaptive Image Receive Coil for Lung MR Imaging Using Zero TE Sequence: Comparison with Conventional Anterior Array Coil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Image Acquisition
2.3. Quantitative Analysis
2.4. Qualitative Analysis
2.5. Lesion Evaluation
2.6. Assessment of Patients’ Comfort
2.7. Statistical Analysis
3. Results
3.1. Quantitative Evaluation
3.2. Qualitative Evaluation
3.3. Lesion Detection
3.4. Patients’ Comfort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauczor, H.-U.; Kreitner, K.-F. MRI of the pulmonary parenchyma. Eur. Radiol. 1999, 9, 1755–1764. [Google Scholar] [CrossRef]
- Larson, P.E.Z.; Han, M.; Krug, R.; Jakary, A.; Nelson, S.J.; Vigneron, D.B.; Henry, R.G.; McKinnon, G.; Kelley, D.A.C. Ultrashort echo time and zero echo time MRI at 7T. MAGMA 2016, 29, 359–370. [Google Scholar] [CrossRef]
- Bae, K.; Jeon, K.N.; Hwang, M.J.; Lee, J.S.; Ha, J.Y.; Ryu, K.H.; Kim, H.C. Comparison of lung imaging using three-dimensional ultrashort echo time and zero echo time sequences: Preliminary study. Eur. Radiol. 2019, 29, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Dournes, G.; Menut, F.; Macey, J.; Fayon, M.; Chateil, J.-F.; Salel, M.; Corneloup, O.; Montaudon, M.; Berger, P.; Laurent, F. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur. Radiol. 2016, 26, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Hatabu, H.; Ohno, Y.; Gefter, W.B.; Parraga, G.; Madore, B.; Lee, K.S.; Altes, T.A.; Lynch, D.A.; Mayo, J.R.; Seo, J.B.; et al. Expanding Applications of Pulmonary MRI in the Clinical Evaluation of Lung Disorders: Fleischner Society Position Paper. Radiology 2020, 297, 286–301. [Google Scholar] [CrossRef]
- Alibek, S.; Vogel, M.; Sun, W.; Winkler, D.; Baker, C.A.; Burke, M.; Gloger, H. Acoustic noise reduction in MRI using Silent Scan: An initial experience. Diagn. Interv. Radiol. 2014, 20, 360–363. [Google Scholar] [CrossRef]
- Bae, K.; Jeon, K.N.; Hwang, M.J.; Lee, J.S.; Park, S.E.; Kim, H.C.; Menini, A. Respiratory motion–resolved four-dimensional zero echo time (4D ZTE) lung MRI using retrospective soft gating: Feasibility and image quality compared with 3D ZTE. Eur. Radiol. 2020, 30, 5130–5138. [Google Scholar] [CrossRef]
- Feng, L.; Delacoste, J.; Smith, D.; Weissbrot, J.; Flagg, E.; Moore, W.H.; Chandarana, H.; Girvin, F.; Raad, R.; Piccini, D.; et al. Simultaneous Evaluation of Lung Anatomy and Ventilation Using 4D Respiratory-Motion-Resolved Ultrashort Echo Time Sparse MRI. J. Magn. Reson. Imaging 2019, 49, 411–422. [Google Scholar] [CrossRef]
- Winkler, S.A.; Corea, J.; Lechêne, B.; O’Brien, K.; Bonanni, J.R.; Chaudhari, A.; Alley, M.; Taviani, V.; Grafendorfer, T.; Robb, F.; et al. Evaluation of a Flexible 12-Channel Screen-printed Pediatric MRI Coil. Radiology 2019, 291, 180–185. [Google Scholar] [CrossRef]
- Corea, J.R.; Lechene, P.B.; Lustig, M.; Arias, A.C. Materials and methods for higher performance screen-printed flexible MRI receive coils. Magn. Reson. Med. 2017, 78, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Nordmeyer-Massner, J.A.; De Zanche, N.; Pruessmann, K.P. Stretchable coil arrays: Application to knee imaging under varying flexion angles. Magn. Reson. Med. 2012, 67, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Malko, A.J.; McClees, E.C.; Braun, I.F.; Davis, P.C.; Hoffman, J.C. A flexible mercury-filled surface coil for MR imaging. Am. J. Neuroradiol. 1986, 7, 246–247. [Google Scholar] [PubMed]
- McGee, K.P.; Stormont, R.S.; Lindsay, A.S.; Taracila, V.; Savitskij, D.; Robb, F.; Witte, R.J.; Kaufmann, T.J.; Huston, J.; Riederer, S.J.; et al. Characterization and evaluation of a flexible MRI receive coil array for radiation therapy MR treatment planning using highly decoupled RF circuits. Phys. Med. Biol. 2018, 63, 08NT02. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Trzasko, J.D.; Gray, E.M.; Campeau, N.G.; Rossman, P.J.; Kang, D.; Robb, F.; Stormont, R.S.; Lindsay, S.A.; Bernstein, M.A.; et al. Application of Adaptive Image Receive Coil Technology for Whole-Brain Imaging. Am. J. Roentgenol. 2021, 216, 552–559. [Google Scholar] [CrossRef]
- Rossman, P.; Stormont, R.; Lindsay, S.; Robb, F.; Savitskij, D.; Stanley, D.; Huston, J.; Kaufmann, T.; McGee, K. Characterization of a new ultra-flexible, low profile RF receive coil technology. In Proceedings of the ISMRM 25th Annual Meeting & Exhibition, Honolulu, HI, USA, 22–27 April 2017; p. 0763. [Google Scholar]
- Crete, F.; Dolmiere, T.; Ladret, P.; Nicolas, M. The blur effect: Perception and estimation with a new no-reference perceptual blur metric. In Proceedings of the SPIE Electronic Imaging Symposium Conference Human Vision and Electronic Imaging, San Jose, CA, USA, 28 January–1 February 2007; p. 64920I. [Google Scholar]
- Ferzli, R.; Karam, L.J. A No-Reference Objective Image Sharpness Metric Based on the Notion of Just Noticeable Blur (JNB). IEEE Trans. Image Process. 2009, 18, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Narvekar, N.D.; Karam, L.J. A No-Reference Image Blur Metric Based on the Cumulative Probability of Blur Detection (CPBD). IEEE Trans. Image Process. 2011, 20, 2678–2683. [Google Scholar] [CrossRef]
- Zhang, S.; Li, P.; Xu, X.; Li, L.; Chang, C.-C. No-Reference Image Blur Assessment Based on Response Function of Singular Values. Symmetry 2018, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Gruber, B.; Ms, M.F.; Leiner, T.; Klomp, D.W. RF coils: A practical guide for nonphysicists. J. Magn. Reson. Imaging 2018, 48, 590–604. [Google Scholar] [CrossRef]
- Zeng, F.; Nogami, M.; Ueno, Y.R.; Kanda, T.; Sofue, K.; Kubo, K.; Kurimoto, T.; Murakami, T. Diagnostic performance of zero-TE lung MR imaging in FDG PET/MRI for pulmonary malignancies. Eur. Radiol. 2020, 30, 4995–5003. [Google Scholar] [CrossRef]
- Serai, S.D.; Rapp, J.B.; States, L.J.; Andronikou, S.; Ciet, P.; Lee, E.Y. Pediatric Lung MRI: Currently Available and Emerging Techniques. Am. J. Roentgenol. 2021, 216, 781–790. [Google Scholar] [CrossRef]
- Burris, N.; Johnson, K.M.; Larson, P.E.Z.; Hope, M.D.; Nagle, S.; Behr, S.C.; Hope, T.A. Detection of Small Pulmonary Nodules with Ultrashort Echo Time Sequences in Oncology Patients by Using a PET/MR System. Radiology 2016, 278, 239–246. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Niisato, E.; Su, M.-Y.M.; Benkert, T.; Hsu, H.-H.; Shih, J.-Y.; Chen, J.-S.; Chang, Y.-C. Detecting small pulmonary nodules with spiral ultrashort echo time sequences in 1.5 T MRI. Magma Magn. Reson. Mater. Phys. Biol. Med. 2020, 34, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Tibiletti, M.; Kjørstad, Å.; Birk, G.; Schad, L.R.; Stierstorfer, B.; Rasche, V.; Stiller, D. Three-dimensional accurate detection of lung emphysema in rats using ultra-short and zero echo time MRI. NMR Biomed. 2015, 28, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Koyama, H.; Yoshikawa, T.; Seki, S.; Takenaka, D.; Yui, M.; Lu, A.; Miyazaki, M.; Sugimura, K. Pulmonary high-resolution ultrashort TE MR imaging: Comparison with thin-section standard- and low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J. Magn. Reson. Imaging 2016, 43, 512–532. [Google Scholar] [CrossRef]
- Wielputz, M.; Kauczor, H.U. MRI of the lung: State of the art. Diagn. Interv. Radiol. 2012, 18, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sheikh, K.; Svenningsen, S.; Pike, D.; Guo, F.; Etemad-Rezai, R.; Leipsic, J.; Coxson, H.O.; McCormack, D.G.; Parraga, G. Ultra-short echo-time pulmonary MRI: Evaluation and reproducibility in COPD subjects with and without bronchiectasis. J. Magn. Reson. Imaging 2015, 41, 1465–1474. [Google Scholar] [CrossRef]
- Dournes, G.; Walkup, L.L.; Benlala, I.; Willmering, M.M.; Macey, J.; Bui, S.; Laurent, F.; Woods, J.C. The Clinical Use of Lung MRI in Cystic Fibrosis: What, Now, How? Chest 2021, 159, 2205–2217. [Google Scholar] [CrossRef]
- Polverino, F.; Hysinger, E.B.; Gupta, N.; Willmering, M.; Olin, T.; Abman, S.H.; Woods, J.C. Lung MRI as a Potential Complementary Diagnostic Tool for Early COPD. Am. J. Med. 2020, 133, 757–760. [Google Scholar] [CrossRef]

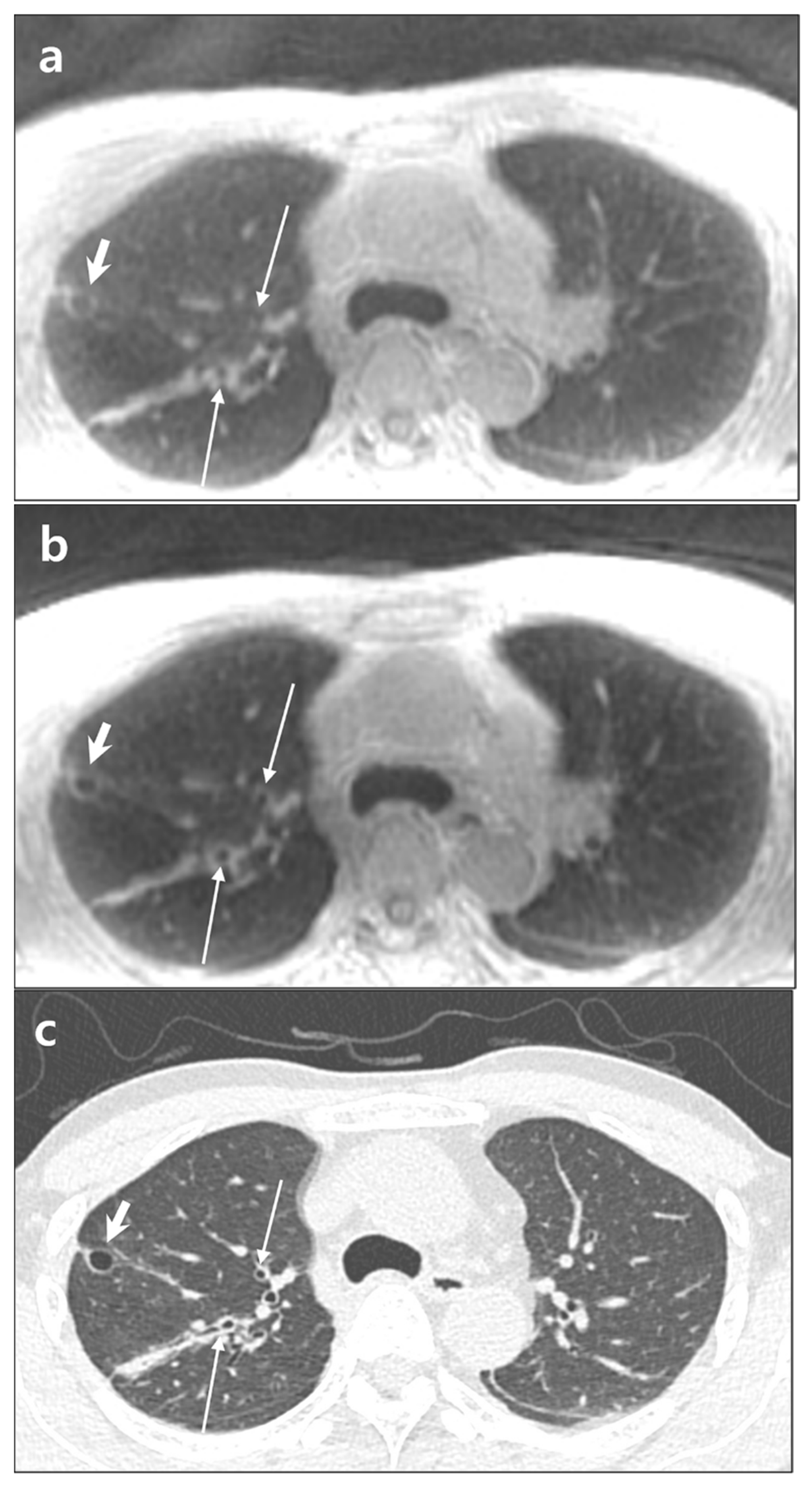
| Visualization of intrapulmonary vessels |
| 1, indistinguishable segmental vessels |
| 2, blurred visualization of segmental vessels |
| 3, clear visualization of segmental vessels |
| 4, visualization of subsegmental vessels |
| 5, visualization of sub-subsegmental vessels |
| Visualization of the bronchi |
| 1, indistinguishable lobar bronchus |
| 2, visualization of lobar bronchus |
| 3, visualization of segmental bronchus |
| 4, visualization of subsegmental bronchus |
| 5, visualization of sub-subsegmental bronchus |
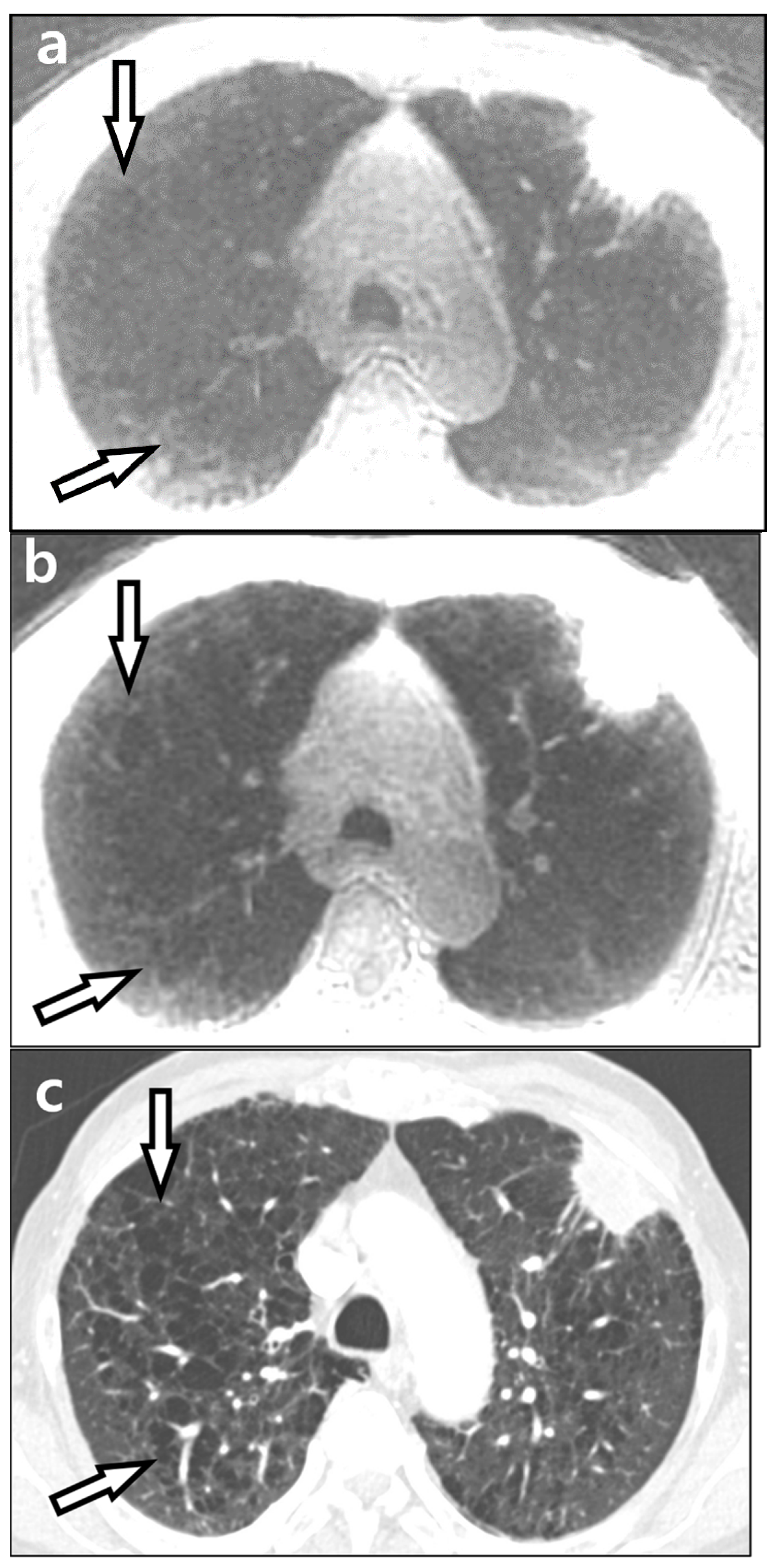
| Visualization of fissures |
| 1, no visualization |
| 2, partial visualization of one fissure |
| 3, partial visualization of two fissures |
| 4, visualization of near whole course of one fissure |
| 5, visualization of near whole course of two fissures |
| Noise and artifacts |
| 1, unacceptable noise/artifacts |
| 2, above-average noise/artifacts |
| 3, average and acceptable noise/artifacts |
| 4, less-than average noise/artifacts |
| 5, minimum or nothing |
| Overall acceptability |
| 1, unacceptable |
| 2, suboptimal |
| 3, satisfactory |
| 4, good |
| 5, excellent |
| ZTE-CAA | ZTE-AIR | p-Value | Effect Size | |
|---|---|---|---|---|
| SNR | 7.47 ± 2.81 | 11.79 ± 5.75 | <0.001 | 0.368 |
| CNR | 1.17 ± 1.25 | 2.10 ± 1.84 | <0.001 | 0.147 |
| Image sharpness * | 0.49 ± 0.37 * | 0.44 ± 0.32 * | <0.001 | 0.378 |
| Reader 1 | Reader 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| ZTE-CAA | ZTE-AIR | p-Value | Effect Size | ZTE-CAA | ZTE-AIR | p-Value | Effect Size | |
| Vessels | 4.74 ± 0.56 | 4.89 ± 0.31 | 0.003 | 0.063 | 4.73 ± 0.54 | 4.83 ± 0.41 | 0.007 | 0.053 |
| Bronchi | 3.59 ± 0.68 | 3.98 ± 0.64 | <0.001 | 0.183 | 3.52 ± 0.64 | 4.82 ± 0.70 | <0.001 | 0.156 |
| Fissures | 2.45 ± 1.13 | 2.79 ± 1.00 | <0.001 | 0.130 | 2.53 ± 1.03 | 2.77 ± 0.87 | <0.001 | 0.137 |
| Noise/Artifacts | 3.36 ± 1.11 | 3.89 ± 0.86 | <0.001 | 0.153 | 3.14 ± 1.12 | 3.50 ± 1.04 | <0.001 | 0.175 |
| Overall Acceptability | 3.5 ± 1.04 | 3.89 ± 0.88 | <0.001 | 0.128 | 3.29 ± 1.12 | 3.83 ± 0.92 | <0.001 | 0.125 |
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
| ZTE-CAA | ZTE-AIR | p-Value | ZTE-CAA | ZTE-AIR | p-Value | |
| Nodules | ||||||
| Sensitivity | 87.0 (47/54) | 88.9 (48/54) | 1.00 | 85.2 (46/54) | 88.9 (48/54) | 0.625 |
| Specificity | 98.1 (206/210) | 98.6 (207/210) | 1.00 | 98.1 (206/210) | 98.6 (207/210) | 1.00 |
| Accuracy | 95.8 (253/264) | 96.6 (255/264) | 0.625 | 95.5 (252/264) | 96.6 (255/264) | 0.375 |
| Emphysema/cysts | ||||||
| Sensitivity | 78.3 (36/46) | 91.3 (42/46) | 0.031 | 73.9 (34/46) | 89.1 (41/46) | 0.039 |
| Specificity | 98.6 (215/218) | 98.6 (215/218) | 1.00 | 98.6 (215/218) | 99.5 (217/218) | 0.500 |
| Accuracy | 95.1 (251/264) | 97.3 (257/264) | 0.031 | 94.3 (249/264) | 97.7 (258/264) | 0.012 |
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
| ZTE-CAA | ZTE-AIR | p-Value | ZTE-CAA | ZTE-AIR | p-Value | |
| All | 80.0 (64/80) | 88.8 (71/80) | 0.065 | 80.0 (64/80) | 87.5 (70/80) | 0.070 |
| 1–3 cm | 93.1 (27/29) | 93.1 (27/29) | 1.000 | 96.8 (28/29) | 93.1 (27/29) | 1.000 |
| <1 cm | 72.5 (37/51) | 86.3 (44/51) | 0.039 | 70.6 (36/51) | 84.3 (43/51) | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.; Jeon, K.N.; Hwang, M.J.; Jung, Y.; Lee, J. Application of Highly Flexible Adaptive Image Receive Coil for Lung MR Imaging Using Zero TE Sequence: Comparison with Conventional Anterior Array Coil. Diagnostics 2022, 12, 148. https://doi.org/10.3390/diagnostics12010148
Bae K, Jeon KN, Hwang MJ, Jung Y, Lee J. Application of Highly Flexible Adaptive Image Receive Coil for Lung MR Imaging Using Zero TE Sequence: Comparison with Conventional Anterior Array Coil. Diagnostics. 2022; 12(1):148. https://doi.org/10.3390/diagnostics12010148
Chicago/Turabian StyleBae, Kyungsoo, Kyung Nyeo Jeon, Moon Jung Hwang, Yunsub Jung, and Joonsung Lee. 2022. "Application of Highly Flexible Adaptive Image Receive Coil for Lung MR Imaging Using Zero TE Sequence: Comparison with Conventional Anterior Array Coil" Diagnostics 12, no. 1: 148. https://doi.org/10.3390/diagnostics12010148






