Imaging for the Initial Staging and Post-Treatment Surveillance of Penile Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Ultrasound
2.1. Primary Staging
2.2. Restaging and Post-Treatment Surveillance
3. Computed Tomography
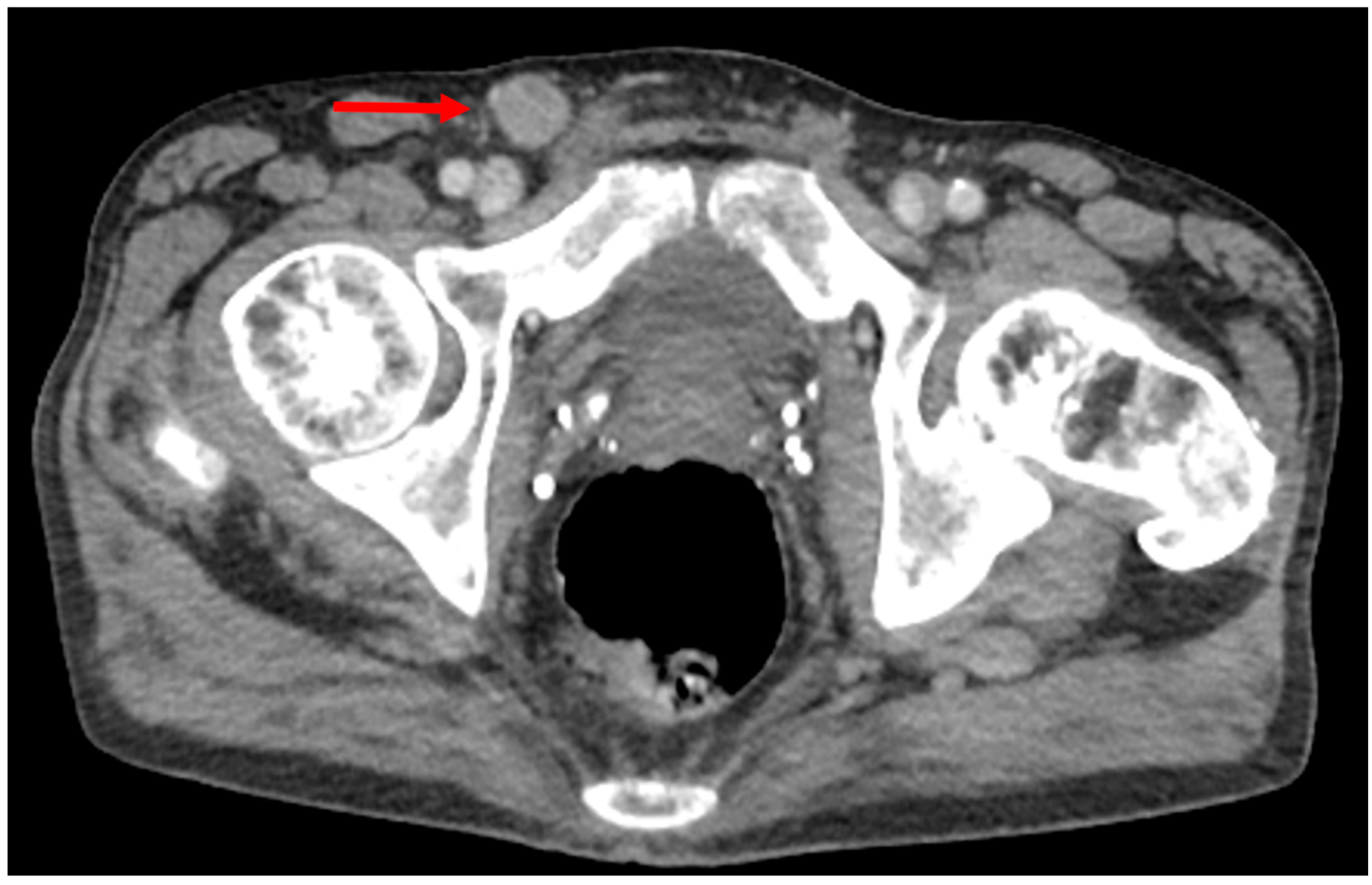
3.1. Primary Staging
3.2. Restaging and Post-Treatment Surveillance
4. Magnetic Resonance Imaging
4.1. Primary Staging
4.2. Restaging and Post-Treatment Surveillance
5. Positron Emission Tomography
5.1. Primary Staging
5.2. Restaging and Post-Treatment Surveillance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.-P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Deutsches Ärzteblatt Int. 2018, 115, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Baheti, A.; Tirumani, S.H.; Rosenthal, M.H.; Kim, K.W.; Ramaiya, N.H.; Shinagare, A.B. Multimodality imaging of penile cancer: What radiologists need to know. Abdom. Imaging 2014, 40, 424–435. [Google Scholar] [CrossRef]
- Eich, M.L.; Pena, M.D.C.R.; Schwartz, L.; Granada, C.P.; Rais-Bahrami, S.; Giannico, G.; Amador, B.M.; Matoso, A.; Gordetsky, J.B. Morphology, p16, HPV, and outcomes in squamous cell carcinoma of the penis: A multi-institutional study. Hum. Pathol. 2020, 96, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Chang, S.S. Penile Cancer: Clinical Presentation, Diagnosis, and Staging. Urol. Clin. N. Am. 2010, 37, 343–352. [Google Scholar] [CrossRef]
- Iorga, L.; Marcu, R.D.; Diaconu, C.C.; Stanescu, A.M.A.; Stoian, A.P.; Mischianu, D.L.D.; Surcel, M.; Bungau, S.; Constantin, T.; Boda, D.; et al. Penile carcinoma and HPV infection (Review). Exp. Ther. Med. 2019, 20, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Schoen, E.J.; Oehrli, M.; Ctr; Colby, C.J.; Machin, G. The Highly Protective Effect of Newborn Circumcision Against Invasive Penile Cancer. Pediatrics 2000, 105, e36. [Google Scholar] [CrossRef] [Green Version]
- Larke, N.L.; Thomas, S.L.; dos Santos Silva, I.; Weiss, H.A. Male circumcision and penile cancer: A systematic review and meta-analysis. Cancer Causes Control 2011, 22, 1097–1110. [Google Scholar] [CrossRef] [Green Version]
- Douglawi, A.; Masterson, T.A. Updates on the epidemiology and risk factors for penile cancer. Transl. Androl. Urol. 2017, 6, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Heyns, C.F.; Mendoza-Valdés, A.; Pompeo, A.C. Diagnosis and Staging of Penile Cancer. Urology 2010, 76, S15–S23. [Google Scholar] [CrossRef]
- Hernandez, B.Y.; Barnholtz-Sloan, J.; German, R.R.; Giuliano, A.; Goodman, M.T.; King, J.B.; Negoita, S.; Villalon-Gomez, J.M. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer 2008, 113, 2883–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiess, P.E. New Treatment Guidelines for Penile Cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Leijte, J.; Shabbir, M.; Watkin, N.; Horenblas, S. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J. Urol. 2008, 27, 197–203. [Google Scholar] [CrossRef]
- Gupta, N.; Goyal, P.; Sharma, K.; Bansal, I.; Gupta, S.; Li, S.; Zinn, K.; Kumar, Y. Penile fracture: Role of ultrasound. Transl. Androl. Urol. 2017, 6, 580–584. [Google Scholar] [CrossRef] [Green Version]
- McCauley, J.F.; Dean, R.C. Diagnostic utility of penile ultrasound in Peyronie’s disease. World J. Urol. 2019, 38, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Dorak, A.C.; Ozkan, G.A.; Tamac, N.I.; Saray, A. Ultrasonography in the recognition of penile cancer. J. Clin. Ultrasound 1992, 20, 624–626. [Google Scholar] [CrossRef]
- Yamashita, T.; Ogawa, A. Ultrasound in penile cancer. Urol. Radiol. 1989, 11, 174–177. [Google Scholar] [CrossRef]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, T.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, definitions and measurements to describe sonographic features of lymph nodes: Consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obstet. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef]
- Kochhar, R.; Taylor, B.; Sangar, V. Imaging in primary penile cancer: Current status and future directions. Eur. Radiol. 2009, 20, 36–47. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Penile Cancer (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf (accessed on 10 December 2021).
- Brody, A.S.; Guillerman, R.P. Don’t let radiation scare trump patient care: 10 ways you can harm your patients by fear of radia-tion-induced cancer from diagnostic imaging. Thorax 2014, 69, 782–784. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.A.; Pennington, C.W.; Sacks, B. Subjecting Radiologic Imaging to the Linear No-Threshold Hypothesis: A Non Sequitur of Non-Trivial Proportion. J. Nucl. Med. 2017, 58, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graafland, N.M.; Teertstra, H.J.; Besnard, A.P.E.; van Boven, H.H.; Horenblas, S. Identification of High Risk Pathological Node Positive Penile Carcinoma: Value of Preoperative Computerized Tomography Imaging. J. Urol. 2011, 185, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Shanbhogue, K.P.; Schieda, N.; Morbeck, F.; Hadas, B.; Kulkarni, G.; McInnes, M.D.; Baroni, R.H. Role of MRI in Staging of Penile Cancer. J. Magn. Reson. Imaging 2020, 51, 1612–1629. [Google Scholar] [CrossRef]
- Ghosh, P.; Chandra, A.; Mukhopadhyay, S.; Chatterjee, A.; Lingegowda, D.; Gehani, A.; Gupta, B.; Gupta, S.; Midha, D.; Sen, S. Accuracy of MRI without intracavernosal prostaglandin E1 injection in staging, preoperative evaluation, and operative planning of penile cancer. Abdom. Radiol. 2021, 46, 4984–4994. [Google Scholar] [CrossRef]
- Gupta, S.; Rajesh, A. Magnetic Resonance Imaging of Penile Cancer. Magn. Reson. Imaging Clin. N. Am. 2014, 22, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Scher, B.; Seitz, M.; Reiser, M.; Hungerhuber, E.; Hahn, K.; Tiling, R.; Herzog, P.; Reiser, M.; Schneede, P.; Dresel, S. 18F-FDG PET/CT for staging of penile cancer. J. Nucl. Med. 2005, 46, 1460–1465. [Google Scholar]
- Schlenker, B.; Scher, B.; Tiling, R.; Siegert, S.; Hungerhuber, E.; Gratzke, C.; Tilki, D.; Reich, O.; Schneede, P.; Bartenstein, P.; et al. Detection of inguinal lymph node involvement in penile squamous cell carcinoma by 18F-fluorodeoxyglucose PET/CT: A prospective single-center study. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 55–59. [Google Scholar] [CrossRef]
- Leijte, J.A.; Graafland, N.M.; Olmos, R.A.V.; Van Boven, H.H.; Hoefnagel, C.A.; Horenblas, S. Prospective evaluation of hybrid18F-fluorodeoxyglucose positron emission tomography/computed tomography in staging clinically node-negative patients with penile carcinoma. BJU Int. 2009, 104, 640–644. [Google Scholar] [CrossRef]
- Dräger, D.L.; Heuschkel, M.; Protzel, C.; Erbersdobler, A.; Krause, B.J.; Hakenberg, O.W.; Schwarzenböck, S.M. [18F]FDG PET/CT for assessing inguinal lymph nodes in patients with penile cancer—Correlation with histopathology after inguinal lymphadenectomy. Nuklearmedizin 2018, 57, 26–30. [Google Scholar]
- Sadeghi, R.; Gholami, H.; Zakavi, S.R.; Kakhki, V.R.; Horenblas, S. Accuracy of 18F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: Systematic review and meta-analysis of the literature. Clin. Nucl. Med. 2012, 37, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, J.K.; Nielsen, T.F.; Ipsen, P.; Albrecht-Beste, E.; Costa, J.C.; Alslev, L.; Krarup, K.P.; Toft, B.G.; Høyer, S.; Bouchelouche, K.; et al. DaPeCa-7: Comparative assessment of fluorodeoxyglucose positron emission tomography/computed tomography (CT) and conventional diagnostic CT in diagnosis of lymph node metastases, distant metastases and incidental findings in patients with invasive penile cancer. BJU Int. 2021, 127, 254–262. [Google Scholar] [CrossRef]
- Jakobsen, J.K.; Alslev, L.; Ipsen, P.; Costa, J.C.; Krarup, K.P.; Sommer, P.; Nerstrøm, H.; Toft, B.G.; Høyer, S.; Bouchelouche, K.; et al. DaPeCa-3: Promising results of sentinel node biopsy combined with (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in clinically lymph node-negative patients with penile cancer—A national study from Denmark. BJU Int. 2016, 118, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Júnior, E.P.; Salles, P.G.O.; Silva-Filho, R.; Reis, E.A.; Mamede, M. (18)F-FDG PET/CT as a prognostic factor in penile cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Albersen, M.; Chipollini, J.; Pederzoli, F.; Zhu, Y.; Ye, D.-W.; Ornellas, A.A.; Watkin, N.; Ager, M.; Hakenberg, O.W.; et al. Optimising the selection of candidates for neoadjuvant chemotherapy amongst patients with node-positive penile squamous cell carcinoma. BJU Int. 2020, 125, 867–875. [Google Scholar] [CrossRef] [PubMed]


| Stage | Description |
|---|---|
| Tumor (T) | |
| Tx | Cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Ta | Noninvasive localized SCC |
| T1 | Invasion of subepithelial connective tissue (varies by location) |
| T2 | Invasion of corpus spongiosum with or without urethral invasion |
| T3 | Invasion of corpora cavernosum with or without urethral invasion |
| T4 | Invasion of other adjacent structures (scrotum, prostate, bone) |
| Lymph node (N) | |
| Nx | Cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | ≤2 unilateral inguinal nodal metastases, no extranodal extension |
| N2 | ≥3 unilateral or bilateral inguinal nodal metastases |
| N3 | Extranodal extension of any lymph node metastasis |
| Metastasis (M) | |
| Mx | Cannot be assessed |
| M0 | No evidence of distant metastasis |
| M1 | Distant metastasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, S.J.; Norton, J.C.; Porter, K.K.; West, J.T.; Rais-Bahrami, S. Imaging for the Initial Staging and Post-Treatment Surveillance of Penile Squamous Cell Carcinoma. Diagnostics 2022, 12, 170. https://doi.org/10.3390/diagnostics12010170
Galgano SJ, Norton JC, Porter KK, West JT, Rais-Bahrami S. Imaging for the Initial Staging and Post-Treatment Surveillance of Penile Squamous Cell Carcinoma. Diagnostics. 2022; 12(1):170. https://doi.org/10.3390/diagnostics12010170
Chicago/Turabian StyleGalgano, Samuel J., John C. Norton, Kristin K. Porter, Janelle T. West, and Soroush Rais-Bahrami. 2022. "Imaging for the Initial Staging and Post-Treatment Surveillance of Penile Squamous Cell Carcinoma" Diagnostics 12, no. 1: 170. https://doi.org/10.3390/diagnostics12010170
APA StyleGalgano, S. J., Norton, J. C., Porter, K. K., West, J. T., & Rais-Bahrami, S. (2022). Imaging for the Initial Staging and Post-Treatment Surveillance of Penile Squamous Cell Carcinoma. Diagnostics, 12(1), 170. https://doi.org/10.3390/diagnostics12010170







