Abstract
Circulating biomarkers have been recently investigated among patients undergoing endovascular aortic aneurysm repair (EVAR) for abdominal aortic aneurysm (AAA). Considering the plethora of small descriptive studies reporting potential associations between biomarkers and clinical outcomes, this review aims to summarize the current literature considering both the treated disease (post EVAR) and the untreated disease (AAA before EVAR). All studies describing outcomes of tissue biomarkers in patients undergoing EVAR and in patients with AAA were included, and references were checked for additional sources. In the EVAR scenario, circulating interleukin-6 (IL-6) is a marker of inflammatory reaction which might predict postoperative morbidity; cystatin C is a promising early marker of post-procedural acute kidney injury; plasma matrix metalloproteinase-9 (MMP-9) concentration after 3 months from EVAR might help in detecting post-procedural endoleak. This review also summarizes the current gaps in knowledge and future direction of this field of research. Among markers used in patients with AAA, galectin and granzyme appear to be promising and should be carefully investigated even in the EVAR setting. Larger prospective trials are required to establish and evaluate prognostic models with highest values with these markers.
1. Introduction
Abdominal aortic aneurysm (AAA) is a multifactorial disease and a potentially life-threatening condition. Pharmacological approaches to slow aneurysm progression and limit the near-fatal risk of acute ruptures are currently under investigation to reduce the negative impact on the healthcare system [1]. Endovascular aortic aneurysm repair (EVAR) has now become the standard of care which has been shown to reduce morbidity and mortality [2]. However, short-term and long-term complications still hamper procedural success, and the most common complication is the residual perfusion of the aneurysmal sac (i.e., endoleak). Considering their frequency, patients undergo long-term surveillance screening with computed tomographic angiography or vascular ultrasound, which are limited by contrast administration or poor accuracy, respectively. Post-procedural complications and their assessment might impact the cost-effectiveness of EVAR.
Therefore, the study of circulating biomarkers has been progressively introduced in the medical literature and in the near future, these biomarkers might help in identifying conditions prone to develop complications, for both post-EVAR patients and patients with aortic aneurysm in follow up. This review will discuss the current biomarkers in the setting of AAA, with particular emphasis on patients undergoing EVAR.
2. Materials and Methods
An electronic database search through PubMed and Scopus was performed in October 2021. All studies describing outcomes of tissue biomarkers in patients undergoing EVAR were included, and references were checked for additional sources. Case reports, opinions, and editorials were excluded. Pre-clinical studies (non-human studies) were considered only for the last paragraph of the results.
3. Results
Pre-clinical evidence was considered only if directly connected with human results, and potential implications for future research are discussed separately. In the presentation of results, the treated disease (i.e., EVAR) and the untreated disease (i.e., AAA) will be separated.
4. Biomarkers and Compounds in EVAR (Treated Disease)
After AAA repair, the hormonal and metabolic stress-related inflammatory cascade, clinically referred to as “post-implantation syndrome”, is rapidly activated [3] by surgical trauma, ischemia–reperfusion injury and local cellular interactions [3]. Although endovascular repair reduces tissue manipulation compared with open surgery, intra-luminal manipulation of the thrombus using catheters is sufficient to initiate and sustain this strong systemic inflammatory response. Inflammatory cytokines and their regulatory activities have been extensively investigated in recent years, and a systematic review [3] concluded that IL-6 and IL-8 were particularly involved in the post-implantation syndrome and their role is greater in open surgery. IL-1b, IL-10, and TNF-a are other final common pathways of the post-implantation syndrome with no differences between open and EVAR techniques [3].
After EVAR, the most common and serious complication is endoleak, which is generally diagnosed with computed tomography during surveillance follow up or in case of symptoms. Assessment of markers released from the aneurysm wall into the bloodstream might potentially be an alternative for early endoleak detection [1]. Among those biomarkers, matrix metalloproteinases (MMPs) are soluble enzymes with lytic activity produced by macrophages and vascular smooth muscle cells. In particular, MMP-9 has been implicated in aneurysm growth through structural changes in the aortic wall and ECM remodeling, and its soluble release in the bloodstream has been advocated as a marker for endoleak, as the continuous perfusion of the aneurysm sac promotes MMP-9 release in the bloodstream [1]. A meta-analysis by Ng et al. [1] concluded that patients with endoleak have higher 3-month values of plasma MMP-9 levels compared to patients without endoleak (SMD 1.42, 95%CI 0.48-2.36, p < 0.003) [1], echoing previous studies [4].
Another feared complication of EVAR is perioperative renal dysfunction, related to contrast medium or reduced flow to renal arteries [2]. Anuria is a tardive event in acute kidney disease and urine output cannot be reliably considered the unique indicator. A recent systematic review [2] concluded that neutrophil gelatinase associated lipocalin (NGAL), cystatin C, and liver-type fatty acid binding protein (FABP-L) were the most promising for assessing postoperative renal failure after EVAR [2]. Considering “classic” biomarkers, serum creatinine >1.5 mg/dL remains a strong predictor of increased 30-day mortality (RR 3.0, 95% CI 2.3-4.1, p < 0.001) [5].
Another aspect recently reviewed in literature is the coagulation cascade. As aneurysm leads to increased thrombin generation and fibrin turnover, EVAR could produce similar results. A recent review [6] concluded that EVAR increased thrombin activation and fibrinolysis up to one year after procedure, suggesting that this period might be associated with increased risk of cardiovascular events and confirming previous findings [7]. Moreover, elevated levels of fibrinogen degradation products (FDP) were correlated with endoleak. However, the impact of these changes in the coagulation and fibrinolysis on the outcomes of EVAR should be still investigated.
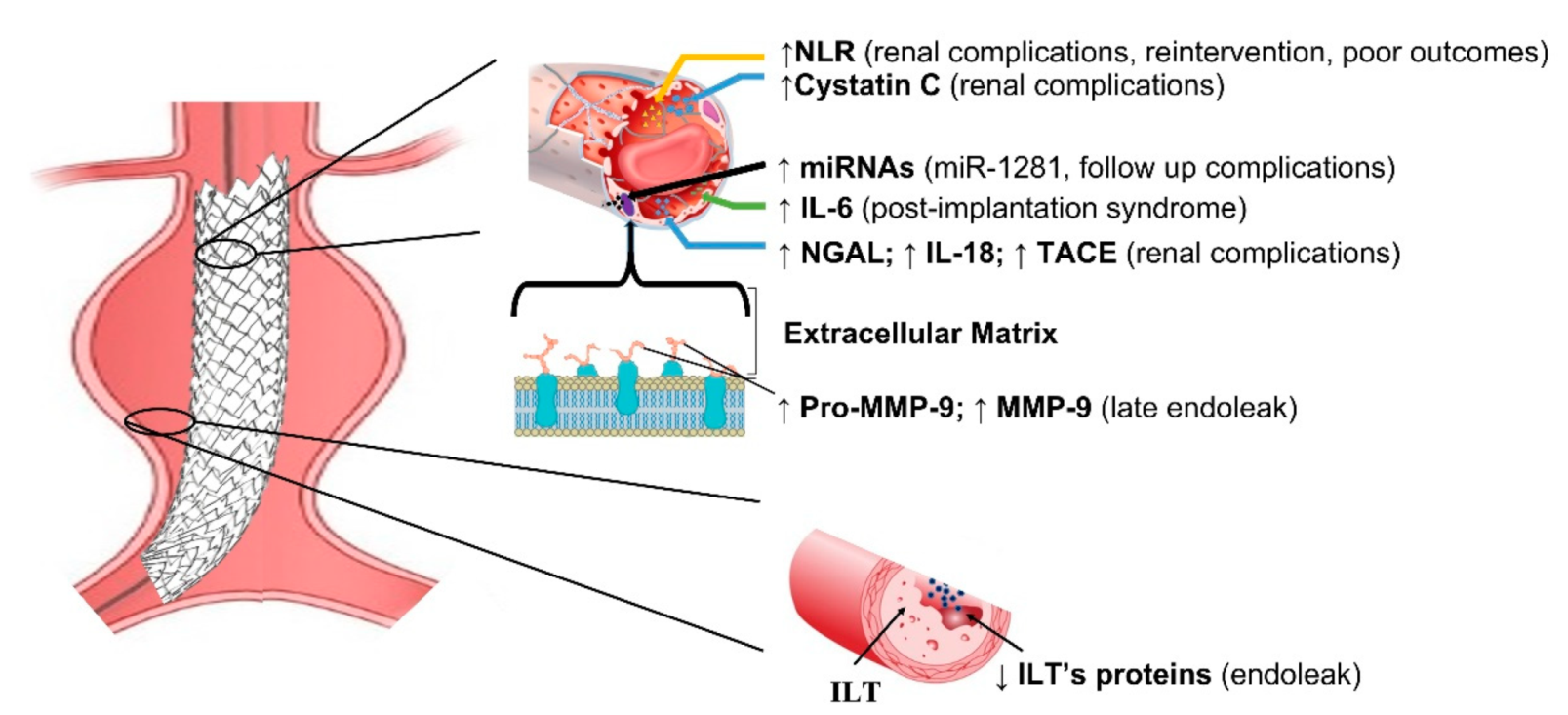
Studies investigating biomarkers in EVAR disease are summarized in Table 1. Due to their genetic regulatory function and their high stability in biological fluids, plasmatic miRNAs have been highlighted as being optimal candidates as non-invasive biomarkers. For instance, a recent study found that elevated miRNA-1281 levels might identify patients with follow-up complications [8]. Most of the current literature is based on predicting post-operative renal disease, with Cystatin C being the recognized early marker of renal failure [9] (Figure 1).

Table 1.
Biomarkers and compounds in EVAR (treated disease).
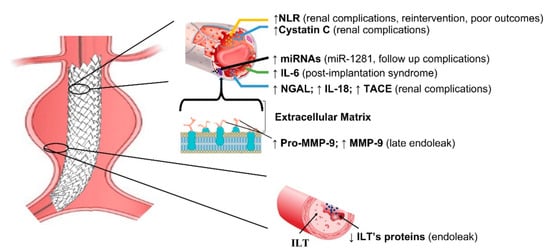
Figure 1.
Schematic representation of main biomarkers in EVAR. NLR = Neutrophil–to–Lymphocyte ratio; IL-6 = interleukin 6; IL-18 = interleukin 18; NGAL = Neutrophil gelatin-associated lipocalin; TACE = tumor necrosis factor –α converting enzyme; ILT = intraluminal thrombus; ILT’s proteins = intraluminal thrombus proteins; MMP-9 = matrix metalloproteinase and tissue inhibitors; miRNAs = micro RNA.
5. Biomarkers and Compounds in Abdominal Aortic Aneurysms (Untreated Disease)
The thorough understanding of AAA pathophysiology has been clarified by proteomic analysis; specific proteins associated with AAA might be released from vascular tissue, intraluminal thrombus, tissue secretome, blood, and cells [10,11]. Proteomic analysis found biomarkers of complications, proteins related to pathogenic mechanisms, and potential therapeutic targets for AAA to be confirmed by tailored studies.
Differently from EVAR, in the setting of AAA there are known predictors of complications which have been investigated through the years such as serum elastin peptides (SEP) and plasmin–antiplasmin (PAP) complexes, MMP-9, IL-6, C-reactive protein (CRP), antitrypsin and IFN-gamma for AAA size, expansion rate or rupture [12,13,14,15,16]. Circulating, biomechanical, and genetic markers for AAA growth and rupture were reviewed in recent years [17,18,19,20,21,22].
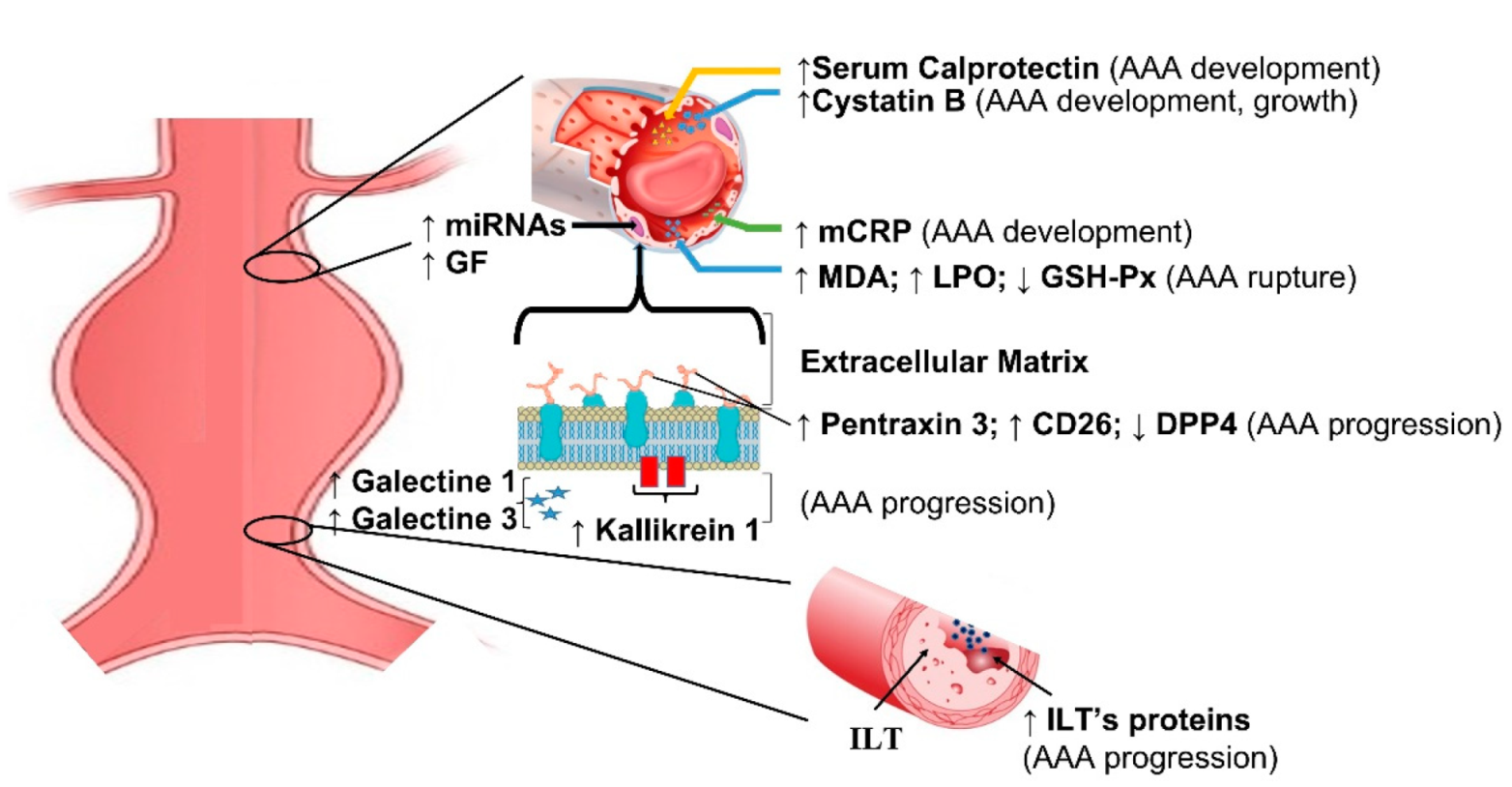
Current biomarkers in aortic aneurysm are summarized in Table 2. Genetic features of AAA have been extensively investigated in recent years using microarray, and genes involved in apoptosis, proteolysis, and humoral immune response yielded the most promising results. ALOX5, PTGIS, and CX3CL1 genes are potentially related with diagnosis of AAA [23,24]. Similarly, miRNA profiling produced under-expressed and overexpressed sequences suggesting the role in regulatory mechanisms [25,26,27]. Tissue factors related with inflammatory infiltrates and matrix degradation, such as pentraxin [28], galectin [29], calprotectin [30,31], kallikrein [32], and granzyme [33], correlate with the presence of AAA and might help in identifying patients at risk of AAA rupture (Figure 2).

Table 2.
Biomarkers and compounds in thoracoabdominal aortic aneurysms (untreated disease).
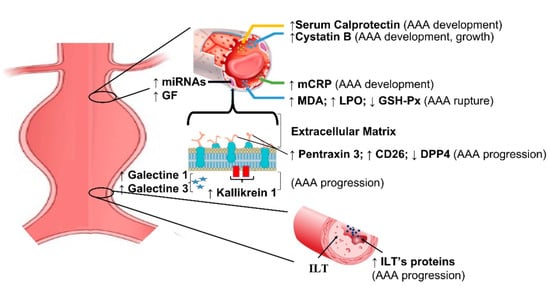
Figure 2.
Schematic representation of main biomarkers in abdominal aortic aneurysms. mCRP = monomeric form of C-reactive protein; MDA = malondialdehyde; LPO = lipid hydroperoxide; GSH-Px = glutathione peroxidase; DPP4 = dipeptidyl peptidase–4; ILT = intraluminal thrombus; ILT’s proteins = intraluminal thrombus proteins; miRNAs = micro RNA; GF = genetic factors.
Some recent reviews summarized the impact of miRNA and long non coding RNA (lncRNA) in cellular processed involved in abdominal aortic aneurysm [34,35,36,37,38,39]. Although some lncRNAs have been described as dysregulated in models or human tissue of AAA, comparatively few studies exist to date that have established their functional roles in pathologic disease.
6. The Common Background between EVAR and Untreated AAA: Extracellular Matrix
The pathogenesis of abdominal aortic aneurysm (AAA) is characterized by medial degeneration, manifesting with elastic fiber fragmentation, collagen fiber disorganization, and proteoglycan accumulation, as well as vascular smooth muscle cells (VSMC) loss [40]. The destruction of aortic connective tissue in AAA is led by a severe inflammatory reaction causing excessive degradation of the aortic extracellular matrix (ECM), which plays a pivotal role in AAA pathogenesis [40].
Vascular smooth muscle cells (VSMCs) play an important role in aorta homeostasis by secreting metalloproteinases (MMPs) and their inhibitors (TIMPs). As previously reported, MMPs, in particular MMP-9, have been implicated in aneurysm growth through structural changes in the aortic wall and ECM remodeling [40]. Previous studies have reported that excessive MMP secretion in the aortic wall leads to abnormal ECM degradation [41,42]. This important degradation of the ECM induces the release of cytokines which are involved in the regulation of ECM homeostasis [43]. All of these pathological events lead to weakening of the aortic wall, overexposing it to the biomechanical forces of pulsatile blood flow and blood pressure. Recent studies have reported a link between genetic defects in several collagen-encoding genes (i.e., COL1A1, COL1A2, COL3A1, COL5A1, and COL4A1/A2) and the development of AAA, thoracic abdominal aneurysm (TAA), and aortic dissection [44,45]. Moreover, mutation in COL3A1 can be found in more than 95% of patients with Ehlers-Danlos syndrome (EDS) with aortic complications [46]. An important role in the pathogenesis of AAA is represented by the degradation of elastin. The template for elastin is provided by fibrillin, which is a large extracellular glycoprotein that assembles to form microfibrils that are key components of most of the ECM. Fibrillin-1 (FBN1) and fibrillin-2 (FBN2) are the main structural components of the microfibril scaffold [45].
Pathologically increased of TGF-ß signaling was first implicated in the pathogenesis of TAA in the context of Marfan syndrome [47]. Furthermore, the role of dysregulated TGF- ß signaling in the pathogenesis of syndromic TAA is explained by the identification of loss-of-function mutations in TGF-ß receptors (TGFßR1 and TGFßR2), ligands (TGFß2 and TGFß3), and downstream effectors (SMAD2 and SMAD3) in patients with Loeys-Dietz syndrome [47,48]. Recently, new studies have proposed proteomics methods for a more systematic analysis of extracellular proteins [48,49]. One of the newest ECM proteomics approaches provides an activity-based proteomics method to relate the activity of specific proteases to ECM degradation components and to identify novel protease targets [49]. Furthermore, the latest glycoproteomics technologies allow an analysis of the glycosylation changes of ECM proteins in the arterial wall [50]. A recent finding, with a study on glycoproteomics, revealed an increase in MFAP4 in patients with MFS compared to control aneurysmatic patients [50]. Furthermore, TGF- ß was observed to induce MFAP4 expression in both human and mouse VSMCs, and MFAP4 was upregulated in aortic specimens from patients with a predisposition to AAA [50].
7. Evidence from Pre-Clinical Studies
Potential new biomarkers from recent pre-clinical studies are summarized in Table 3. Epigenetic mediators such as citrullinated histone H3 appear to be a promising therapeutic target for AAA [51]. Similarly, miRNA silencing and the PTEN pathway will have a role in modulating AAA progression and changing cell viability [52,53,54].

Table 3.
Potential new biomarkers from recent pre-clinical studies.
8. Future Directions
Despite the great interest and the flourishing literature in recent years, at present, the use of circulating biomarkers to detect complications of EVAR is still limited in clinical practice due to restricted availability and lack of recommendations from guidelines. To overcome those limitations, the methodology used to study the development and progression of aortic aneurysm should be translated into the EVAR scenario to support the use of biomarkers in the early diagnosis of complications. A potential first-in-clinic biomarker for EVAR would improve surveillance programs after hospital discharge, with tailored use of CT scan. Derivation and validation of a predictive model, according to age and sex, could be performed with a tailored registry analysis, with a synthetic evaluation of the most promising biomarkers such as IL-6, IL-18, cystatin C, MMP-9, and NGAL, similarly to point-of-care testing for platelet function.
An accurate analysis of the postoperative immune response will help in creating a more predictive biomarker panel for post-procedural morbidity. Future large prospective studies are required to identify the exact mechanisms of the cytokine interaction in the post-EVAR setting. Similarly, MMP-9 testing sensitivity and specificity should be evaluated in a real-life cohort before being considered as a surveillance test. Specific serum biomarkers could potentially form the basis of a tailored follow-up for patients with AAA or post-EVAR. Larger prospective trials are required to establish and evaluate prognostic models with highest values with these markers.
9. Conclusions
Circulating IL-6 is a marker of inflammatory reaction after EVAR and might act as a useful predictor of postoperative morbidity. Cystatin C is a promising early marker of post-procedural acute kidney injury after EVAR. Plasma MMP-9 concentration after three months from EVAR might help in detecting post-procedural endoleak. miRNAs are promising, but still limited in their clinical practice. Biomarkers for postoperative renal failure after EVAR are extremely debated in the literature, with some having strong references (NGAL, cystatin C) and others having weaker data to support their use (retinol binding protein, IL-18, N-acetyle-b-D-glocosaminidase).
In the setting of AAA, biomarkers have been more extensively investigated. Genetic factors and specific miRNA showed great association with AAA development and progression. Considering point-of-care testing, Galectin-1 and Galectin-3 might be extremely useful in clinical practice as biomarker of AAA progression, while Granzyme K and malondialdehyde are potential indicators of AAA rupture.
Author Contributions
Conceptualization, F.S. (Francesco Stilo), V.C., A.N.; methodology, F.S. (Francesco Stilo), V.C., A.N., T.G., N.M; data curation, F.S. (Francesco Stilo), V.C., A.N., M.J., E.V.; writing—original draft preparation, F.S. (Francesco Stilo), V.C., M.J., E.V., T.G., N.M., F.A.C.; writing—review and editing, A.N., M.C.; supervision, M.C., F.S. (Francesco Spinelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
PubMed: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 1 November 2021); Scopus: https://www.scopus.com/home.uri (accessed on 1 November 2021).
Acknowledgments
We would like to thank our friend and colleague David Rose for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ng, E.; Morris, D.R.; Golledge, J. The association between plasma matrix metalloproteinase-9 concentration and endoleak after endovascular aortic aneurysm repair: A meta-analysis. Atherosclerosis 2015, 242, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Karaolanis, G.; Williams, Z.F.; Bakoyiannis, C.; Hadjis, D.; Cox, M.W.; Moris, D. The Clinical Utility and Assessment of Renal Biomarkers in Acute Kidney Injury After Abdominal Endovascular Aneurysm Repair. A Systematic Review. Curr. Pharm. Des. 2019, 25, 4695–4701. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sigala, F.; Karaolanis, G.; Ntanasis-Stathopoulos, I.; Spartalis, E.; Spartalis, M.; Patelis, N.; Papalampros, A.; Long, C.; Moris, D. Cytokines as biomarkers of inflammatory response after open versus endovascular repair of abdominal aortic aneurysms: A systematic review. Acta Pharmacol. Sin. 2018, 39, 1164–1175. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Murray, D.; Smyth, J.V.; Serracino-Inglott, F.; Paraskevas, K.I. Plasma matrix metalloproteinase 9 levels may predict endoleaks after endovascular aortic aneurysm repair. Angiology 2013, 64, 49–56. [Google Scholar] [CrossRef]
- Huddle, M.G.; Schlosser, F.J.; Dewan, M.C.; Indes, J.; Muhs, B.E. Can laboratory tests predict the prognosis of patients after endovascular aneurysm repair? Current status and future directions. Vascular 2009, 17, 129–137. [Google Scholar] [CrossRef]
- Kapetanios, D.M.; Karkos, C.D.; Papazoglou, K.O. Changes in circulating markers of coagulation and fibrinolysis after EVAR. Int. Angiol. A J. Int. Union Angiol. 2018, 37, 444–450. [Google Scholar] [CrossRef]
- Davies, R.S.; Abdelhamid, M.; Wall, M.L.; Vohra, R.K.; Bradbury, A.W.; Adam, D.J. Coagulation, fibrinolysis, and platelet activation in patients undergoing open and endovascular repair of abdominal aortic aneurysm. J. Vasc. Surg. 2011, 54, 865–878. [Google Scholar] [CrossRef] [Green Version]
- Missae, L.; Rossoni, B.; Tenorio, E.J.R.; Ribeiro, M.S.; Tirapelli, D.; Joviliano, E.E. Expression of MicroRNA-1281, C-Reactive Protein, and Renal Function in Individuals with Abdominal Aortic Aneurysm and their Clinical Correlation after Endovascular Repair. Braz. J. Cardiovasc. Surg. 2021, 36, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.F.; Davies, R.S.; Vohra, R.K.; Adam, D.J.; Bradbury, A.W. Assessment of renal function by means of cystatin C following standard and fenestrated endovascular aneurysm repair. Ann. Vasc. Surg. 2013, 27, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, W.; Chen, Z.; Xu, F.; Zheng, Y. Proteomics applications in biomarker discovery and pathogenesis for abdominal aortic aneurysm. Expert Rev. Proteom. 2021, 18, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Bylund, D.; Henriksson, A.E. Proteomic approaches to identify circulating biomarkers in patients with abdominal aortic aneurysm. Am. J. Cardiovasc. Dis. 2015, 5, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Urbonavicius, S.; Urbonaviciene, G.; Honore, B.; Henneberg, E.W.; Vorum, H.; Lindholt, J.S. Potential circulating biomarkers for abdominal aortic aneurysm expansion and rupture—A systematic review. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2008, 36, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stather, P.W.; Sidloff, D.A.; Dattani, N.; Gokani, V.J.; Choke, E.; Sayers, R.D.; Bown, M.J. Meta-analysis and meta-regression analysis of biomarkers for abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 1358–1372. [Google Scholar] [CrossRef]
- Nana, P.; Dakis, K.; Brodis, A.; Spanos, K.; Kouvelos, G. Circulating Biomarkers for the Prediction of Abdominal Aortic Aneurysm Growth. J. Clin. Med. 2021, 10, 1718. [Google Scholar] [CrossRef]
- Moris, D.; Mantonakis, E.; Avgerinos, E.; Makris, M.; Bakoyiannis, C.; Pikoulis, E.; Georgopoulos, S. Novel biomarkers of abdominal aortic aneurysm disease: Identifying gaps and dispelling misperceptions. BioMed Res. Int. 2014, 2014, 925840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Zheng, Y. Challenges of applying circulating biomarkers for abdominal aortic aneurysm progression. Exp. Biol. Med. 2021, 246, 1054–1059. [Google Scholar] [CrossRef]
- Groeneveld, M.E.; Meekel, J.P.; Rubinstein, S.M.; Merkestein, L.R.; Tangelder, G.J.; Wisselink, W.; Truijers, M.; Yeung, K.K. Systematic Review of Circulating, Biomechanical, and Genetic Markers for the Prediction of Abdominal Aortic Aneurysm Growth and Rupture. J. Am. Heart Assoc. 2018, 7, e007791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.M.; Rateri, D.L.; Daugherty, A. Abdominal aortic aneurysm: Novel mechanisms and therapies. Curr. Opin. Cardiol. 2015, 30, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Jalalzadeh, H.; Indrakusuma, R.; Planken, R.N.; Legemate, D.A.; Koelemay, M.J.; Balm, R. Inflammation as a Predictor of Abdominal Aortic Aneurysm Growth and Rupture: A Systematic Review of Imaging Biomarkers. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2016, 52, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lv, Z.; Jing, J.J.; Yang, J.; Yuan, Y. Matrix metalloproteinase family polymorphisms and the risk of aortic aneurysmal diseases: A systematic review and meta-analysis. Clin. Genet. 2018, 93, 15–32. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Martinez-Lopez, D.; Roldan-Montero, R.; Gomez-Guerrero, C.; Blanco-Colio, L.M. Role of complement system in pathological remodeling of the vascular wall. Mol. Immunol. 2019, 114, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Klopf, J.; Brostjan, C.; Neumayer, C.; Eilenberg, W. Neutrophils as Regulators and Biomarkers of Cardiovascular Inflammation in the Context of Abdominal Aortic Aneurysms. Biomedicines 2021, 9, 1236. [Google Scholar] [CrossRef]
- Araujo, N.N.F.; Lin-Wang, H.T.; Germano, J.F.; Farsky, P.S.; Feldman, A.; Rossi, F.H.; Izukawa, N.M.; Higuchi, M.L.; Savioli Neto, F.; Hirata, M.H.; et al. Dysregulation of microRNAs and target genes networks in human abdominal aortic aneurysm tissues. PLoS ONE 2019, 14, e0222782. [Google Scholar] [CrossRef]
- Butt, H.Z.; Sylvius, N.; Salem, M.K.; Wild, J.B.; Dattani, N.; Sayers, R.D.; Bown, M.J. Microarray-based Gene Expression Profiling of Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2016, 52, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Plana, E.; Galvez, L.; Medina, P.; Navarro, S.; Fornes-Ferrer, V.; Panadero, J.; Miralles, M. Identification of Novel microRNA Profiles Dysregulated in Plasma and Tissue of Abdominal Aortic Aneurysm Patients. Int. J. Mol. Sci. 2020, 21, 4600. [Google Scholar] [CrossRef]
- Zalewski, D.P.; Ruszel, K.P.; Stepniewski, A.; Galkowski, D.; Bogucki, J.; Kolodziej, P.; Szymanska, J.; Plachno, B.J.; Zubilewicz, T.; Feldo, M.; et al. Identification of Transcriptomic Differences between Lower Extremities Arterial Disease, Abdominal Aortic Aneurysm and Chronic Venous Disease in Peripheral Blood Mononuclear Cells Specimens. Int. J. Mol. Sci. 2021, 22, 3200. [Google Scholar] [CrossRef]
- Zalewski, D.P.; Ruszel, K.P.; Stepniewski, A.; Galkowski, D.; Bogucki, J.; Komsta, L.; Kolodziej, P.; Chmiel, P.; Zubilewicz, T.; Feldo, M.; et al. Dysregulation of microRNA Modulatory Network in Abdominal Aortic Aneurysm. J. Clin. Med. 2020, 9, 1974. [Google Scholar] [CrossRef] [PubMed]
- Blassova, T.; Tonar, Z.; Tomasek, P.; Hosek, P.; Hollan, I.; Treska, V.; Molacek, J. Inflammatory cell infiltrates, hypoxia, vascularization, pentraxin 3 and osteoprotegerin in abdominal aortic aneurysms—A quantitative histological study. PLoS ONE 2019, 14, e0224818. [Google Scholar] [CrossRef]
- Chiang, M.T.; Chen, I.M.; Hsu, F.F.; Chen, Y.H.; Tsai, M.S.; Hsu, Y.W.; Leu, H.B.; Huang, P.H.; Chen, J.W.; Liu, F.T.; et al. Gal-1 (Galectin-1) Upregulation Contributes to Abdominal Aortic Aneurysm Progression by Enhancing Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 331–345. [Google Scholar] [CrossRef]
- Hauzer, W.; Ferenc, S.; Rosinczuk, J.; Gnus, J. The Role of Serum Calprotectin as a New Marker in Abdominal Aortic Aneurysms—A Preliminary Report. Curr. Pharm. Biotechnol. 2021, 22, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Hauzer, W.; Witkiewicz, W.; Gnus, J. Calprotectin and Receptor for Advanced Glycation End Products as a Potential Biomarker in Abdominal Aortic Aneurysm. J. Clin. Med. 2020, 9, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, C.S.; Biros, E.; Krishna, S.M.; Morton, S.K.; Sexton, D.J.; Golledge, J. Kallikrein-1 Blockade Inhibits Aortic Expansion in a Mouse Model and Reduces Prostaglandin E2 Secretion From Human Aortic Aneurysm Explants. J. Am. Heart Assoc. 2021, 10, e019372. [Google Scholar] [CrossRef]
- Li, T.; Yang, C.; Jing, J.; Sun, L.; Yuan, Y. Granzyme K—A novel marker to identify the presence and rupture of abdominal aortic aneurysm. Int. J. Cardiol. 2021, 338, 242–247. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Trenner, M.; Boon, R.A.; Spin, J.M.; Maegdefessel, L. Long noncoding RNAs in key cellular processes involved in aortic aneurysms. Atherosclerosis 2020, 292, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Raffort, J.; Lareyre, F.; Clement, M.; Mallat, Z. Micro-RNAs in abdominal aortic aneurysms: Insights from animal models and relevance to human disease. Cardiovasc. Res. 2016, 110, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Boon, R.A.; Maegdefessel, L.; Dimmeler, S.; Jo, H. Role of Noncoding RNAs in the Pathogenesis of Abdominal Aortic Aneurysm. Circ. Res. 2019, 124, 619–630. [Google Scholar] [CrossRef]
- Borek, A.; Drzymala, F.; Botor, M.; Augusciak-Duma, A.M.; Sieron, A.L. Roles of microRNAs in abdominal aortic aneurysm pathogenesis and the possibility of their use as biomarkers. Kardiochirurgia I Torakochirurgia Pol. = Pol. J. Cardio-Thorac. Surg. 2019, 16, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Rowbotham, S.; Biros, E.; Bingley, J.; Golledge, J. A systematic review investigating the association of microRNAs with human abdominal aortic aneurysms. Atherosclerosis 2017, 261, 78–89. [Google Scholar] [CrossRef]
- Knappich, C.; Spin, J.M.; Eckstein, H.H.; Tsao, P.S.; Maegdefessel, L. Involvement of Myeloid Cells and Noncoding RNA in Abdominal Aortic Aneurysm Disease. Antioxid. Redox Signal. 2020, 33, 602–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovsepian, D.M.; Ziporin, S.J.; Sakurai, M.K.; Lee, J.K.; Curci, J.A.; Thompson, R.W. Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: A circulating marker of degenerative aneurysm disease. J. Vasc. Interv. Radiol. JVIR 2000, 11, 1345–1352. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Wang, X.; Wilks, J.A.; Carter, S.A.; Wen, S.; Won, T.; Leonardelli, D.; Anand, G.; Conklin, L.D.; Wang, X.L.; et al. Matrix metalloproteinases in ascending aortic aneurysms: Bicuspid versus trileaflet aortic valves. J. Surg. Res. 2005, 123, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Koullias, G.J.; Ravichandran, P.; Korkolis, D.P.; Rimm, D.L.; Elefteriades, J.A. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2004, 78, 2106–2110. [Google Scholar] [CrossRef]
- Ramirez, F.; Rifkin, D.B. Extracellular microfibrils: Contextual platforms for TGFbeta and BMP signaling. Curr. Opin. Cell Biol. 2009, 21, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffensen, L.B.; Stubbe, J.; Lindholt, J.S.; Beck, H.C.; Overgaard, M.; Bloksgaard, M.; Genovese, F.; Holm Nielsen, S.; Tha, M.L.T.; Bang-Moeller, S.K.; et al. Basement membrane collagen IV deficiency promotes abdominal aortic aneurysm formation. Sci. Rep. 2021, 11, 12903. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, N.P.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, U.; Schievink, W.I.; Petty, E.; Jaff, M.R.; Babovic-Vuksanovic, D.; Cherry, K.J.; Pepin, M.; Byers, P.H. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am. J. Hum. Genet. 2001, 69, 989–1001. [Google Scholar] [CrossRef] [Green Version]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Didangelos, A.; Yin, X.; Mandal, K.; Baumert, M.; Jahangiri, M.; Mayr, M. Proteomics characterization of extracellular space components in the human aorta. Mol. Cell. Proteom. MCP 2010, 9, 2048–2062. [Google Scholar] [CrossRef] [Green Version]
- Barallobre-Barreiro, J.; Loeys, B.; Mayr, M.; Rienks, M.; Verstraeten, A.; Kovacic, J.C. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2189–2203. [Google Scholar] [CrossRef]
- Yin, X.; Wanga, S.; Fellows, A.L.; Barallobre-Barreiro, J.; Lu, R.; Davaapil, H.; Franken, R.; Fava, M.; Baig, F.; Skroblin, P.; et al. Glycoproteomic Analysis of the Aortic Extracellular Matrix in Marfan Patients. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Zagrapan, B.; Bleichert, S.; Ibrahim, N.; Knobl, V.; Brandau, A.; Martelanz, L.; Grasl, M.T.; Hayden, H.; Nawrozi, P.; et al. Histone citrullination as a novel biomarker and target to inhibit progression of abdominal aortic aneurysms. Transl. Res. J. Lab. Clin. Med. 2021, 233, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Zhang, J.; Xu, P.; Wang, H. MicroRNA-29a-3p regulates abdominal aortic aneurysm development and progression via direct interaction with PTEN. J. Cell. Physiol. 2020, 235, 9414–9423. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y.; Siu, K.L.; Zhang, Y.; Cai, H. Targeting feed-forward signaling of TGFbeta/NOX4/DHFR/eNOS uncoupling/TGFbeta axis with anti-TGFbeta and folic acid attenuates formation of aortic aneurysms: Novel mechanisms and therapeutics. Redox Biol. 2021, 38, 101757. [Google Scholar] [CrossRef]
- Huang, T.; Liu, S.; Liu, R.; Pan, B.; Wang, W. Inhibition of miR-188-5p Suppresses Progression of Experimental Abdominal Aortic Aneurysms. J. Cardiovasc. Pharmacol. 2021, 77, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.S.; Opacic, D.; Mutavdzic, P.; Koncar, I.; Dragas, M.; Jovicic, S.; Markovic, M.; Davidovic, L. Evaluation of the renal function using serum Cystatin C following open and endovascular aortic aneurysm repair. Vascular 2018, 26, 132–141. [Google Scholar] [CrossRef]
- Arnaoutoglou, E.; Kouvelos, G.; Papa, N.; Karamoutsios, A.; Bouris, V.; Vartholomatos, G.; Matsagkas, M. Platelet activation after endovascular repair of abdominal aortic aneurysm. Vascular 2016, 24, 287–294. [Google Scholar] [CrossRef]
- De Haro, J.; Bleda, S.; Acin, F. C-reactive protein predicts aortic aneurysmal disease progression after endovascular repair. Int. J. Cardiol. 2016, 202, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Courtois, A.; Makrygiannis, G.; El Hachemi, M.; Hultgren, R.; Allaire, E.; Namur, G.; Hustinx, R.; Defraigne, J.O.; Sakalihasan, N. Positron Emission Tomography/Computed Tomography Predicts and Detects Complications After Endovascular Repair of Abdominal Aortic Aneurysms. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2019, 26, 520–528. [Google Scholar] [CrossRef]
- Ascoli Marchetti, A.; Pratesi, G.; Di Giulio, L.; Battistini, M.; Massoud, R.; Ippoliti, A. EVAR and OPEN treatment of abdominal aortic aneurysm: What is the role of MMP-9 in the follow-up? J. Med. Vasc. 2017, 42, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, G.S.; Antoniou, G.A.; Argyriou, C.; Schoretsanitis, N.; Nikolopoulos, E.; Kapoulas, K.; Lazarides, M.K.; Tentes, I. Correlation of Baseline Plasma and Inguinal Connective Tissue Metalloproteinases and Their Inhibitors With Late High-Pressure Endoleak After Endovascular Aneurysm Repair: Long-term Results. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2019, 26, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, F.A.; Ten Bosch, J.A.; Pulinx, B.; Wodzig, W.K.; de Haan, M.W.; Prins, M.H.; Welten, R.J.; Teijink, J.A.; Schurink, G.W. Plasma levels of matrix metalloproteinase-9: A possible diagnostic marker of successful endovascular aneurysm repair. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2012, 43, 171–172. [Google Scholar] [CrossRef] [Green Version]
- Moxon, J.V.; Ng, E.; Lazzaroni, S.M.; Boult, M.; Velu, R.; Fitridge, R.A.; Golledge, J. Circulating biomarkers are not associated with endoleaks after endovascular repair of abdominal aortic aneurysms. J. Vasc. Surg. 2018, 67, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Filis, K.; Martinakis, V.; Galyfos, G.; Sigala, F.; Theodorou, D.; Andreadou, I.; Zografos, G. Osteopontin and Osteoprotegerin as Potential Biomarkers in Abdominal Aortic Aneurysm before and after Treatment. Int. Sch. Res. Not. 2014, 2014, 461239. [Google Scholar] [CrossRef]
- Ikoma, A.; Nakai, M.; Sato, M.; Sato, H.; Takeuchi, H.; Tanaka, F.; Sanda, H.; Nakata, K.; Minamiguchi, H.; Sonomura, T.; et al. Changes in inflammatory, coagulopathic, and fibrinolytic responses after endovascular repair of an abdominal aortic aneurysm: Relationship between fibrinogen degradation product levels and endoleaks. Jpn. J. Radiol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Inagaki, E.; Farber, A.; Eslami, M.H.; Kalish, J.; Rybin, D.V.; Doros, G.; Peacock, M.R.; Siracuse, J.J. Preoperative hypoalbuminemia is associated with poor clinical outcomes after open and endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2017, 66, 53–63.e51. [Google Scholar] [CrossRef] [Green Version]
- Wohlauer, M.; Brier, C.; Kuramochi, Y.; Eagleton, M. Preoperative Hypoalbuminemia is a Risk Factor for Early and Late Mortality in Patients Undergoing Endovascular Juxtarenal and Thoracoabdominal Aortic Aneurysm Repair. Ann. Vasc. Surg. 2017, 42, 198–204. [Google Scholar] [CrossRef]
- Kapetanios, D.; Karkos, C.D.; Pliatsios, I.; Mitka, M.; Giagtzidis, I.T.; Konstantinidis, K.; Papazoglou, K.O. Association Between Perioperative Fibrinogen Levels and the Midterm Outcome in Patients Undergoing Elective Endovascular Repair of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2019, 56, 202–208. [Google Scholar] [CrossRef]
- Lecumberri, E.; Ruiz-Carmona, C.; Mateos, E.; Galarza, A.; Subirana, I.; Clara, A. Prognostic Value of Inflammatory Biomarkers in 5-Year Survival After Endovascular Repair of Abdominal Aortic Aneurysms in a Predominantly Male Cohort: Implications for Practice. World J. Surg. 2021, 45, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Ntalouka, M.P.; Nana, P.; Kouvelos, G.N.; Stamoulis, K.; Spanos, K.; Giannoukas, A.; Matsagkas, M.; Arnaoutoglou, E. Association of Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratio with Adverse Events in Endovascular Repair for Abdominal Aortic Aneurysm. J. Clin. Med. 2021, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Octeau, D.; Faries, C.; Barnes, H.; Nakazawa, K.R.; Rao, A.J.; Ting, W.; Marin, M.L.; Vouyouka, A.G.; Faries, P.L.; Tadros, R.O. Neutrophil-to-Lymphocyte Ratio Associated With Adverse Events After Endovascular Aneurysm Repair (EVAR). Ann. Vasc. Surg. 2021, 75, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, J.H.; Kim, E.J. Volume of mural thrombus plays a role in the elevation of inflammatory markers after endovascular aortic repair. J. Cardiothorac. Surg. 2018, 13, 27. [Google Scholar] [CrossRef]
- Lee, R.; Cassimee, I.; Huang, H.; Lapolla, P.; Ngetich, E.; Chandrashekar, A.; Charles, P.; Kessler, B.; Fischer, R.; Handa, A. Integrated Plasma and Tissue Proteomics Reveals Attractin Release by Intraluminal Thrombus of Abdominal Aortic Aneurysms and Improves Aneurysm Growth Prediction in Humans. Ann. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, F.; Lindstrom, D.; Gillgren, P.; Ternhag, A. The role of procalcitonin in postimplantation syndrome after EVAR: A pilot study. Ann. Vasc. Surg. 2014, 28, 866–873. [Google Scholar] [CrossRef]
- Nessvi Otterhag, S.; Gottsater, A.; Acosta, S.; Palmqvist, B.; Lindblad, B. Inflammatory mediators after endovascular aortic aneurysm repair. Cytokine 2014, 70, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Noorani, A.; Sadat, U.; Rollins, K.E.; Chowdhury, M.M.; Tang, T.Y.; Harrison, S.C.; Usman, A.; Burling, K.; Nordon, A.; Boyle, J.R. Assessment of Renal Injury in Patients Undergoing Elective EVAR Using Urinary Neutrophil Gelatin-Associated Lipocalin, Interleukin 18, and Retinol-Binding Protein. Angiology 2017, 68, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, B.; Tessarolo, S.; Giubbilini, P.; Gaia, P.; Corino, S.D.; Mazza, S.; Rigolini, R.; Poli, M.D.; Vianello, E.; Romanelli, M.M.C.; et al. Neutrophil gelatinase-associated lipocalin and acute kidney injury in endovascular aneurysm repair or open aortic repair: A pilot study. Biochem. Med. 2018, 28, 010904. [Google Scholar] [CrossRef]
- Obata, Y.; Kamijo-Ikemori, A.; Ichikawa, D.; Sugaya, T.; Kimura, K.; Shibagaki, Y.; Tateda, T. Clinical usefulness of urinary liver-type fatty-acid-binding protein as a perioperative marker of acute kidney injury in patients undergoing endovascular or open-abdominal aortic aneurysm repair. J. Anesth. 2016, 30, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obata, Y.; Kamijo-Ikemori, A.; Inoue, S. Clinical Utility of Urinary Biomarkers for Prediction of Acute Kidney Injury and Chronic Renal Dysfunction After Open Abdominal Aortic Aneurysm Repair. Int. J. Nephrol. Renov. Dis. 2021, 14, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Ousaka, D.; Fujii, Y.; Oozawa, S.; Nishibori, M.; Kuroko, Y.; Masuda, Z.; Sano, S. Decreased Serum Levels of High Mobility Group Box 1 (HMGB-1) after Graft Replacement or Stenting of Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2017, 41, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Pirgakis, K.M.; Makris, K.; Dalainas, I.; Lazaris, A.M.; Maltezos, C.K.; Liapis, C.D. Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann. Vasc. Surg. 2014, 28, 1649–1658. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, W.; Niu, L.; Yu, W.; Li, C.; Wang, H. Combined Detection of Plasma Tumor Necrosis Factor-alpha Converting Enzyme and Notch1 is Valuable in Screening Endoleak After Endovascular Abdominal Aortic Aneurysms Repair. Ann. Vasc. Surg. 2021, 76, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Whaley, Z.L.; Cassimjee, I.; Novak, Z.; Rowland, D.; Lapolla, P.; Chandrashekar, A.; Pearce, B.J.; Beck, A.W.; Handa, A.; Lee, R. The Spatial Morphology of Intraluminal Thrombus Influences Type II Endoleak after Endovascular Repair of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2020, 66, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, C.M.; Abdelhamid, M.; Adam, D.J.; Nash, G.B.; Bradbury, A.W.; Rainger, G.E. Endovascular aneurysm repair reverses the increased titer and the inflammatory activity of interleukin-1alpha in the serum of patients with abdominal aortic aneurysm. J. Vasc. Surg. 2011, 54, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Zettervall, S.L.; Dansey, K.; Swerdlow, N.J.; Soden, P.; Evenson, A.; Schermerhorn, M.L. Aspartate transaminase to platelet ratio index and Model for End-Stage Liver Disease scores are associated with morbidity and mortality after endovascular aneurysm repair among patients with liver dysfunction. J. Vasc. Surg. 2020, 72, 904–909. [Google Scholar] [CrossRef]
- Zettervall, S.L.; Ultee, K.H.J.; Soden, P.A.; Deery, S.E.; Shean, K.E.; Pothof, A.B.; Wyers, M.; Schermerhorn, M.L. Predictors of renal dysfunction after endovascular and open repair of abdominal aortic aneurysms. J. Vasc. Surg. 2017, 65, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Lesiak, M.; Augusciak-Duma, A.; Stepien, K.L.; Fus-Kujawa, A.; Botor, M.; Sieron, A.L. Searching for new molecular markers for cells obtained from abdominal aortic aneurysm. J. Appl. Genet. 2021, 62, 487–497. [Google Scholar] [CrossRef]
- Xie, X.; Wang, E.C.; Xu, D.; Shu, X.; Zhao, Y.F.; Guo, D.; Fu, W.; Wang, L. Bioinformatics Analysis Reveals the Potential Diagnostic Biomarkers for Abdominal Aortic Aneurysm. Front. Cardiovasc. Med. 2021, 8, 656263. [Google Scholar] [CrossRef]
- Gan, S.; Pan, Y.; Mao, J. miR-30a-GNG2 and miR-15b-ACSS2 Interaction Pairs May Be Potentially Crucial for Development of Abdominal Aortic Aneurysm by Influencing Inflammation. DNA Cell Biol. 2019, 38, 1540–1556. [Google Scholar] [CrossRef]
- Gan, S.; Shi, W.; Tang, J. miRNAs regulating the expressions of NTF3, GNG2 and ITGA7 are involved in the pathogenesis of abdominal aortic aneurysm in mice. Gen. Physiol. Biophys. 2021, 40, 1–16. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Li, R.; Zhang, P.; Wang, Y.; Gopinath, S.C.B.; Gong, K.; Wan, P. High-performance detection of an abdominal aortic aneurysm biomarker by immunosensing. Biotechnol. Appl. Biochem. 2020, 67, 383–388. [Google Scholar] [CrossRef]
- Cersit, S.; Ocal, L.; Keskin, M.; Gursoy, M.O.; Kalcik, M.; Bayam, E.; Karaduman, A.; Uysal, S.; Uslu, A.; Kup, A.; et al. Association of C-Reactive Protein-to-Albumin Ratio With the Presence and Progression of Abdominal Aortic Aneurysm. Angiology 2021, 72, 153–158. [Google Scholar] [CrossRef]
- Lu, H.Y.; Shih, C.M.; Sung, S.H.; Wu, A.T.H.; Cheng, T.M.; Lin, Y.C.; Shih, C.C. Galectin-3 as a Biomarker for Stratifying Abdominal Aortic Aneurysm Size in a Taiwanese Population. Front. Cardiovasc. Med. 2021, 8, 663152. [Google Scholar] [CrossRef]
- Jablonska, A.; Zagrapan, B.; Neumayer, C.; Klinger, M.; Eilenberg, W.; Nanobachvili, J.; Paradowska, E.; Brostjan, C.; Huk, I. TLR2 2029C/T and TLR3 1377C/T and -7C/A Polymorphisms Are Associated with the Occurrence of Abdominal Aortic Aneurysm. J. Immunol. 2020, 204, 2900–2909. [Google Scholar] [CrossRef]
- Jeong, S.J.; Cho, M.J.; Ko, N.Y.; Kim, S.; Jung, I.H.; Min, J.K.; Lee, S.H.; Park, J.G.; Oh, G.T. Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm. Exp. Mol. Med. 2020, 52, 1587–1601. [Google Scholar] [CrossRef]
- Kim, E.N.; Yu, J.; Lim, J.S.; Jeong, H.; Kim, C.J.; Choi, J.S.; Kim, S.R.; Ahn, H.S.; Kim, K.; Oh, S.J. CRP immunodeposition and proteomic analysis in abdominal aortic aneurysm. PLoS ONE 2021, 16, e0245361. [Google Scholar] [CrossRef]
- Li, L.; Shao, J.; Niu, W.; Che, H.; Song, F.; Liu, G.; Lu, S. Neutrophil Gelatinase-Associated Lipocalin as an Early Predictor of Contrast-Induced Nephropathy Following Endovascular Aortic Repair for Abdominal Aortic Aneurysm. Clin. Appl. Thromb./Hemost. Off. J. Int. Acad. Clin. Appl. Thromb./Hemost. 2021, 27, 10760296211025618. [Google Scholar] [CrossRef]
- Li, T.; Wang, T.; Zhao, X. Profiles of immune infiltration in abdominal aortic aneurysm and their associated marker genes: A gene expression-based study. Braz. J. Med. Biol. Res. = Rev. Bras. De Pesqui. Med. E Biol. 2021, 54, e11372. [Google Scholar] [CrossRef]
- Lieberg, J.; Wanhainen, A.; Ottas, A.; Vahi, M.; Zilmer, M.; Soomets, U.; Bjorck, M.; Kals, J. Metabolomic Profile of Abdominal Aortic Aneurysm. Metabolites 2021, 11, 555. [Google Scholar] [CrossRef]
- Lindquist Liljeqvist, M.; Eriksson, L.; Villard, C.; Lengquist, M.; Kronqvist, M.; Hultgren, R.; Roy, J. Dipeptidyl peptidase-4 is increased in the abdominal aortic aneurysm vessel wall and is associated with aneurysm disease processes. PLoS ONE 2020, 15, e0227889. [Google Scholar] [CrossRef] [Green Version]
- Maitiseyiti, A.; Ci, H.; Fang, Q.; Guan, S.; Shawuti, A.; Wang, H.; Ge, X. Identification of Novel Long Noncoding RNAs and Their Role in Abdominal Aortic Aneurysm. BioMed Res. Int. 2020, 2020, 3502518. [Google Scholar] [CrossRef]
- Memon, A.A.; Zarrouk, M.; Agren-Witteschus, S.; Sundquist, J.; Gottsater, A.; Sundquist, K. Identification of novel diagnostic and prognostic biomarkers for abdominal aortic aneurysm. Eur. J. Prev. Cardiol. 2020, 27, 132–142. [Google Scholar] [CrossRef]
- Shi, F.; Ma, C.; Ji, C.; Li, M.; Liu, X.; Han, Y. Serum Lipid Oxidative Stress Products as Risk Factors Are the Candidate Predictive Biomarkers for Human Abdominal Aortic Aneurysms. Clin. Appl. Thromb. /Hemost. Off. J. Int. Acad. Clin. Appl. Thromb. /Hemost. 2020, 26, 1076029620932226. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Pope, A.; Dear, A.E. Point of care ankle pulse waveform: A biomarker for abdominal aortic aneurysm? Vascular 2021, 17085381211013976. [Google Scholar] [CrossRef]
- Xie, T.; Yin, L.; Guo, D.; Zhang, Z.; Chen, Y.; Liu, B.; Wang, W.; Zheng, Y. The potential role of plasma fibroblast growth factor 21 as a diagnostic biomarker for abdominal aortic aneurysm presence and development. Life Sci. 2021, 274, 119346. [Google Scholar] [CrossRef]
- Liu, P.; Sun, Z.; Zhang, Y.; Guo, W. Myeloid related protein 8/14 is a new candidate biomarker and therapeutic target for abdominal aortic aneurysm. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 118, 109229. [Google Scholar] [CrossRef]
- Xu, W.; Chao, Y.; Liang, M.; Huang, K.; Wang, C. CTRP13 Mitigates Abdominal Aortic Aneurysm Formation via NAMPT1. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 324–337. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Chen, S.; Li, F.; Cui, L.; Liu, Z.; Shao, J.; Chen, Y.; Liu, B.; Zheng, Y. Identification of potential proteases for abdominal aortic aneurysm by weighted gene coexpression network analysis. Genome 2020, 63, 561–575. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).