Differentiation Model for Insomnia Disorder and the Respiratory Arousal Threshold Phenotype in Obstructive Sleep Apnea in the Taiwanese Population Based on Oximetry and Anthropometric Features
Abstract
:1. Introduction
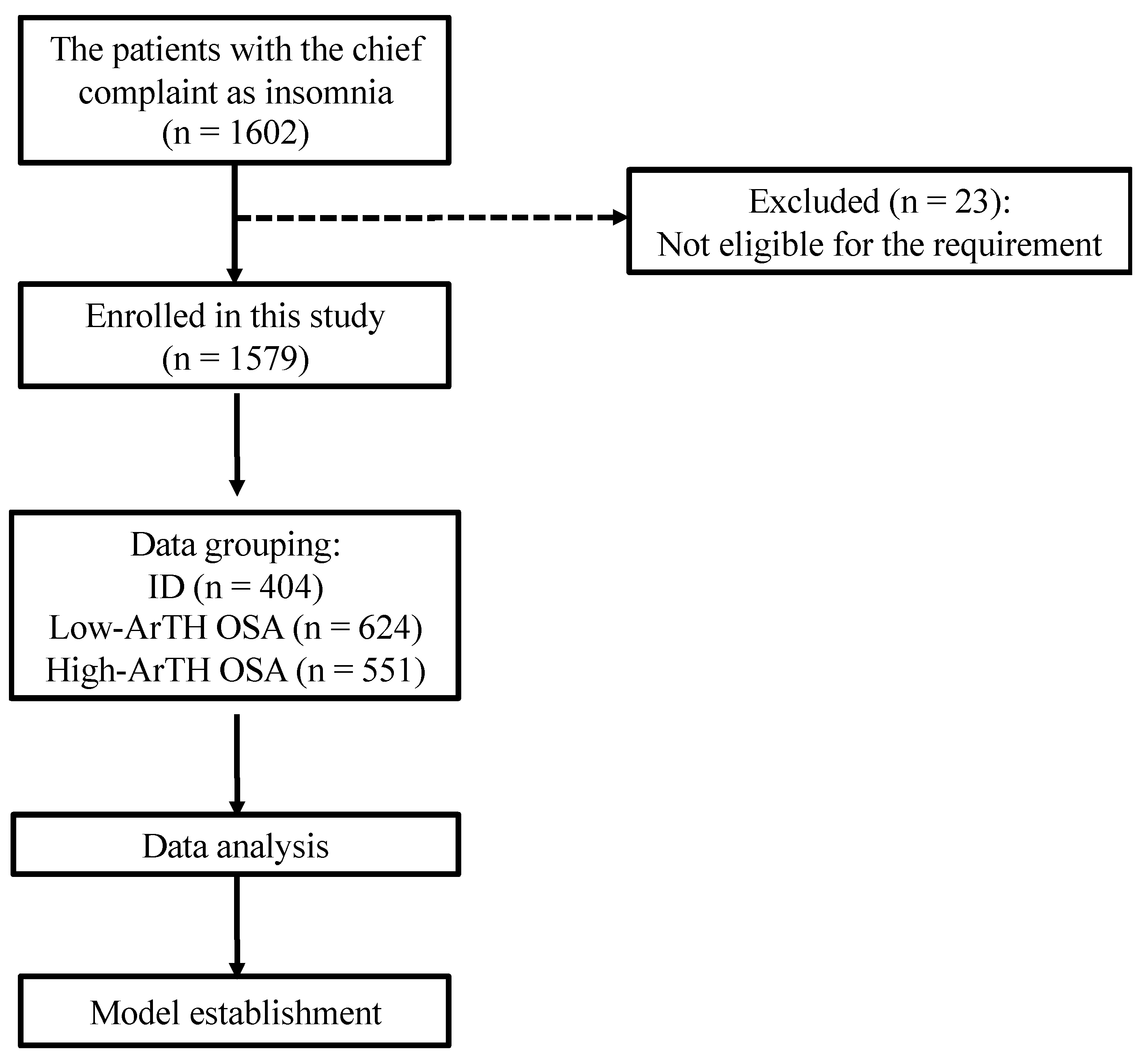
2. Materials and Methods
2.1. Study Population
2.2. PSG Results
2.3. Statistical Analysis
2.4. Prediction Model
2.5. Performance Evaluation
3. Results
3.1. Demographics of Study Participants
3.2. Sleep Architecture and Oximetry
3.3. Model Construction and Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ID | insomnia disorder |
| OSA | obstructive sleep apnea |
| AASM | American Association of Sleep Medicine |
| AHI | apnea–hypopnea index |
| ArI | arousal index |
| ArTH | respiratory arousal threshold |
| AUC | area under the curve |
| BMI | body mass index |
| CART | multiple classification and regression trees |
| CPAP | continuous positive airway pressure |
| ESS | Epworth Sleepiness Scale |
| F-hypopnea | hypopnea events / hypopnea events + apnea events |
| HST | home sleep test |
| ISI | Insomnia Severity Index |
| I-SpO2-<90% | the ratio of accumulation time for an SpO2 below 90% and total recording time |
| kNN | k-nearest neighbors |
| lab-PSG | in-laboratory polysomnography |
| LMArI | limb movement-related arousal index |
| LR | logistic regression |
| NB | naive Bayes |
| NREM | nonrapid eye movement |
| ODI-3% | oxygen desaturation index ≥ 3% |
| ODI-3%-TRT | the total recording time ODI-3% |
| PSG | polysomnography |
| PSQI | Pittsburgh Sleep Quality Index |
| RArI | respiratory arousal index |
| RBD | rapid eye movement sleep behavior disorder |
| REM | rapid eye movement |
| RF | random forest |
| SnArI | snoring arousal index |
| SpO2 | peripheral arterial oxygen saturation measured using pulse oximetry |
| SPT | sleep period of time |
| SVM | support vector machine |
| TST | total sleep time |
| TMU-JIRB | Taipei Medical University–Joint Institutional Review Board |
| WASO | wake after sleep onset |
References
- Cho, Y.W.; Kim, K.T.; Moon, H.-J.; Korostyshevskiy, V.R.; Motamedi, G.K.; Yang, K.I. Comorbid Insomnia with Obstructive Sleep Apnea: Clinical Characteristics and Risk Factors. J. Clin. Sleep Med. 2018, 14, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ren, R.; Lei, F.; Zhou, J.; Zhang, J.; Wing, Y.-K.; Sanford, L.D.; Tang, X. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 45, 1–17. [Google Scholar] [CrossRef]
- Chung, K.F.; Yeung, W.F.; Ho, F.Y.; Yung, K.P.; Yu, Y.M.; Kwok, C.W. Cross-cultural and comparative epidemiology of insomnia: The Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and International classification of sleep disorders (ICSD). Sleep Med. 2015, 16, 477–482. [Google Scholar] [CrossRef]
- Hnin, K.; Mukherjee, S.; Antic, N.A.; Catcheside, P.; Chai-Coetzer, C.L.; McEvoy, D.; Vakulin, A. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med. Rev. 2018, 41, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Vaishali, K.; Gatty, A.; Srivastav, P.; Amin, R.R. Coping strategies for obese individuals with obstructive sleep apnea during COVID-19 pandemic: A narrative review. Obes. Med. 2021, 22, 100324. [Google Scholar] [CrossRef]
- Kokou-Kpolou, C.K.; Megalakaki, O.; Laimou, D.; Kousouri, M. Insomnia during COVID-19 pandemic and lockdown: Prevalence, severity, and associated risk factors in French population. Psychiatry Res. 2020, 290, 113128. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Sands, S.A.; Edwards, B.A.; Azarbarzin, A.; Marques, M.; de Melo, C.; Eckert, D.J.; White, D.P.; Wellman, A. Effects of Tiagabine on Slow Wave Sleep and Arousal Threshold in Patients with Obstructive Sleep Apnea. Sleep 2017, 40, zsw047. [Google Scholar] [CrossRef]
- Sweetman, A.M.; Lack, L.C.; Catcheside, P.G.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.S.; Douglas, J.A.; McEvoy, R.D. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med. Rev. 2017, 33, 28–38. [Google Scholar] [CrossRef]
- Edwards, B.A.; Eckert, D.J.; McSharry, D.G.; Sands, S.A.; Desai, A.; Kehlmann, G.; Bakker, J.P.; Genta, P.R.; Owens, R.L.; White, D.P. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1293–1300. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Sands, S.A.; Terrill, P.I.; Edwards, B.A.; Taranto Montemurro, L.; Azarbarzin, A.; Marques, M.; de Melo, C.M.; Loring, S.H.; Butler, J.P.; White, D.P.; et al. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018, 41, zsx183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Reply: Arousal threshold in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 373–374. [Google Scholar] [CrossRef] [Green Version]
- Winkelman, J.W. Insomnia disorder. N. Engl. J. Med. 2015, 373, 1437–1444. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; Volume 21, pp. 362–364. [Google Scholar]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Krystal, A.D.; Prather, A.A.; Ashbrook, L.H. The assessment and management of insomnia: An update. World Psychiatry 2019, 18, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Dolly, F.R.; Block, A.J. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am. J. Med. 1982, 73, 239–243. [Google Scholar] [CrossRef]
- Matheson, E.; Hainer, B.L. Insomnia: Pharmacologic Therapy. Am. Fam. Physician 2017, 96, 29–35. [Google Scholar]
- Riedel, B.W.; Winfield, C.F.; Lichstein, K.L. First night effect and reverse first night effect in older adults with primary insomnia: Does anxiety play a role? Sleep Med. 2001, 2, 125–133. [Google Scholar] [CrossRef]
- Newell, J.; Mairesse, O.; Verbanck, P.; Neu, D. Is a one-night stay in the lab really enough to conclude? First-night effect and night-to-night variability in polysomnographic recordings among different clinical population samples. Psychiatry Res. 2012, 200, 795–801. [Google Scholar] [CrossRef]
- Littner, M.; Hirshkowitz, M.; Kramer, M.; Kapen, S.; Anderson, W.M.; Bailey, D.; Berry, R.B.; Davila, D.; Johnson, S.; Kushida, C. Practice parameters for using polysomnography to evaluate insomnia: An update. Sleep 2003, 26, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [Green Version]
- Rotenberg, B.W.; George, C.F.; Sullivan, K.M.; Wong, E. Wait times for sleep apnea care in Ontario: A multidisciplinary assessment. Can. Respir. J. 2010, 17, 170–174. [Google Scholar] [CrossRef]
- Wallace, D.M.; Wohlgemuth, W.K. Predictors of Insomnia Severity Index profiles in United States veterans with obstructive sleep apnea. J. Clin. Sleep Med. 2019, 15, 1827–1837. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Jan, Y.-W.; Yang, C.-M. Are the Insomnia Severity Index and Pittsburgh Sleep Quality Index valid outcome measures for cognitive behavioral therapy for insomnia? Inquiry from the perspective of response shifts and longitudinal measurement invariance in their Chinese versions. Sleep Med. 2017, 35, 35–40. [Google Scholar] [CrossRef]
- Sanford, S.D.; Lichstein, K.L.; Durrence, H.H.; Riedel, B.W.; Taylor, D.J.; Bush, A.J. The influence of age, gender, ethnicity, and insomnia on Epworth sleepiness scores: A normative US population. Sleep Med. 2006, 7, 319–326. [Google Scholar] [CrossRef]
- Kram, J.A.; Hu, H. Home sleep testing for sleep disorders. In Primary Care Sleep Medicine; Springer: New York, USA, 2014; pp. 43–54. [Google Scholar] [CrossRef]
- Collop, N.A.; Anderson, W.M.; Boehlecke, B.; Claman, D.; Goldberg, R.; Gottlieb, D.J.; Hudgel, D.; Sateia, M.; Schwab, R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J. Clin. Sleep Med. 2007, 3, 737–747. [Google Scholar] [CrossRef]
- Kundel, V.; Shah, N. Impact of portable sleep testing. Sleep Med. Clin. 2017, 12, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Thurzo, A.; Kosnáčová, H.S.; Kurilová, V.; Kosmeľ, S.; Beňuš, R.; Moravanský, N.; Kováč, P.; Kuracinová, K.M.; Palkovič, M.; Varga, I. Use of Advanced Artificial Intelligence in Forensic Medicine, Forensic Anthropology and Clinical Anatomy. Healthcare 2021, 9, 1545. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C. Machine Learning-Based Definition of Symptom Clusters and Selection of Antidepressants for Depressive Syndrome. Diagnostics 2021, 11, 1631. [Google Scholar] [CrossRef]
- Urtnasan, E.; Joo, E.Y.; Lee, K.H. AI-Enabled Algorithm for Automatic Classification of Sleep Disorders Based on Single-Lead Electrocardiogram. Diagnostics 2021, 11, 2054. [Google Scholar] [CrossRef]
- Uçar, M.K.; Bozkurt, M.R.; Bilgin, C.; Polat, K. Automatic detection of respiratory arrests in OSA patients using PPG and machine learning techniques. Neural Comput. Appl. 2017, 28, 2931–2945. [Google Scholar] [CrossRef]
- Buettner, R.; Grimmeisen, A.; Gotschlich, A. High-performance Diagnosis of Sleep Disorders: A Novel, Accurate and Fast Machine Learning Approach Using Electroencephalographic Data. In Proceedings of the 53rd Hawaii International Conference on System Sciences, Honolulu, HI, USA, 7 January 2020; The University of Hawaii at Mānoa: Honolulu, HI, USA, 2020; pp. 3246–3255. [Google Scholar] [CrossRef] [Green Version]
- Crönlein, T.; Langguth, B.; Busch, V.; Rupprecht, R.; Wetter, T.C. Severe chronic insomnia is not associated with higher body mass index. J. Sleep Res. 2015, 24, 514–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhou, J.; Sun, Y.; Li, Z.; Lei, F.; Zhou, G.; Tang, X. Polysomnographically determined sleep and body mass index in patients with insomnia. Psychiatry Res. 2013, 209, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Roux, F. The relationship of obesity and obstructive sleep apnea. Clin. Chest Med. 2009, 30, 455–465. [Google Scholar] [CrossRef]
- Li, R.; Wing, Y.; Ho, S.; Fong, S. Gender differences in insomnia—a study in the Hong Kong Chinese population. J. Psychosom. Res. 2002, 53, 601–609. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Sooronbaev, T.; Mirrakhimov, E.M. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm. Med. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichstein, K.L.; Riedel, B.W.; Lester, K.W.; Aguillard, R.N. Occult sleep apnea in a recruited sample of older adults with insomnia. J. Consult. Clin. Psychol. 1999, 67, 405. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Gehrman, P.R.; Nkwuo, J.E.; Bellamy, S.L.; Schutte-Rodin, S.; Dinges, D.F.; Pack, A.I. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch. Intern. Med. 2006, 166, 1732–1738. [Google Scholar] [CrossRef]
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis. Primers 2015, 1, 15026. [Google Scholar] [CrossRef]
- Wali, S.O.; Abaalkhail, B.; AlQassas, I.; Alhejaili, F.; Spence, D.W.; Pandi-Perumal, S.R. The correlation between oxygen saturation indices and the standard obstructive sleep apnea severity. Ann. Thorac. Med. 2020, 15, 70–75. [Google Scholar] [CrossRef]
- Oeverland, B.; Skatvedt, O.; Kvaerner, K.J.; Akre, H. Pulseoximetry: Sufficient to diagnose severe sleep apnea. Sleep Med. 2002, 3, 133–138. [Google Scholar] [CrossRef]
- Lee, R.W.; Vasudavan, S.; Hui, D.S.; Prvan, T.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep 2010, 33, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Lee, R.W.; Cistulli, P.A. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: Impact of ethnicity. Respirology 2012, 17, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Schorr, F.; Kayamori, F.; Hirata, R.P.; Danzi-Soares, N.J.; Gebrim, E.M.; Moriya, H.T.; Malhotra, A.; Lorenzi-Filho, G.; Genta, P.R. Different Craniofacial Characteristics Predict Upper Airway Collapsibility in Japanese-Brazilian and White Men. Chest 2016, 149, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sert Kuniyoshi, F.H.; Covassin, N.; Singh, P.; Gami, A.S.; Wang, S.; Chahal, C.A.; Wei, Y.; Somers, V.K. Nocturnal Hypoxemia due to Obstructive Sleep Apnea Is an Independent Predictor of Poor Prognosis After Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003162. [Google Scholar] [CrossRef] [Green Version]
- Mencar, C.; Gallo, C.; Mantero, M.; Tarsia, P.; Carpagnano, G.E.; Foschino Barbaro, M.P.; Lacedonia, D. Application of machine learning to predict obstructive sleep apnea syndrome severity. Health Inform. J. 2020, 26, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, F.; Jozani, M.J.; Moussavi, Z. A comparison of regularized logistic regression and random forest machine learning models for daytime diagnosis of obstructive sleep apnea. Med. Biol. Eng. Comput. 2020, 58, 2517–2529. [Google Scholar] [CrossRef]
- Shahin, M.; Ahmed, B.; Hamida, S.T.; Mulaffer, F.L.; Glos, M.; Penzel, T. Deep Learning and Insomnia: Assisting Clinicians with Their Diagnosis. IEEE J. Biomed. Health Inform. 2017, 21, 1546–1553. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, H.; Song, J.; Yang, Y.; Yang, X. Sleep stages classification based on heart rate variability and random forest. Biomed. Signal Process. Control 2013, 8, 624–633. [Google Scholar] [CrossRef]
- Mitchell, M.W. Bias of the Random Forest out-of-bag (OOB) error for certain input parameters. Open J. Stat. 2011, 1, 205. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Altmann, A.; Toloşi, L.; Sander, O.; Lengauer, T. Permutation importance: A corrected feature importance measure. Bioinformatics 2010, 26, 1340–1347. [Google Scholar] [CrossRef]
- Radivojac, P.; Obradovic, Z.; Dunker, A.K.; Vucetic, S. Feature Selection Filters Based on the Permutation Test. In Proceedings of the European Conference on Machine Learning, Pisa, Italy, 20–24 September 2004; Springer: Berlin, Germany, 2004; pp. 334–346. [Google Scholar]
- Thomas, D.R.; Zhu, P.C.; Zumbo, B.D.; Dutta, S. On Measuring the Relative Importance of Explanatory Variables in a Logistic Regression. J. Mod. Appl. Stat. Methods 2008, 7, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Briem, G.J.; Benediktsson, J.A.; Sveinsson, J.R. Multiple classifiers applied to multisource remote sensing data. IEEE Trans. Geosci. Remote Sens. 2002, 40, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Parmar, A.; Katariya, R.; Patel, V. A Review on Random Forest: An Ensemble Classifier, Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things, Coimbatore, India, 7–8 August 2018; Springer: Cham, Switzerland, 2018; pp. 758–763. [Google Scholar]
- Kaja, N.; Shaout, A.; Ma, D. An intelligent intrusion detection system. Appl. Intell. 2019, 49, 3235–3247. [Google Scholar] [CrossRef]
- Krell, S.B.; Kapur, V.K. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005, 9, 104–110. [Google Scholar] [CrossRef]
- Gray, E.L.; McKenzie, D.K.; Eckert, D.J. Obstructive sleep apnea without obesity is common and difficult to treat: Evidence for a distinct pathophysiological phenotype. J. Clin. Sleep Med. 2017, 13, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Hang, L.-W.; Huang, C.-S.; Cheng, W.-J. Clinical characteristics of Asian patients with sleep apnea with low arousal threshold and sleep structure change with continuous positive airway pressure. Sleep Breath. 2020, 25, 1309–1317. [Google Scholar] [CrossRef]
- Crönlein, T. Insomnia and obesity. Curr. Opin. Psychiatry 2016, 29, 409–412. [Google Scholar] [CrossRef]
- Eckert, D.J.; Younes, M.K. Arousal from sleep: Implications for obstructive sleep apnea pathogenesis and treatment. J. Appl. Physiol. 2014, 116, 302–313. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia: State of the science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- De Zambotti, M.; Covassin, N.; De Min Tona, G.; Sarlo, M.; Stegagno, L. Sleep onset and cardiovascular activity in primary insomnia. J. Sleep Res. 2011, 20, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Collop, N.A. Scoring variability between polysomnography technologists in different sleep laboratories. Sleep Med. 2002, 3, 43–47. [Google Scholar] [CrossRef]
- Roepke, S.K.; Ancoli-Israel, S. Sleep disorders in the elderly. Indian J. Med. Res. 2010, 131, 302. [Google Scholar]



| Categorical Variable | ID (n = 404) | Low-ArTH OSA (n = 624) | High-ArTH OSA (n = 551) |
|---|---|---|---|
| Sex (male/female) b | 137/267 | 361/263 | 422/129 |
| Age (years) a | 45.91 ± 13.81 #,△ | 52.42 ± 13.88 # | 53.47 ± 13.02 △ |
| BMI (kg/m2) a | 23.22 ± 4.03 #,△ | 26.06 ± 4.52 #,* | 28.08 ± 4.87 *,△ |
| Neck (cm) a | 34.05 ± 3.37 #,△ | 36.74 ± 3.70 #,* | 38.81 ± 3.90 *,△ |
| Waist (cm) a | 80.51 ± 10.25 #,△ | 89.69 ± 10.73 #,* | 96.13 ± 12.30 *,△ |
| Low-ArTH criteria a | |||
| AHI (events/h) | 2.21 ± 1.48 #,△ | 15.52 ± 7.01 #,* | 49.57 ± 21.71 *,△ |
| SpO2-min (%) | 92.44 ± 2.96 #,△ | 86.74 ± 5.26 #,* | 78.63 ± 8.30 *,△ |
| F-hypopnea (%) | 45.25 ± 10.94 #,△ | 91.21 ± 11.18 #,* | 67.28 ± 29.79 *,△ |
| OSA severity b | |||
| Mild, n (%) | - | 334 (53.53%) | 30 (5.44%) |
| Moderate, n (%) | - | 290 (46.47%) | 38 (6.90%) |
| Severe, n (%) | - | - | 483 (87.66%) |
| Categorical Variable | ID (n = 404) | Low-ArTH OSA (n = 624) | High-ArTH OSA (n = 551) |
|---|---|---|---|
| Sleep architecture | |||
| Sleep onset (min) | 30.42 ± 36.79 #,△ | 26.72 ± 31.6 # | 24.93 ± 34.11 △ |
| WASO (min) | 59.67 ± 55.54 #,△ | 62.81 ± 49.12 #,*, | 72.11 ± 55.46 *,△ |
| Wake (% of SPT) | 17.75 ± 16.35 △ | 18.63 ± 14.58 * | 21.65 ± 17.21 *,△ |
| NREM (% of SPT) | 71.87 ± 14.37 | 70.97 ± 12.85 | 69.84 ± 15.39 |
| REM (% of SPT) | 10.38 ± 6.51 △ | 10.41 ± 6.43 * | 8.52 ± 5.93 *,△ |
| Oximetry parameters | |||
| SpO2-mean (%) | 97.12 ± 1.12 #,△ | 96.01 ± 1.35 #,* | 94.61 ± 2.13 *,△ |
| ODI-3% (events/h) | 1.82 ± 1.54 #,△ | 12.97 ± 7.61 #,* | 46.99 ± 22.28 *,△ |
| Arousal parameters (events/h) | |||
| ArI | 13.67 ± 9.07 #,△ | 17.34 ± 9.12 #,* | 30.89 ± 18.36 *,△ |
| SpArI | 11.34 ± 7.68 #,△ | 9.49 ± 6.41 #,* | 6.80 ± 6.50 *,△ |
| RArI | 0.62 ± 0.97 #,△ | 5.99 ± 4.35 #,* | 22.22 ± 16.62 *,△ |
| SnArI | 0.13 ± 0.65 #,△ | 0.32 ± 1.02 # | 0.40 ± 1.40 △ |
| LMArI | 1.40 ± 3.11 | 1.38 ± 2.06 | 1.35 ± 2.13 |
| Categorical Variable | LR | kNN | NB | RF | SVM |
|---|---|---|---|---|---|
| Training set (n) | ID: 332; Low-ArTH OSA: 416; High-ArTH OSA:515 | ||||
| Oximetry model | |||||
| Accuracy (%) | 77.28 | 77.67 | 77.20 | 79.57 | 77.04 |
| Precision (%) | 77.25 | 78.17 | 79.03 | 80.38 | 77.88 |
| Recall (%) | 78.79 | 78.60 | 78.71 | 80.65 | 77.07 |
| F1-score (%) | 77.59 | 78.16 | 77.79 | 80.11 | 77.31 |
| AUC (%) | 91.82 | 89.60 | 91.79 | 92.52 | 90.99 |
| Combined model | |||||
| Accuracy (%) | 79.65 | 71.65 | 77.75 | 80.60 | 78.70 |
| Precision (%) | 79.57 | 72.89 | 79.35 | 81.88 | 79.73 |
| Recall (%) | 81.05 | 71.73 | 78.94 | 81.48 | 78.66 |
| F1-score (%) | 79.97 | 72.05 | 78.34 | 81.19 | 78.98 |
| AUC (%) | 93.17 | 86.96 | 91.16 | 93.40 | 92.01 |
| Categorical Variable | Oximetry Model | Combined Model |
|---|---|---|
| Testing set (n) | ID: 72; Low-ArTH OSA:109; High-ArTH OSA:135 | |
| Accuracy (%) | 77.53 | 80.06 |
| Precision (%) | 78.72 | 80.17 |
| Recall (%) | 78.17 | 81.24 |
| F1-score (%) | 78.14 | 80.41 |
| AUC (%) | 92.24 | 93.61 |
| Feature importance (%) | ||
| SpO2-mean | 5.43 | 6.46 |
| SpO2-min | 14.89 | 14.69 |
| I-SpO2-<90% | 15.10 | 13.40 |
| ODI-3%-TRT | 64.57 | 49.50 |
| Age | - | 3.25 |
| BMI | - | 4.06 |
| Neck | - | 3.29 |
| Waist | - | 4.51 |
| Sex | - | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-Y.; Kuan, Y.-C.; Hsu, W.-H.; Lin, Y.-T.; Hsu, C.-R.; Lo, K.; Hsu, W.-H.; Majumdar, A.; Liu, Y.-S.; Hsu, S.-M.; et al. Differentiation Model for Insomnia Disorder and the Respiratory Arousal Threshold Phenotype in Obstructive Sleep Apnea in the Taiwanese Population Based on Oximetry and Anthropometric Features. Diagnostics 2022, 12, 50. https://doi.org/10.3390/diagnostics12010050
Tsai C-Y, Kuan Y-C, Hsu W-H, Lin Y-T, Hsu C-R, Lo K, Hsu W-H, Majumdar A, Liu Y-S, Hsu S-M, et al. Differentiation Model for Insomnia Disorder and the Respiratory Arousal Threshold Phenotype in Obstructive Sleep Apnea in the Taiwanese Population Based on Oximetry and Anthropometric Features. Diagnostics. 2022; 12(1):50. https://doi.org/10.3390/diagnostics12010050
Chicago/Turabian StyleTsai, Cheng-Yu, Yi-Chun Kuan, Wei-Han Hsu, Yin-Tzu Lin, Chia-Rung Hsu, Kang Lo, Wen-Hua Hsu, Arnab Majumdar, Yi-Shin Liu, Shin-Mei Hsu, and et al. 2022. "Differentiation Model for Insomnia Disorder and the Respiratory Arousal Threshold Phenotype in Obstructive Sleep Apnea in the Taiwanese Population Based on Oximetry and Anthropometric Features" Diagnostics 12, no. 1: 50. https://doi.org/10.3390/diagnostics12010050
APA StyleTsai, C.-Y., Kuan, Y.-C., Hsu, W.-H., Lin, Y.-T., Hsu, C.-R., Lo, K., Hsu, W.-H., Majumdar, A., Liu, Y.-S., Hsu, S.-M., Ho, S.-C., Cheng, W.-H., Lin, S.-Y., Lee, K.-Y., Wu, D., Lee, H.-C., Wu, C.-J., & Liu, W.-T. (2022). Differentiation Model for Insomnia Disorder and the Respiratory Arousal Threshold Phenotype in Obstructive Sleep Apnea in the Taiwanese Population Based on Oximetry and Anthropometric Features. Diagnostics, 12(1), 50. https://doi.org/10.3390/diagnostics12010050








