Sleep Treatments in Disorders of Consciousness: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
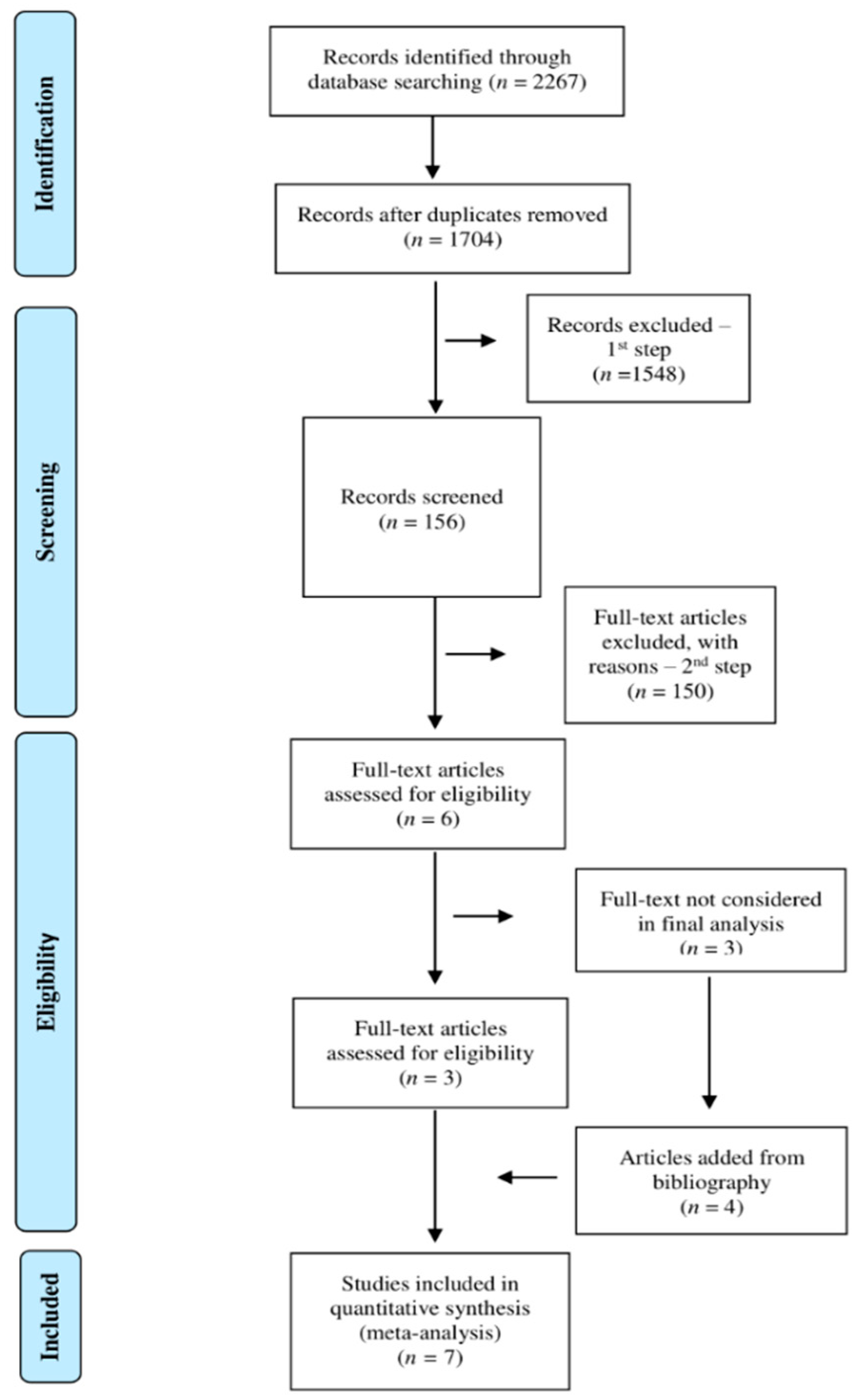
2.3. Screening
2.4. Data Extraction
3. Results
3.1. Literature Search Results
3.2. Studies’ Design of the Articles Analyzed
3.3. Clinical Populations Considered in the Included Studies
3.4. Sleep Disorders and Treatments
3.4.1. Treatments for Sleep-Related Breathing Disorders
3.4.2. Treatments for Sleep Rhythms Dysregulation
4. Discussion
4.1. Limits
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Gosseries, O.; Pistoia, F.; Charland-Verville, V.; Carolei, A.; Sacco, S.; Laureys, S. The Role of Neuroimaging Techniques in Establishing Diagnosis, Prognosis and Therapy in Disorders of Consciousness. Open Neuroimag. J. 2016, 10, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Gosseries, O.; Di, H.; Laureys, S.; Boly, M. Measuring consciousness in severely damaged brains. Annu. Rev. Neurosci. 2014, 37, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Marino, S.; Bonanno, L.; Giorgio, A. Functional connectivity in disorders of consciousness: Methodological aspects and clinical relevance. Brain Imaging Behav. 2016, 10, 604–608. [Google Scholar] [CrossRef]
- Schiff, N.D. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef]
- Mura, E.; Pistoia, F.; Sarà, M.; Sacco, S.; Carolei, A.; Govoni, S. Pharmacological modulation of the state of awareness in patients with disorders of consciousness: An overview. Curr. Pharm. Des. 2014, 20, 4121–4139. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.-P. Experimental and theoretical approaches to conscious processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Changeux, J.-P.; Naccache, L.; Sackur, J.; Sergent, C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn. Sci. 2006, 10, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Tononi, G. An information integration theory of consciousness. BMC Neurosci. 2004, 5, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Tononi, G. The integrated information theory of consciousness: An updated account. Arch. Ital. Biol. 2012, 150, 56–90. [Google Scholar]
- Yeo, B.T.T.; Tandi, J.; Chee, M.W.L. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 2015, 111, 147–158. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, L.; Ye, E.; Jin, X.; Ni, W.; Yang, Y.; Wen, B.; Hu, D.; Yang, Z. Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: Evidence from resting state FMRI. PLoS ONE 2013, 8, e78830. [Google Scholar]
- Miraglia, F.; Tomino, C.; Vecchio, F.; Gorgoni, M.; De Gennaro, L.; Rossini, P.M. The brain network organization during sleep onset after deprivation. Clin. Neurophysiol. 2021, 132, 36–44. [Google Scholar] [CrossRef]
- Pavlova, M.K.; Latreille, V. Sleep disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, M.; Haswell, J.D.; De Vivo, L.; Marshall, W.; Roseboom, P.H.; Tononi, G.; Cirelli, C. Myelin modifications after chronic sleep loss in adolescent mice. Sleep 2018, 41, zsy034. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hauglund, N.L.; Pavan, C.; Nedergaard, M. Cleaning the sleeping brain–the potential restorative function of the glymphatic system. Curr. Opin. Physiol. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Zada, D.; Bronshtein, I.; Lerer-Goldshtein, T.; Garini, Y.; Appelbaum, L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kusztor, A.; Raud, L.; Juel, B.E.; Nilsen, A.S.; Storm, J.F.; Huster, R.J. Sleep deprivation differentially affects subcomponents of cognitive control. Sleep 2019, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Drummond, S.P.A.; Walker, M.; Almklov, E.; Campos, M.; Anderson, D.E.; Straus, L.D. Neural correlates of working memory performance in primary insomnia. Sleep 2013, 36, 1307–1316. [Google Scholar] [CrossRef]
- Chee, M.W.L.; Choo, W.C. Functional imaging of working memory after 24 hr of total sleep deprivation. J. Neurosci. 2004, 24, 4560–4567. [Google Scholar] [CrossRef]
- Verweij, I.M.; Romeijn, N.; Smit, D.J.A.; Piantoni, G.; Van Someren, E.J.W.; van der Werf, Y.D. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.-J.; Liu, C.-L.; Zhou, R.-L.; Gong, H.-H.; Wu, B.; Gao, L.; Wang, Y.-X.J. Long-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: A resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2015, 11, 761. [Google Scholar] [CrossRef] [Green Version]
- Wislowska, M.; Del Giudice, R.; Lechinger, J.; Wielek, T.; Heib, D.P.J.; Pitiot, A.; Pichler, G.; Michitsch, G.; Donis, J.; Schabus, M. Night and day variations of sleep in patients with disorders of consciousness. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Mertel, I.; Pavlov, Y.G.; Barner, C.; Müller, F.; Diekelmann, S.; Kotchoubey, B. Sleep in disorders of consciousness: Behavioral and polysomnographic recording. BMC Med. 2020, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sebastiano, D.R.; Visani, E.; Panzica, F.; Sattin, D.; Bersano, A.; Nigri, A.; Ferraro, S.; Parati, E.; Leonardi, M.; Franceschetti, S. Sleep patterns associated with the severity of impairment in a large cohort of patients with chronic disorders of consciousness. Clin. Neurophysiol. 2018, 129, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Del Giudice, R.; Wislowska, M.; Lechinger, J.; Schabus, M. Across the consciousness continuum—from unresponsive wakefulness to sleep. Front. Hum. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Song, C.; Yuan, F.; Zhao, J.; Jiang, Y.; Yang, F.; Kang, X.; Jiang, W. Prognostic roles of sleep electroencephalography pattern and circadian rhythm biomarkers in the recovery of consciousness in patients with coma: A prospective cohort study. Sleep Med. 2020, 69, 204–212. [Google Scholar] [CrossRef]
- Duclos, C.; Dumont, M.; Blais, H.; Paquet, J.; Potvin, M.-J.; Menon, D.K.; Bernard, F.; Gosselin, N. Severe sleep-wake disturbances in acute and post-acute traumatic brain injury: A case report. Brain Inj. 2014, 28, 657. [Google Scholar] [CrossRef]
- Arnaldi, D.; Terzaghi, M.; Cremascoli, R.; De Carli, F.; Maggioni, G.; Pistarini, C.; Nobili, F.; Moglia, A.; Manni, R. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin. Neurophysiol. 2016, 127, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Placidi, F.; Oliveira, A.J.; Bigagli, A.; Morghen, I.; Proietti, R.; Gigli, G.L. Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma. Clin. Neurophysiol. 2002, 113, 1798–1805. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottshall, J.L.; Rossi Sebastiano, D. Sleep in disorders of consciousness: Diagnostic, prognostic, and therapeutic considerations. Curr. Opin. Neurol. 2020, 33, 684–690. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Gardani, M.; Morfiri, E.; Thomson, A.; O’Neill, B.; McMillan, T.M. Evaluation of Sleep Disorders in Patients with Severe Traumatic Brain Injury During Rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, 1691–1697.e3. [Google Scholar] [CrossRef]
- Mathias, J.L.; Alvaro, P.K. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: A meta-analysis. Sleep Med. 2012, 13, 898–905. [Google Scholar] [CrossRef]
- Bukhari, M.A.A.; Alghtani, M.A.M.; Sultan, Z.; Aljohani, A.A.A.; Alhazmi, I.H.M. Diagnosis and treatment of sleep disorders: A brief review. Int. J. Med. Dev. Ctries. 2021, 5, 364–369. [Google Scholar] [CrossRef]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, M.E.; Groet, E.; Daams, J.G.; Geurtsen, G.J.; Van Bennekom, C.A.M.; Van Someren, E.J.W. Non-pharmacological treatment for insomnia following acquired brain injury: A systematic review. Sleep Med. Rev. 2020, 50, 101255. [Google Scholar] [CrossRef] [PubMed]
- Pilon, L.; Frankenmolen, N.; Bertens, D. Treatments for sleep disturbances in individuals with acquired brain injury: A systematic review. Clin. Rehabil. 2021, 35, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, Z.M.; Forgacs, P.B.; Conte, M.M.; Nauvel, T.J.; Drover, J.D.; Schiff, N.D. Late and progressive alterations of sleep dynamics following central thalamic deep brain stimulation (CT-DBS) in chronic minimally conscious state. Clin. Neurophysiol. 2016, 127, 3086. [Google Scholar] [CrossRef] [Green Version]
- Formica, F.; Pozzi, M.; Avantaggiato, P.; Molteni, E.; Arrigoni, F.; Giordano, F.; Clementi, E.; Strazzer, S. Disordered consciousness or disordered wakefulness? The importance of prolonged polysomnography for the diagnosis, drug therapy, and rehabilitation of an unresponsive patient with brain injury. J. Clin. Sleep Med. 2017, 13, 1477–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottshall, J.L.; Adams, Z.M.; Forgacs, P.B.; Schiff, N.D. Daytime central thalamic deep brain stimulation modulates sleep dynamics in the severely injured brain: Mechanistic insights and a novel framework for alpha-delta sleep generation. Front. Neurol. 2019, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, F.; Formica, F.; Galbiati, S.; Avantaggiato, P.; Beretta, E.; Carnovale, C.; Pozzi, M.; Clementi, E.; Strazzer, S. Polysomnographic analysis of a pediatric case of baclofen-induced central sleep apnea. J. Clin. Sleep Med. 2019, 15, 351–354. [Google Scholar] [CrossRef]
- Silva, M.A.; Schwartz, D.J.; Nakase-Richardson, R. Functional improvement after severe brain injury with disorder of consciousness paralleling treatment for comorbid obstructive sleep apnoea: A case report. Int. J. Rehabil. Res. 2019, 42, 285–288. [Google Scholar] [CrossRef]
- Blume, C.; Lechinger, J.; Santhi, N.; Del Giudice, R.; Gnjezda, M.-T.; Pichler, G.; Scarpatetti, M.; Donis, J.; Michitsch, G.; Schabus, M. Significance of circadian rhythms in severely brain-injured patients: A clue to consciousness? Neurology 2017, 88, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Dhamapurkar, S.K.; Wilson, B.A.; Rose, A.; Watson, P.; Shiel, A. Does Modafinil improve the level of consciousness for people with a prolonged disorder of consciousness? A retrospective pilot study. Disabil. Rehabil. 2017, 39, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Cologan, V.; Drouot, X.; Parapatics, S.; Delorme, A.; Gruber, G.; Moonen, G.; Laureys, S. Sleep in the unresponsive wakefulness syndrome and minimally conscious state. J. Neurotrauma 2013, 30, 339–346. [Google Scholar] [CrossRef]
- Yaron, I.; Melloni, L.; Pitts, M.; Mudrik, L. How are theories of consciousness empirically tested? The Consciousness Theories Studies (ConTraSt) database. J. Vis. 2021, 21, 2195. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Pack, A.I.; Black, J.E.; Schwartz, J.R.L.; Matheson, J.K. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 164, 1675–1681. [Google Scholar] [CrossRef]
- Moldofsky, H.; Broughton, R.J.; Hill, J.D. A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy. Sleep Med. 2000, 1, 109–116. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Walsh, J.K.; Roth, T.; Hughes, R.J.; Wright, K.P.; Kingsbury, L.; Arora, S.; Schwartz, J.R.L.; Niebler, G.E.; Dinges, D.F. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N. Engl. J. Med. 2005, 353, 476–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, T.; Schwartz, J.R.L.; Hirshkowitz, M.; Erman, M.K.; Dayno, J.M.; Arora, S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J. Clin. Sleep Med. 2007, 3, 595–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasinski, D.R.; Kovacevic-Ristanovic, R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin. Neuropharmacol. 2000, 23, 149–156. [Google Scholar] [CrossRef]
- Lees, J.; Michalopoulou, P.G.; Lewis, S.W.; Preston, S.; Bamford, C.; Collier, T.; Kalpakidou, A.; Wykes, T.; Emsley, R.; Pandina, G. Modafinil and cognitive enhancement in schizophrenia and healthy volunteers: The effects of test battery in a randomised controlled trial. Psychol. Med. 2017, 47, 2358–2368. [Google Scholar] [CrossRef]
- Rasmussen, N.-A.; Schrøder, P.; Olsen, L.R.; Brødsgaard, M.; Undén, M.; Bech, P. Modafinil augmentation in depressed patients with partial response to antidepressants: A pilot study on self-reported symptoms covered by the Major Depression Inventory (MDI) and the Symptom Checklist (SCL-92). Nord. J. Psychiatry 2005, 59, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.C.; Robbins, T.W.; Clark, L.; Aron, A.R.; Dowson, J.; Sahakian, B.J. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology 2003, 165, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.J. Cognitive dysfunction in individuals with cocaine use disorder: Potential moderating factors and pharmacological treatments. Exp. Clin. Psychopharmacol. 2019, 27, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kalechstein, A.D.; Mahoney III, J.J.; Yoon, J.H.; Bennett, R.; De La Garza II, R. Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology 2013, 64, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Cilli, F.; Pieramico, V.; Ferretti, A.; Macchia, A.; Tommasi, M.; Saggino, A.; Ciavardelli, D.; Manna, A.; Navarra, R. Acute effects of modafinil on brain resting state networks in young healthy subjects. PLoS ONE 2013, 8, e69224. [Google Scholar] [CrossRef]
- Ciurleo, R.; Bramanti, P.; Calabrò, R.S. Pharmacotherapy for disorders of consciousness: Are ‘awakening’drugs really a possibility? Drugs 2013, 73, 1849–1862. [Google Scholar] [CrossRef]
- Georgiopoulos, M.; Katsakiori, P.; Kefalopoulou, Z.; Ellul, J.; Chroni, E.; Constantoyannis, C. Vegetative state and minimally conscious state: A review of the therapeutic interventions. Stereotact. Funct. Neurosurg. 2010, 88, 199–207. [Google Scholar] [CrossRef]
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012, 366, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Noormandi, A.; Shahrokhi, M.; Khalili, H. Potential benefits of zolpidem in disorders of consciousness. Expert Rev. Clin. Pharmacol. 2017, 10, 983–992. [Google Scholar] [CrossRef]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Margetis, K.; Korfias, S.I.; Gatzonis, S.; Boutos, N.; Stranjalis, G.; Boviatsis, E.; Sakas, D.E. Intrathecal baclofen associated with improvement of consciousness disorders in spasticity patients. Neuromodulation Technol. Neural Interface 2014, 17, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sacco, S.; Sara, M.; Franceschini, M.; Carolei, A. Intrathecal Baclofen: Effects on Spasticity, Pain, and Consciousness in Disorders of Consciousness and Locked-in Syndrome. Curr. Pain Headache Rep. 2015, 19, 466. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, V.; Scifo, P.; Castellano, A.; Aloia, M.S.; Iadanza, A.; Marelli, S.; Cappa, S.F.; Strambi, L.F.; Falini, A. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014, 37, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Arendt, J.; Broadway, J. Light and melatonin as zeitgebers in man. Chronobiol. Int. 1987, 4, 273–282. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Gehrman, P.; Martin, J.L.; Shochat, T.; Marler, M.; Corey-Bloom, J.; Levi, L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav. Sleep Med. 2003, 1, 22–36. [Google Scholar] [CrossRef]
- Beaven, C.M.; Ekström, J. A comparison of blue light and caffeine effects on cognitive function and alertness in humans. PLoS ONE 2013, 8, e76707. [Google Scholar] [CrossRef] [Green Version]
- Videnovic, A.; Klerman, E.B.; Wang, W.; Marconi, A.; Kuhta, T.; Zee, P.C. Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: A randomized clinical trial. JAMA Neurol. 2017, 74, 411–418. [Google Scholar] [CrossRef]
- Cajochen, C. Alerting effects of light. Sleep Med. Rev. 2007, 11, 453–464. [Google Scholar] [CrossRef]
- Kundu, B.; Brock, A.A.; Englot, D.J.; Butson, C.R.; Rolston, J.D. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: A review. Neurosurg. Focus 2018, 45, E14. [Google Scholar] [CrossRef] [Green Version]
- Royal College of Physicians. Prolonged Disorders of Consciousness Following Sudden Onset Brain Injury: National Clinical Guidelines; Royal College of Physicians: London, UK, 2020. [Google Scholar]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch. Phys. Med. Rehabil. 2018, 99, 1699–1709. [Google Scholar] [CrossRef]
- Pratap-Chand, R.; Gourie-Devi, M. Bruxism: Its significance in coma. Clin. Neurol. Neurosurg. 1985, 87, 113–117. [Google Scholar] [CrossRef]
- Pennestri, M.H.; Montplaisir, J.; Colombo, R.; Lavigne, G.; Lanfranchi, P.A. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology 2007, 68, 1213–1218. [Google Scholar] [CrossRef]
- Galbiati, A.; Marelli, S.; Giora, E.; Zucconi, M.; Oldani, A.; Ferini-Strambi, L. Neurocognitive function in patients with idiopathic Restless Legs Syndrome before and after treatment with dopamine-agonist. Int. J. Psychophysiol. 2015, 95, 304–309. [Google Scholar] [CrossRef]
- de Alencar, N.A.; Leão, C.S.; Leão, A.T.T.; Luiz, R.R.; Fonseca-Gonçalves, A.; Maia, L.C. Sleep bruxism and anxiety impacts in quality of life related to oral health of Brazilian children and their families. J. Clin. Pediatr. Dent. 2017, 41, 179–185. [Google Scholar] [CrossRef]
- Silvestri, R.; Gagliano, A.; Aricò, I.; Calarese, T.; Cedro, C.; Bruni, O.; Condurso, R.; Germanò, E.; Gervasi, G.; Siracusano, R. Sleep disorders in children with Attention-Deficit/Hyperactivity Disorder (ADHD) recorded overnight by video-polysomnography. Sleep Med. 2009, 10, 1132–1138. [Google Scholar] [CrossRef]
- Frohlich, J.; Toker, D.; Monti, M.M. Consciousness among delta waves: A paradox. Brain 2021, 144, 2257–2277. [Google Scholar] [CrossRef]
- Scheinin, A.; Kantonen, O.; Alkire, M.; Långsjö, J.; Kallionpää, R.E.; Kaisti, K.; Radek, L.; Johansson, J.; Sandman, N.; Nyman, M. Foundations of human consciousness: Imaging the twilight zone. J. Neurosci. 2021, 41, 1769–1778. [Google Scholar] [CrossRef]
| Follow Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Patients | Etiology | Sleep Disorder | Intervention | Dose | Treatment Duration | Behavioral Measures | Time | Sleep Measures |
| Dhamapurkar et al., 2017 [52] | VS = 16 MCS = 8 | TBI = 12 n-TBI = 12 | Excessive daytime sleepiness | Modafinil | From 100 mg up to 300 mg daily or the maximal tolerated dose | 24 weeks (in average) | WHIM; CRS-r | From 4 to 72 weeks | Sleep–wake cycle behavioral charts |
| Formica et al., 2017 [47] | VS = 1 | n-TBI | Circadian sleep disorders | Modafinil (Baclofen, Delorazepam, Melatonin as concomitant therapies) | 100 mg bid | 4 weeks | DRS; CNCS; LOCFAS | 1 day | 24-h PSG; presence and features of sleep stages |
| Blume et al., 2019 [51] | MCS = 4 VS = 3 eMCS = 1 | TBI = 1 n-TBI = 7 | Cyrcadian rhythms regulation | Habitual light; bright light | HL: below 500 lux at eye level from 7 a.m. to 9 p.m. daily; BL: around 2000 lux at eye level for 1 h three times a day | 2 weeks | CRS-r | 1 week | n/a |
| Locatelli et al., 2019 [49] | MCS = 1 | n-TBI | Central Sleep Apnea | Baclofen | Initial: 450 μg/d; 1st increase: 600 μg/d; 2nd increase: 700 μg/d; 3th decrease: 650 μg/d; 4th decrease: 100 μg/d | 30 weeks and 2 day | n/a | 10 weeks | Apnoea hypopnea index; oxygen saturation |
| Silva et al., 2019 [50] | MCS = 1 | n-TBI | Obstructive Sleep Apnea | PAP | Nightly | 35 weeks | CRS-r; DRS; FIM | between 10 and 45 weeks | PAP compliance monitoring using propriety software that measures devise usage. |
| Adams et al., 2016 [46] | MCS = 1 | n-TBI | Unusual mixing of sleep features (as revealed by EEG) | CT-DBS | 240 weeks | CRS-r | 240 weeks | Background EEG activity in awake; presence and features of sleep stages | |
| Gottshall et al., 2019 [48] | 48 weeks (discontinuation of therapy) | CRS-r | 288 weeks | Background EEG activity in awake; presence and features of sleep stages | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciatore, M.; Magnani, F.G.; Leonardi, M.; Rossi Sebastiano, D.; Sattin, D. Sleep Treatments in Disorders of Consciousness: A Systematic Review. Diagnostics 2022, 12, 88. https://doi.org/10.3390/diagnostics12010088
Cacciatore M, Magnani FG, Leonardi M, Rossi Sebastiano D, Sattin D. Sleep Treatments in Disorders of Consciousness: A Systematic Review. Diagnostics. 2022; 12(1):88. https://doi.org/10.3390/diagnostics12010088
Chicago/Turabian StyleCacciatore, Martina, Francesca G. Magnani, Matilde Leonardi, Davide Rossi Sebastiano, and Davide Sattin. 2022. "Sleep Treatments in Disorders of Consciousness: A Systematic Review" Diagnostics 12, no. 1: 88. https://doi.org/10.3390/diagnostics12010088
APA StyleCacciatore, M., Magnani, F. G., Leonardi, M., Rossi Sebastiano, D., & Sattin, D. (2022). Sleep Treatments in Disorders of Consciousness: A Systematic Review. Diagnostics, 12(1), 88. https://doi.org/10.3390/diagnostics12010088







