Radiomic Analysis for Pretreatment Prediction of Recurrence Post-Radiotherapy in Cervical Squamous Cell Carcinoma Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
2.3. Treatment
2.3.1. Radiotherapy
2.3.2. Chemotherapy
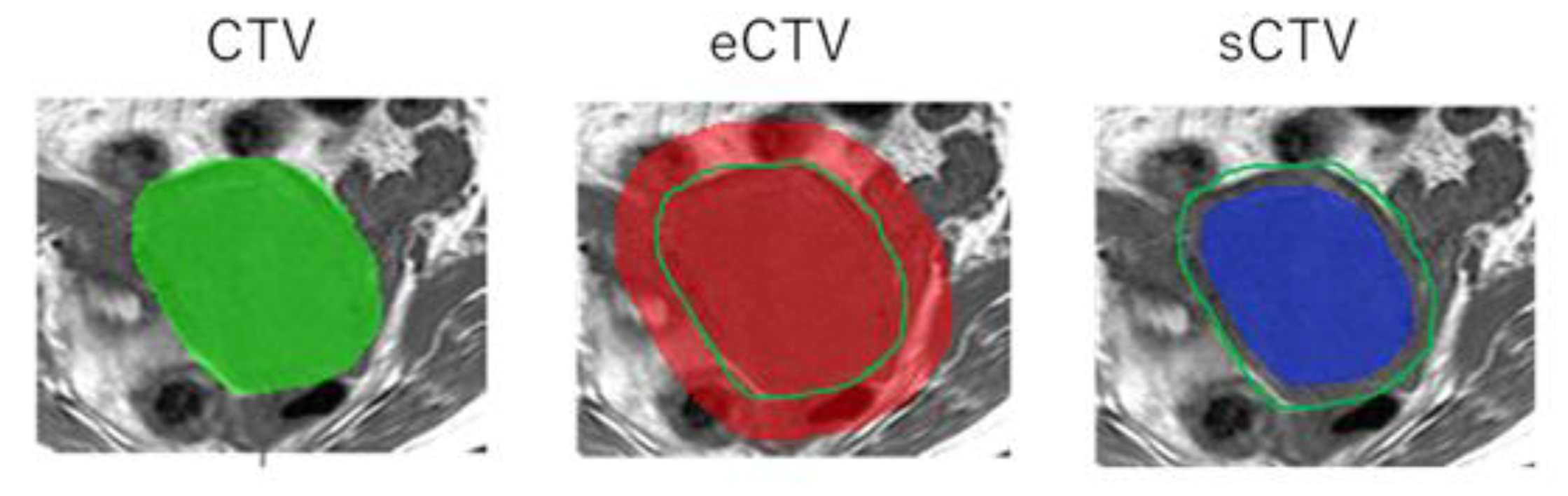
2.4. Radiomics Analysis
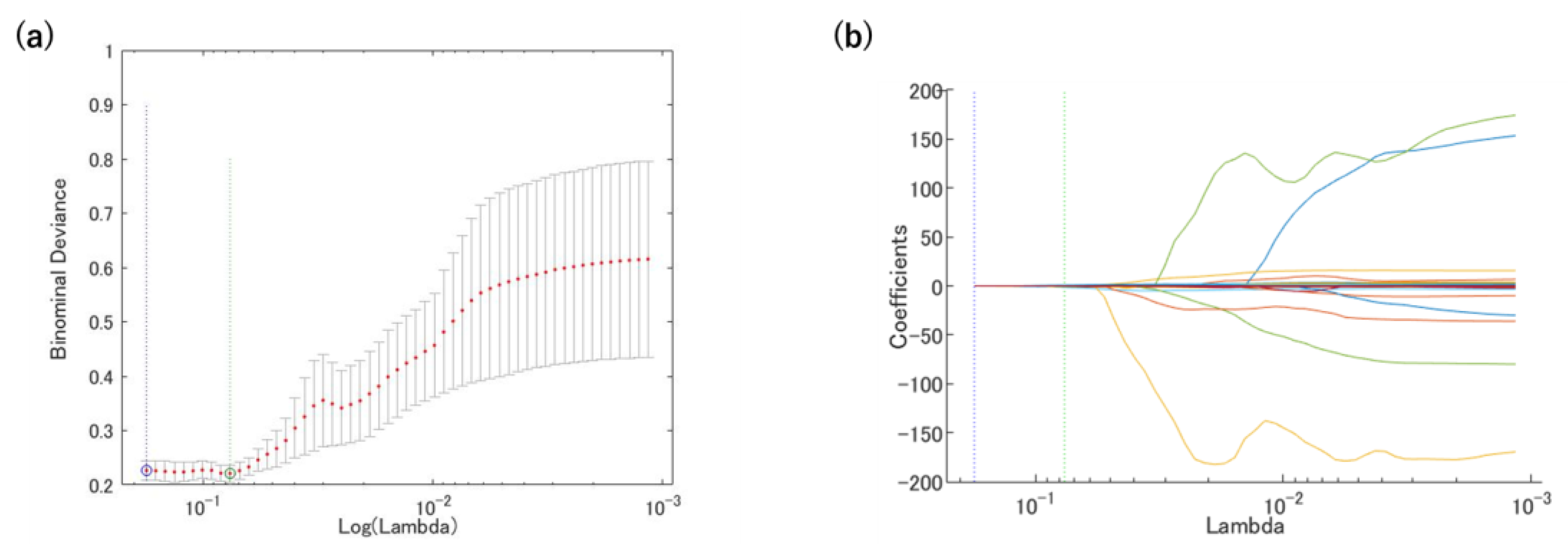
2.5. Prediction Model
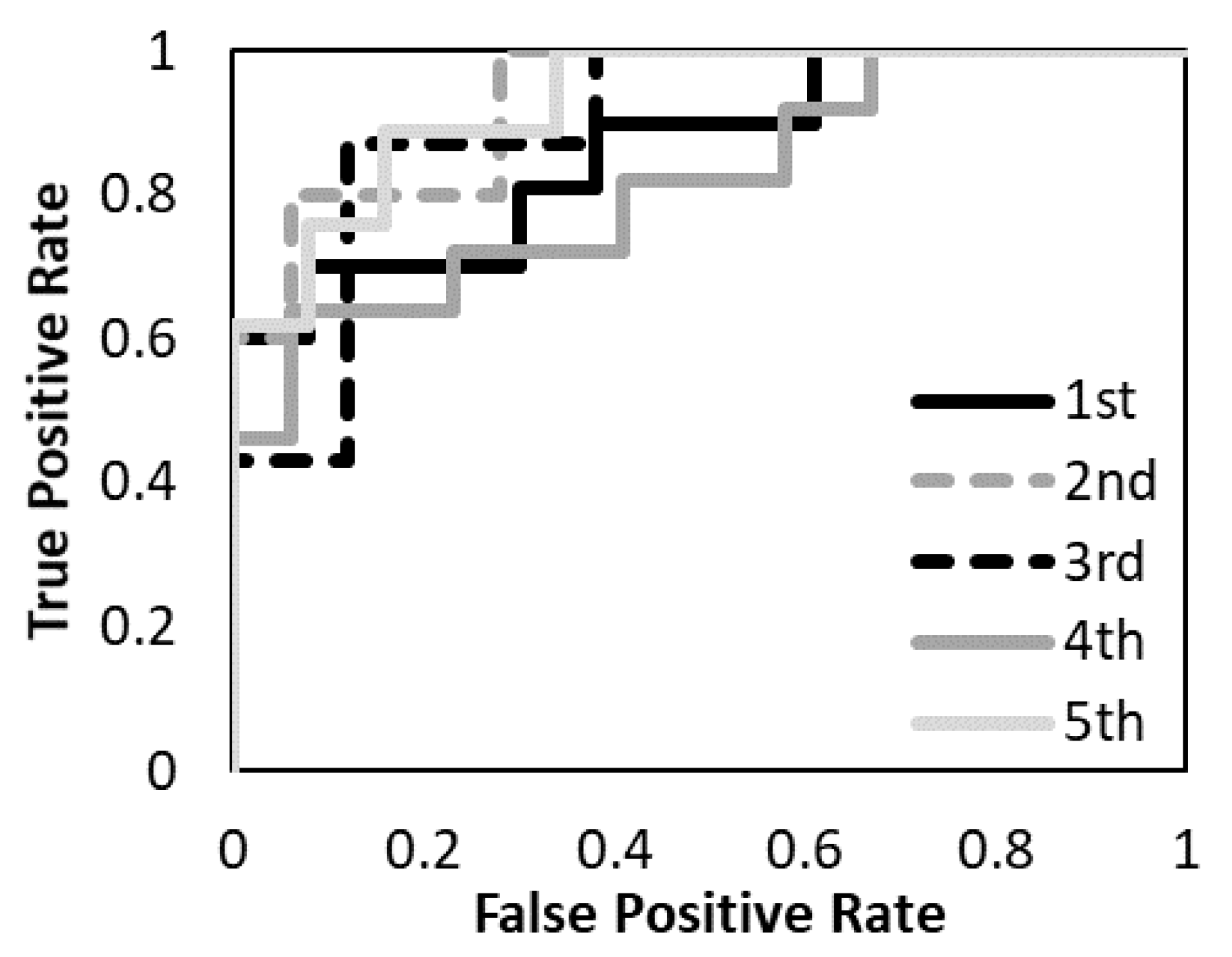
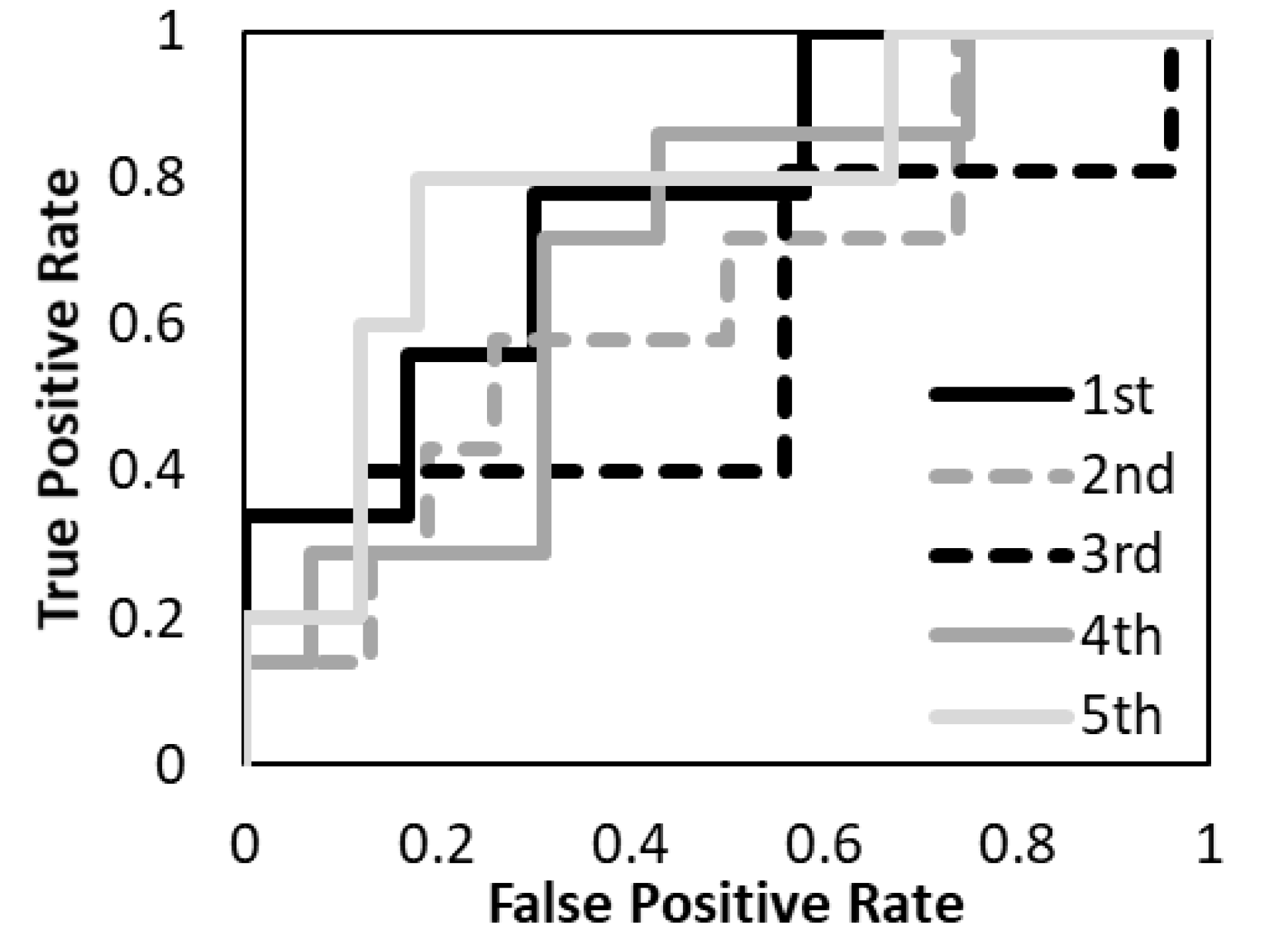
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Wakely, S.; Senior, E.; Lomas, D. MRI of Malignant Neoplasms of the Uterine Corpus and Cervix. Am. J. Roentgenol. 2007, 188, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Liu, X.; Du, Y.; Tang, Z.; Wang, K.; Tian, J. Radiomics analysis allows for precise prediction of epilepsy in patients with low-grade gliomas. Neuroimage Clin. 2018, 19, 271–278. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Shen, C.; Li, Z.; Yan, F.; Tian, J.; Xian, J. MR-based radiomics signature in differentiating ocular adnexal lymphoma from idiopathic orbital inflammation. Eur. Radiol. 2018, 28, 3872–3881. [Google Scholar] [CrossRef]
- Shen, C.; Liu, Z.; Wang, Z.; Guo, J.; Zhang, H.; Wang, Y.; Tian, J. Building CT radiomics based nomogram for preoperative esophageal cancer patients lymph node metastasis prediction. Transl. Oncol. 2018, 11, 815–824. [Google Scholar] [CrossRef]
- Lovinfosse, P.; Janvary, Z.L.; Coucke, P.; Jodogne, S.; Bernard, C.; Hatt, M.; Visvikis, D.; Jansen, N.; Duysinx, B.; Hustinx, R. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur. J. Nucl. Med. Mol. Imaging. 2016, 43, 1453–1460. [Google Scholar] [CrossRef]
- Reuzé, S.; Orlhac, F.; Chargari, C.; Nioche, C.; Limkin, E.; Riet, F.; Escande, A.; Haie-Meder, C.; Dercle, L.; Gouy, S.; et al. Prediction of cervical cancer recurrence using textural features extracted from 18F-FDG PET images acquired with different scanners. Oncotarget 2017, 8, 43169–43179. [Google Scholar] [CrossRef]
- Meng, J.; Liu, S.; Zhu, L.; Zhu, L.; Wang, H.; Xie, L.; Guan, Y.; He, J.; Yang, X.; Zhou, Z. Texture Analysis as Imaging Biomarker for recurrence in advanced cervical cancer treated with CCRT. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Xie, C.; Yang, P.; Zhang, X.; Xu, L.; Wang, X.; Li, X.; Zhang, L.; Xie, R.; Yang, L.; Jing, Z.; et al. Sub-region based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. eBioMedicine 2019, 44, 289–297. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for indi-vidual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso: A retrospective. J. R. Stat. Soc. Ser. B Stat Methodol. 2011, 73, 267–288. [Google Scholar] [CrossRef]
- Zhang, J.-X.; Song, W.; Chen, Z.-H.; Wei, J.-H.; Liao, Y.-J.; Lei, J.; Hu, M.; Chen, G.-Z.; Liao, B.; Lu, J.; et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: A microRNA expression analysis. Lancet Oncol. 2013, 14, 1295–1306. [Google Scholar] [CrossRef]
- Ho, K.-C.; Fang, Y.-H.D.; Chung, H.-W.; Yen, T.-C.; Ho, T.-Y.; Chou, H.-H.; Hong, J.-H.; Huang, Y.-T.; Wang, C.-C.; Lai, C.-H. A preliminary investigation into textural features of intratumoral metabolic heterogeneity in (18)F-FDG PET for overall survival prognosis in patients with bulky cervical cancer treated with definitive concurrent chemoradiotherapy. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 166–175. [Google Scholar]
- Sun, C.; Tian, X.; Liu, Z.; Li, W.; Li, P.; Chen, J.; Zhang, W.; Fang, Z.; Du, P.; Duan, H.; et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. eBioMedicine 2019, 46, 160–169. [Google Scholar] [CrossRef]
- Nie, P.; Yang, G.; Guo, J.; Chen, J.; Li, X.; Ji, Q.; Xu, W. A CT-based radiomics nomogram for differentiation of focal nodular hyperplasia from hepatocellular carcinoma in the non-cirrhotic liver. Cancer Imaging 2020, 20, 20. [Google Scholar] [CrossRef]
- Mayr, N.A.; Yuh, W.T.; Arnholt, J.C.; Ehrhardt, J.C.; Sorosky, J.I.; Magnotta, V.A.; Berbaum, K.S.; Zhen, W.; Paulino, A.C.; Oberley, L.W.; et al. Pixel analysis of MR perfusion imaging in predicting radiation therapy outcome in cervical cancer. J. Magn. Reson. Imaging 2000, 12, 1027–1033. [Google Scholar] [CrossRef]
- Haider, M.A.; Milosevic, M.; Fyles, A.; Sitartchouk, I.; Yeung, I.; Henderson, E.; Lockwood, G.; Lee, T.Y.; Roberts, T.P. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1100–1107. [Google Scholar] [CrossRef]
- Loncaster, J.A.; Carrington, B.M.; Sykes, J.R.; Jones, A.P.; Todd, S.M.; Cooper, R.; Buckley, D.L.; E Davidson, S.; Logue, J.P.; Hunter, R.D.; et al. Prediction of radiotherapy outcome using dynamic contrast enhanced MRI of carcinoma of the cervix. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 759–767. [Google Scholar] [CrossRef]
- Lyng, H.; Vorren, A.O.; Sundfør, K.; Taksdal, I.; Lien, H.H.; Kaalhus, O.; Rofstad, E.K. Assessment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J. Magn. Reson. Imaging 2001, 14, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Hallac, R.R.; Ding, Y.; Yuan, Q.; McColl, R.W.; Lea, J.; Sims, R.D.; Weatherall, P.T.; Mason, R.P. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T. NMR Biomed. 2012, 25, 1321–1330. [Google Scholar] [CrossRef]
- Matsumoto, K.-I.; Bernardo, M.; Subramanian, S.; Choyke, P.; Mitchell, J.B.; Krishna, M.C.; Lizak, M.J. MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn. Reson. Med. 2006, 56, 240–246. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Naish, J.; Parker, G.; Waterton, J.C.; Watson, Y.; Jayson, G.; Buonaccorsi, G.A.; Cheung, S.; Buckley, D.; McGrath, D.; et al. Preliminary Study of Oxygen-Enhanced Longitudinal Relaxation in MRI: A Potential Novel Biomarker of Oxygenation Changes in Solid Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1209–1215. [Google Scholar] [CrossRef]
- Zhou, H.; Hallac, R.R.; Yuan, Q.; Ding, Y.; Zhang, Z.; Xie, X.-J.; Francis, F.; Roehrborn, C.G.; Sims, R.D.; Costa, D.N.; et al. Incorporating Oxygen-Enhanced MRI into Multi-Parametric Assessment of Human Prostate Cancer. Diagnostics 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-J.; Park, K.-R.; Lee, K.-K.; Song, J.-S.; Lee, K.-G.; Lee, J.-Y.; Cha, D.-S.; Choi, H.-I.; Kim, D.-H.; Deung, Y.-K. Prognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 768–779. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Simonsen, T.G.; Lund, K.V.; Hompland, T.; Kristensen, G.B.; Rofstad, E.K. DCE-MRI–Derived Measures of Tumor Hypoxia and Interstitial Fluid Pressure Predict Outcomes in Cervical Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1193–1201. [Google Scholar] [CrossRef]
- Rao, S.X.; Lambregts, D.M.; Schnerr, R.S.; Beckers, R.C.; Maas, M.; Albarello, F.; Beets-Tan, R.G. CT texture analysis in colorectal liver metastases: A better way than size and volume measurements to assess response to chemotherapy? United Eur. Gastroenterol. J. 2016, 4, 257–263. [Google Scholar] [CrossRef]
- Goh, V.; Ganeshan, B.; Nathan, P.; Juttla, J.K.; Vinayan, A.; Miles, K.A. Assessment of Response to Tyrosine Kinase Inhibitors in Metastatic Renal Cell Cancer: CT Texture as a Predictive Biomarker. Radiology 2011, 261, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, A.; Armato, S.G.; Castillo, R.; Pham, N.; Guerrero, T.; Al-Hallaq, H.A. Lung Texture in Serial Thoracic Computed Tomography Scans: Correlation of Radiomics-based Features with Radiation Therapy Dose and Radiation Pneumonitis Development. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1048–1056. [Google Scholar] [CrossRef] [PubMed]


| Age (Years) | Median (Range) | 63 (30–85) |
|---|---|---|
| PS | 0 | 71 |
| 1 | 14 | |
| 2 | 4 | |
| 3 | 0 | |
| Histology | Squamous | 89 |
| T factor (UICC-8th) | 1a | 1 |
| 1b | 8 | |
| 2a | 1 | |
| 2b | 37 | |
| 3a | 0 | |
| 3b | 36 | |
| 4a | 6 | |
| N factor | 0 | 43 |
| 1 | 46 | |
| M factor | 0 | 76 |
| 1 | 13 |
| Feature Type | Methods | Feature Name |
|---|---|---|
| Morphology-based | Shape | Maximum 3D diameter |
| Maximum 2D diameter slice | ||
| Sphericity | ||
| Minor axis | ||
| Elongation | ||
| Surface volume ratio | ||
| Volume | ||
| Major axis | ||
| Surface area | ||
| Flatness | ||
| Least axis | ||
| Maximum 2D diameter column | ||
| Maximum 2D diameter row | ||
| First or-der-based | Histogram | Interquartile range |
| Skewness | ||
| Uniformity | ||
| Median | ||
| Energy | ||
| Robust mean absolute deviation | ||
| Mean absolute deviation | ||
| Total energy | ||
| Maximum | ||
| Root mean squared | ||
| 90 percentile | ||
| Minimum | ||
| Entropy | ||
| Range | ||
| Variance | ||
| 10 percentile | ||
| Kurtosis | ||
| Mean | ||
| Texture-based | GLCM | Joint average |
| Sum average | ||
| Joint entropy | ||
| Cluster shade | ||
| Maximum probability | ||
| Idmn | ||
| Joint energy | ||
| Contrast | ||
| Difference entropy | ||
| Inverse variance | ||
| Difference variance | ||
| Idn | ||
| Idm | ||
| Correlation | ||
| Autocorrelation | ||
| Sum entropy | ||
| Sum squares | ||
| Cluster prominence | ||
| Imc2 | ||
| Imc1 | ||
| MCC | ||
| Difference average | ||
| Id | ||
| Cluster tendency | ||
| GLSZM | Gray level variance | |
| Zone variance | ||
| Gray level non-uniformity normalized | ||
| Size zone non-uniformity normalized | ||
| Size zone non-uniformity | ||
| Gray level non-uniformity | ||
| Large area emphasis | ||
| Small Area high gray level emphasis | ||
| Zone percentage | ||
| Large area low gray level emphasis | ||
| Large area high gray level emphasis | ||
| High gray level zone emphasis | ||
| Small area emphasis | ||
| Low gray level zone emphasis | ||
| Zone entropy | ||
| Small area low gray level emphasis | ||
| Gray level variance | ||
| GLRLM | Short run low gray level emphasis | |
| Gray level variance | ||
| Low gray level run emphasis | ||
| Gray level non-uniformity normalized | ||
| Run variance | ||
| Gray level non-uniformity | ||
| Long run emphasis | ||
| Short Run high gray level emphasis | ||
| Run length non-uniformity | ||
| Short run emphasis | ||
| Long run high gray level emphasis | ||
| Run percentage | ||
| Long run low gray level emphasis | ||
| Run entropy | ||
| High gray level run emphasis | ||
| Run length non-uniformity normalized | ||
| NGTDM | Coarseness | |
| Complexity | ||
| Strength | ||
| Contrast | ||
| Busyness | ||
| GLDM | Gray level variance | |
| High gray level emphasis | ||
| Dependence entropy | ||
| Dependence non-uniformity | ||
| Gray level non-uniformity | ||
| Small dependence emphasis | ||
| Small dependence high gray level emphasis | ||
| Dependence non-uniformity normalized | ||
| Large dependence emphasis | ||
| Large dependence low gray level emphasis | ||
| Dependence variance | ||
| Large dependence high gray level emphasis | ||
| Small dependence low gray level emphasis | ||
| Low gray level emphasis |
| Feature Type | Wavelet-Based |
|---|---|
| Methods | First-order statistic and texture of wavelet decomposition. Decomposition levels: LLL, LLH, LHL, LHH, HLL, HLH, HHL, HHH. |
| Feature name | First-order features |
| GLCM features | |
| GLSZM features | |
| GLRLM features | |
| NGTDM features | |
| GLDM features |
| ROI | Filter | Feature List | |
|---|---|---|---|
| T1-weighted MR image | |||
| CTV | wavelet-LLH | Firstorder | Skewness |
| eCTV5 | wavelet-HLL | GLDM | LargeDependenceHighGrayLevelEmphasis |
| eCTV5 | wavelet-HLH | GLCM | Correlation |
| eCTV20 | Original | Shape | SurfaceVolumeRatio |
| eCTV20 | Original | GLCM | MCC |
| eCTV20 | wavelet-LLH | GLSZM | GrayLevelNonUniformity |
| eCTV20 | wavelet-HLH | Firstorder | Kurtosis |
| eCTV20 | wavelet-HHH | Firstorder | Skewness |
| eCTV20 | wavelet-HHL | Gldm | DependenceNonUniformity |
| eCTV20 | wavelet-HHL | Glcm | Imc1 |
| sCTV5 | wavelet-HLL | Glcm | InverseVariance |
| sCTV5 | wavelet-HLL | Firstorder | Skewness |
| sCTV5 | wavelet-LHL | Glszm | LargeAreaLowGrayLevelEmphasis |
| sCTV5 | wavelet-LLH | Firstorder | Skewness |
| sCTV5 | wavelet-HLH | Firstorder | Median |
| sCTV10 | Original | Glszm | LargeAreaHighGrayLevelEmphasis |
| sCTV10 | wavelet-HLL | Glcm | InverseVariance |
| sCTV10 | wavelet-LHH | Firstorder | Mean |
| sCTV10 | wavelet-LLH | Gldm | SmallDependenceLowGrayLevelEmphasis |
| sCTV10 | wavelet-HLH | Firstorder | Mean |
| sCTV10 | wavelet-HHH | Gldm | SmallDependenceLowGrayLevelEmphasis |
| sCTV10 | wavelet-HHH | Firstorder | Skewness |
| sCTV10 | wavelet-HHL | Firstorder | Skewness |
| sCTV10 | wavelet-HHL | Firstorder | Mean |
| sCTV10 | wavelet-LLL | Gldm | SmallDependenceLowGrayLevelEmphasis |
| T2-weighted MR image | |||
| CTV | wavelet-HHH | Firstorder | Median |
| eCTV20 | wavelet-HLL | Firstorder | Skewness |
| sCTV5 | wavelet-HHH | Firstorder | Median |
| sCTV10 | Original | Gldm | SmallDependenceLowGrayLevelEmphasis |
| T1 | T2 | T1&T2 | ||||
|---|---|---|---|---|---|---|
| Training | Test | Training | Test | Training | Test | |
| Sensitivity | 89.9 | 86.4 | 87.8 | 87.4 | 97.6 | 93.1 |
| Specificity | 81.7 | 74.9 | 31.3 | 38.1 | 92.2 | 81.6 |
| Accuracy | 87.2 | 81.8 | 67.9 | 72.2 | 95.9 | 88.7 |
| AUC | 0.89 | 0.69 | 0.94 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, D.; Nishibuchi, I.; Kawamura, M.; Yoshida, T.; Koh, I.; Tomono, K.; Sekine, M.; Takahashi, H.; Kikuchi, Y.; Kudo, Y.; et al. Radiomic Analysis for Pretreatment Prediction of Recurrence Post-Radiotherapy in Cervical Squamous Cell Carcinoma Cancer. Diagnostics 2022, 12, 2346. https://doi.org/10.3390/diagnostics12102346
Kawahara D, Nishibuchi I, Kawamura M, Yoshida T, Koh I, Tomono K, Sekine M, Takahashi H, Kikuchi Y, Kudo Y, et al. Radiomic Analysis for Pretreatment Prediction of Recurrence Post-Radiotherapy in Cervical Squamous Cell Carcinoma Cancer. Diagnostics. 2022; 12(10):2346. https://doi.org/10.3390/diagnostics12102346
Chicago/Turabian StyleKawahara, Daisuke, Ikuno Nishibuchi, Masashi Kawamura, Takahito Yoshida, Iemasa Koh, Katsuyuki Tomono, Masaki Sekine, Haruko Takahashi, Yutaka Kikuchi, Yoshiki Kudo, and et al. 2022. "Radiomic Analysis for Pretreatment Prediction of Recurrence Post-Radiotherapy in Cervical Squamous Cell Carcinoma Cancer" Diagnostics 12, no. 10: 2346. https://doi.org/10.3390/diagnostics12102346
APA StyleKawahara, D., Nishibuchi, I., Kawamura, M., Yoshida, T., Koh, I., Tomono, K., Sekine, M., Takahashi, H., Kikuchi, Y., Kudo, Y., & Nagata, Y. (2022). Radiomic Analysis for Pretreatment Prediction of Recurrence Post-Radiotherapy in Cervical Squamous Cell Carcinoma Cancer. Diagnostics, 12(10), 2346. https://doi.org/10.3390/diagnostics12102346







