Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study
Abstract
1. Introduction
- −
- They become osteocytes embedded in mineralized ECM and lose most of their cytoplasmic organelles. Osteocytes have low metabolic activity, can be present throughout the patient’s entire life, and represent approximately 90–95% of bone cells in the adult [29].
- −
- They die by apoptosis.
- −
- They turn into BLC (bone lining cells), a line of post-mitotic osteoblasts, with a flat appearance that are found on the surface of the bone. These cells have an important role in regulating bone remodeling processes [30].
2. Materials and Methods
2.1. Patient Selection
2.2. Bone Tissue Harvest
2.3. Bone Explant Cultivation
2.4. Differentiation of Mesenchymal Stem Cells to Osteoblasts under Laser Application
2.5. Differentiation of Mesenchymal Stem Cells into the Osteoblast Phenotype
2.6. Analysis of the Viability and Proliferation of Osteoblasts under the Effect of Laser Application
2.7. Quantification of Cell Proliferation under the Effect of Laser
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Thirumagal, A. Stem cell research: A bibliometric analysis from 1999–2008. Stem Cell Res. 2012, 2, 1–12. [Google Scholar]
- Elliott, M.J.; Butler, C.R.; Varanou-Jenkins, A.; Partington, L.; Carvalho, C.; Samuel, E.; Crowley, C.; Lange, P.; Hamilton, N.J.; Hynds, R.E.; et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl. Med. 2017, 6, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 26, 68. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Plomer, A. The European Group on Ethics: Law, Politics and the Limits of Moral Integration in Europe. Eur. Law J. 2008, 148, 839–859. [Google Scholar] [CrossRef]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for Personalized Medicine: Ethical Challenges, Isolation and Biocompatibility Evaluation of 3D Electrospun and Printed Scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Characteristics and potential applications of human dental tissuederived mesenchymal stem cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Poudineh, M.; Wang, Z.; Labib, M.; Ahmadi, M.; Zhang, L.; Das, J.; Ahmed, S.; Angers, S.; Kelley, S.O. Threedimensional nanostructured architectures enable efficient neural differentiation of Mesenchymal stem cells via Mechanotransduction. Nano Lett. 2018, 18, 7188–7193. [Google Scholar] [CrossRef]
- Dos Santos, J.F.; Borçari, N.R.; da Silva Araújo, M.; Nunes, V.A. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: Towards an in vitro model of human epidermis. J. Cell. Biochem. 2019, 120, 13141–13155. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Ariyanti, A.D.; Luo, Q.; Song, G. Recent Progress in Engineering Mesenchymal Stem Cell Differentiation. Stem Cell Rev. Rep. 2020, 16, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.E.; Bruscia, E.; Krause, D.S. Plasticity of bone marrow-derived stem cells. Stem Cells 2004, 22, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Bossolasco, P.; Corti, S.; Strazzer, S.; Borsotti, C.; Del Bo, R.; Fortunato, F.; Salani, S.; Quirici, N.; Bertolini, F.; Gobbi, A.; et al. Skeletal muscle differentiation potential of human adult bone marow cells. Exp. Cell Res. 2004, 295, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Fermor, B.; Urban, J.; Murray, D.; Pocock, A.; Lim, E.; Francis, M. Proliferation and collagen synthesis of human anterior cruciate ligament cells in vitro: Effects of ascorbate-2-phosphate, dexamethasone, and oxygen tension. Cell Biol. Int. 1998, 22, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Shastri, V.P.; Padera, R.F.; Yang, J.; Mackay, A.J.; Langer, R. Selective differentiation of mammalia bone marrow stromal cells cultured on three-dimensional polymer foams. J. Biomed. Mater. Res. 2001, 55, 229–235. [Google Scholar] [CrossRef]
- Choi, K.M.; Seo, Y.K.; Yoon, H.H.; Song, K.Y.; Kwon, S.Y.; Lee, H.S.; Park, J.K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.; An, S.; Cheng, Q.; Huang, Y.; Wang, Y.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef]
- Bakopoulou, A.; About, I. Stem cells of dental origin: Current research trends and key milestones towards clinical application. Stem Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Q.I.; Yang, T.; Qi, Y.; Fu, M.; Yang, X.I.; Qiao, L.; Ling, Q.; Liu, S.; Zhao, Y. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol. Med. Rep. 2018, 17, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Sordi, M.B.; Curtarelli, R.B.; da Silva, I.T.; Fongaro, G.; Benfatti, C.A.M.; Magini, R.D.S.; da Cruz, A.C.C. Effect of dexamethasone as osteogenic supplementation in in vitro osteogenic differentiation of stem cells from human exfoliated deciduous teeth. J. Mater. Sci. Mater. Med. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, L.; Csöbönyeiová, M. Stem Cells and Their Derivatives —Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef] [PubMed]
- Tetè, G.; D’Orto, B.; Nagni, M.; Agostinacchio, M.; Polizzi, E.; Agliardi, E. Role of induced pluripotent stem cells (IPSCS) in bone tissue regeneration in dentistry: A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 3), 1–10. [Google Scholar]
- Tetè, G.; Capparè, P.; Gherlone, E. New Application of Osteogenic Differentiation from HiPS Stem Cells for Evaluating the Osteogenic Potential of Nanomaterials in Dentistry. Int. J. Environ. Res. Public Health 2020, 17, 1947. [Google Scholar] [CrossRef] [PubMed]
- Hynes, K.; Menicanin, D.; Han, J.; Marino, V.; Mrozik, K.; Gronthos, S.; Bartold, P. Mesenchymal Stem Cells from iPS Cells Facilitate Periodontal Regeneration. J. Dent. Res. 2013, 92, 833–839. [Google Scholar] [CrossRef]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanismsand implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef]
- Everts, V.; Delaissé, J.M.; Korper, W.; Jansen, D.C.; Tigchelaar-Gutter, W.; Saftig, P.; Beertsen, W. The bone lining cell: Its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Miner. Res. 2002, 17, 77–90. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Vignesh, R.C.; Arunakaran, J.; Aruldhas, M.M.; Srinivasan, N. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem. Cell Biol. 2006, 84, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Current Concepts of Laser–Oral Tissue Interaction. Dent. J. 2020, 8, 61. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A.; Cavalcanti, R.; Ronsivalle, V.; Chaurasia, A.; Spagnuolo, G.; Isola, G. Impact of Laser application on Periodontal and Peri-Implant Diseases. Photobiomodul. Photomed. Laser Surg. 2022, 40, 454–462. [Google Scholar]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Martu, A.; Agop-Forna, D.; Luchian, I.; Toma, V.; Picus, M.; Solomon, S. Influence of laser application as an adjunct to periodontaly optimizing orthodontic tooth movement. Rom. J. Oral Rehabil. 2019, 11, 161–169. [Google Scholar]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Hanna, R.; Agas, D.; Benedicenti, S.; Ferrando, S.; Laus, F.; Cuteri, V.; Lacava, G.; Sabbieti, M.G.; Amaroli, A. A comparative study between the effectiveness of 980 nm Photobiomodulation delivered by hand-piece with Gaussian vs. flat-top profiles on osteoblasts maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Kamali, F.; Bayat, M.; Torkaman, G.; Ebrahimi, E.; Salavati, M. The therapeutic effect of lowlevel laser on repair of osteochondral defects in rabbit knee. J. Photochem. Photobiol. B 2007, 88, 11–15. [Google Scholar] [CrossRef]
- Jenkins, P.A.; Carroll, J.D. How to report low-level laser application (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed. Laser Surg. 2011, 29, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser application with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Niimi, H.; Ohsugi, Y.; Katagiri, S.; Watanabe, K.; Hatasa, M.; Shimohira, T.; Tsuchiya, Y.; Maekawa, S.; Hirota, T.; Kadokura, H.; et al. Effects of Low-Level Er:YAG Laser Irradiation on Proliferation and Calcification of Primary Osteoblast-Like Cells Isolated From Rat Calvaria. Front. Cell Dev. Biol. 2020, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Deana, A.M.; de Souza, A.M.; Teixeira, V.P.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Fernandes, K.P.S. The impact of photobiomodulation on osteoblast-like cell: A review. Lasers Med. Sci. 2018, 33, 1147–1158. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Dong, S.; Liu, S.; Zhang, X.; Si, X.; Zhou, Y. Laser irradiation promotes the proliferation of mouse pre-osteoblast cell line MC3T3-E1 through hedgehog signaling pathway. Lasers Med. Sci. 2017, 32, 1489–1496. [Google Scholar] [CrossRef]
- Chang, B.; Qiu, H.; Zhao, H.; Yang, X.; Wang, Y.; Ji, T.; Zhang, Y.; Quan, Q.; Li, Y.; Zeng, J.; et al. The Effects of Photobiomodulation on MC3T3-E1 Cells via 630 nm and 810 nm Light-Emitting Diode. Med. Sci. Monit. 2019, 25, 8744–8752. [Google Scholar] [CrossRef]
- Rosenberg, N.; Gendelman, R.; Noofi, N. Photobiomodulation of human osteoblast-like cells in vitro by low-intensity-pulsed LED light. FEBS Open Bio 2020, 10, 1276–1287. [Google Scholar] [CrossRef]
- Cardoso, M.V.; do Vale Placa, R.; Sant’Ana, A.C.P.; Greghi, S.L.A.; Zangrando, M.S.R.; de Rezende, M.L.R.; Oliveira, R.C.; Damante, C.A. Laser and LED photobiomodulation effects in osteogenic or regular medium on rat calvaria osteoblasts obtained by newly forming bone technique. Lasers Med. Sci. 2021, 36, 541–553. [Google Scholar] [CrossRef]
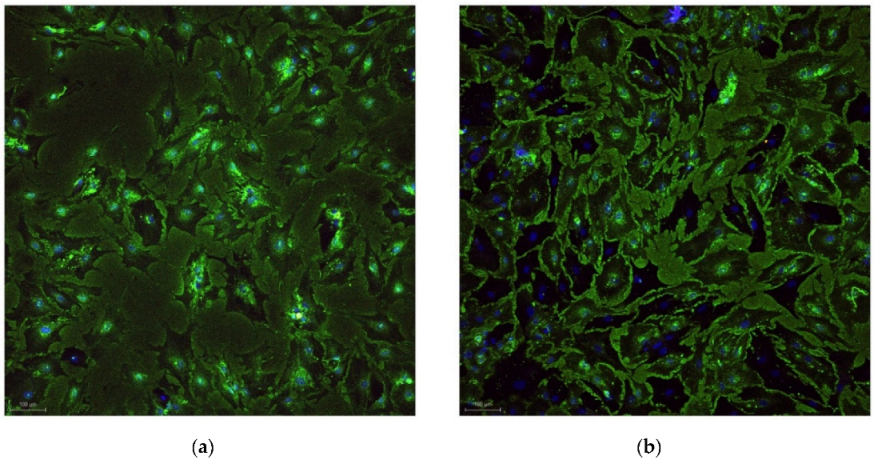
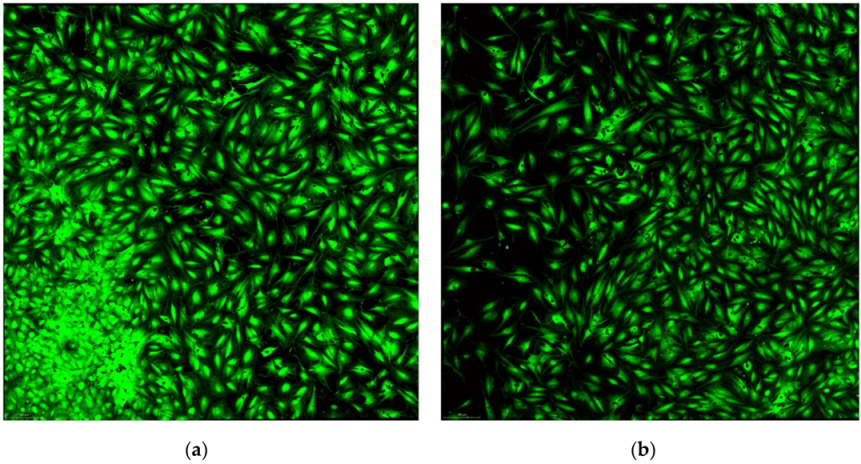
| Fluorochromes | λ Excitation | Biological Sample | Biological Sample | LASER Power | Detector Type | Emission Band |
|---|---|---|---|---|---|---|
| Alexa Fluor 488 | 488 nm | Intracellular alkaline phosphatase | osteoblasts | 7 mW | PMT | 503–580 nm |
| Alexa Fluor 594 | 561 nm | Intracellular osteocalcin | osteoblasts | 35.6 mW | HyD Intern | 590–790 nm |
| DAPI | 405 nm | Nuclear DNA | osteoblasts | 24.5 mW | PMT | 415–475 nm |
| Fluorochromes | λ Excitation | Biological Sample | Biological Sample | LASER Power | Detector Type | Emission Band |
|---|---|---|---|---|---|---|
| Ethidium III Homodimer (EthD-III) | 561 nm | Dead cells’ DNA | osteoblasts | 20 mW | PMT | 575–780 nm |
| Calcein AM | 496 nm | Esterases from living cells | osteoblasts | 8 mW | HyD Intern | 505–611 nm |
| Patient | Age | Gender | Harvest Site |
|---|---|---|---|
| 1 | 28 | female | Upper second premolar (2.5) |
| 2 | 22 | male | Upper second molar (1.7) |
| 3 | 20 | female | Upper first premolar (1.4) |
| 4 | 38 | female | Upper third molar (2.8) |
| 5 | 21 | male | Lower second molar (4.7) |
| 6 | 41 | female | Lower third molar (3.8) |
| 7 | 45 | male | Lower second premolar (3.5) |
| 8 | 49 | female | Upper second molar (1.7) |
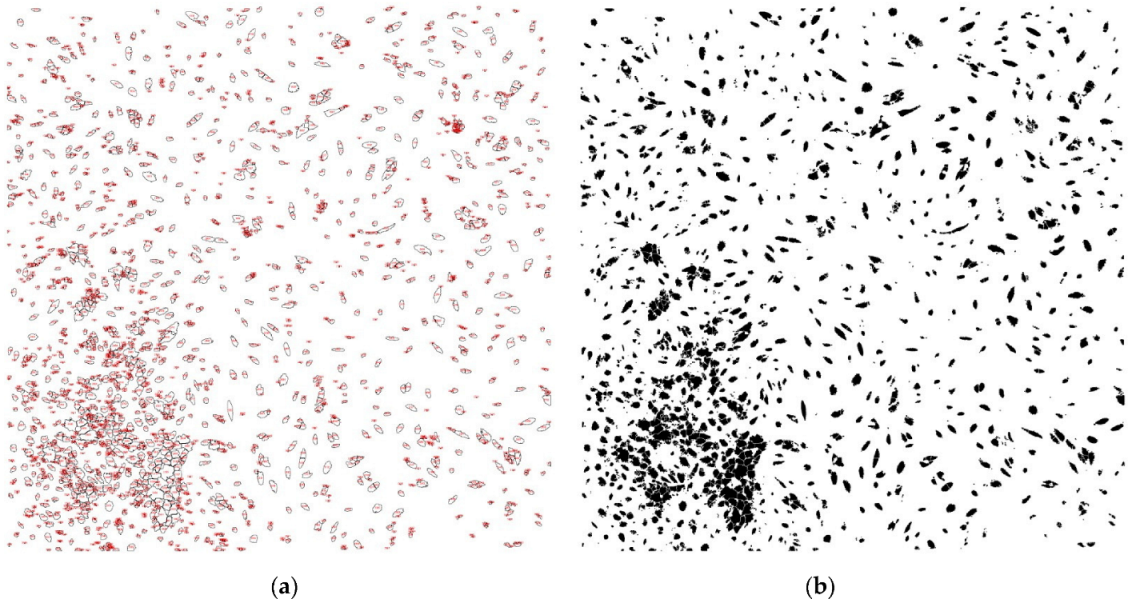
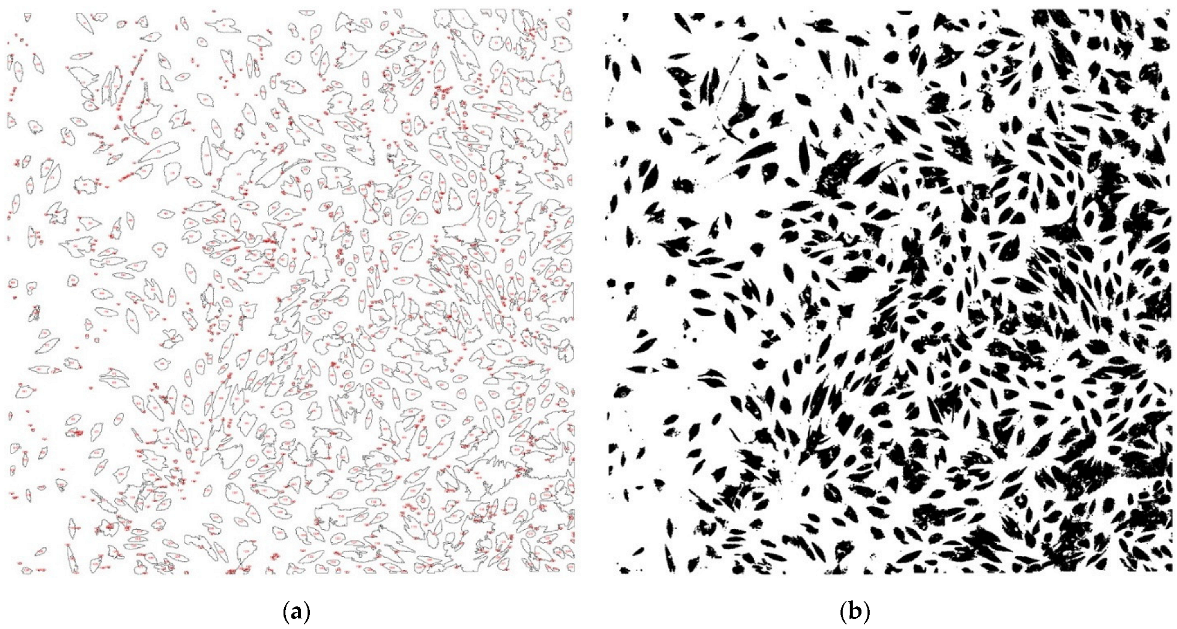
| Slice | Count (n) | Total Area (Pixels) | Average Size (Pixels) | Area (%) |
|---|---|---|---|---|
| Patient 1 laser + | 2309 | 553,123.852 | 239.551 | 10.556 |
| Patient 1 laser - | 1315 | 1,401,762.407 | 1065.979 | 26.777 |
| Patient 2 laser + | 3701 | 645,989.447 | 174.545 | 12.292 |
| Patient 2 laser - | 2508 | 638,447.68 | 254.564 | 12.166 |
| Patient 3 laser + | 4617 | 537,615.553 | 116.443 | 41.039 |
| Patient 3 laser - | 2860 | 480,853.707 | 168.131 | 36.527 |
| Patient 4 laser + | 1537 | 239,764.02 | 155.995 | 18.231 |
| Patient 4 laser - | 482 | 133,835.969 | 277.668 | 10.216 |
| Patient 5 laser + | 4583 | 1,076,814.891 | 234.959 | 20.55 |
| Patient 5 laser - | 2478 | 795,648.215 | 321.085 | 15.206 |
| Patient 6 laser + | 1322 | 620,348.193 | 469.25 | 11.85 |
| Patient 6 laser - | 1156 | 432,137.329 | 373.821 | 8.259 |
| Patient 7 laser + | 529 | 1,772,710.067 | 335.105 | 16.906 |
| Patient 7 laser - | 220 | 489,080.086 | 222.309 | 3.722 |
| Patient 8 laser + | 241 | 739,000.588 | 306.641 | 5.541 |
| Patient 8 laser - | 93 | 2,791,200.064 | 300.129 | 0.665 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, L.; Manu, D.R.; Dako, T.; Mârțu, M.-A.; Suciu, M.; Ormenișan, A.; Păcurar, M.; Lazăr, A.-P. Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study. Diagnostics 2022, 12, 2358. https://doi.org/10.3390/diagnostics12102358
Lazăr L, Manu DR, Dako T, Mârțu M-A, Suciu M, Ormenișan A, Păcurar M, Lazăr A-P. Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study. Diagnostics. 2022; 12(10):2358. https://doi.org/10.3390/diagnostics12102358
Chicago/Turabian StyleLazăr, Luminița, Doina Ramona Manu, Timea Dako, Maria-Alexandra Mârțu, Mircea Suciu, Alina Ormenișan, Mariana Păcurar, and Ana-Petra Lazăr. 2022. "Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study" Diagnostics 12, no. 10: 2358. https://doi.org/10.3390/diagnostics12102358
APA StyleLazăr, L., Manu, D. R., Dako, T., Mârțu, M.-A., Suciu, M., Ormenișan, A., Păcurar, M., & Lazăr, A.-P. (2022). Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study. Diagnostics, 12(10), 2358. https://doi.org/10.3390/diagnostics12102358







