The CHA2DS2-VASc Score Predicts New-Onset Atrial Fibrillation and Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluated Parameters
2.3. Outcome Definitions
2.4. Statistical Analyses
3. Results
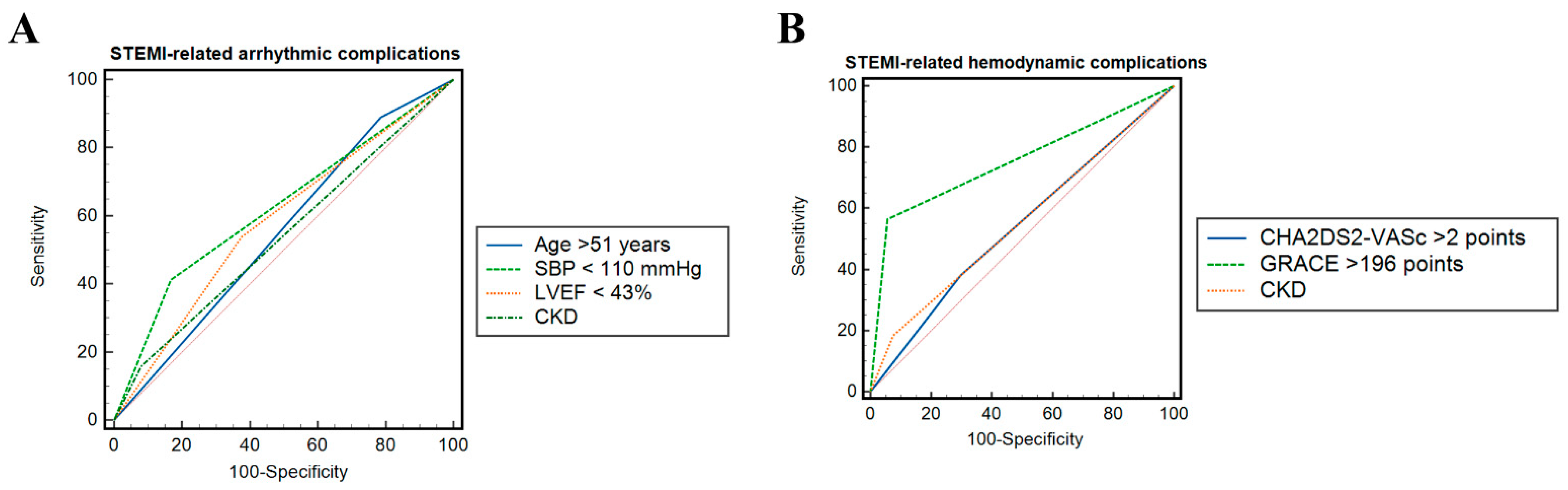
3.1. Predictors of ST-Segment Elevation Myocardial Infarction-Related Arrhythmic Complications
3.2. Predictors of ST-SEGMENT Elevation Myocardial Infarction-Related Hemodynamic Complications
4. Discussion
4.1. Arrhythmic and Hemodynamic Complications Remain Common in the Era of Primary Percutaneous Coronary Interventions
4.2. CHA2DS2-VASc Score above 2 Points Independently Predicts New-Onset Atrial Fibrillation in Patients with ST-Segment Elevation Myocardial Infarction
4.3. CHA2DS2-VASc Score above 2 Points Independently Predicts Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction
4.4. Clinical Implications
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bishu, K.G.; Lekoubou, A.; Kirkland, E.; Schumann, S.O.; Schreiner, A.; Heincelman, M.; Moran, W.P.; Mauldin, P.D. Estimating the Economic Burden of Acute Myocardial Infarction in the US: 12 Year National Data. Am. J. Med Sci. 2020, 359, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Tricarico, L.; Casavecchia, G.; Ieva, R.; Iacoviello, M.; Di Biase, M.; Magnesa, M.; Corbo, M.D.; Vitale, E.; Brunetti, N.D. Revascularization rates with coronary angioplasty and mortality in type 2 myocardial infarction: A meta-regression analysis. Am. J. Emerg. Med. 2021, 47, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Karam, N.; Bataille, S.; Marijon, E.; Tafflet, M.; Benamer, H.; Caussin, C.; Garot, P.; Juliard, J.-M.; Pires, V.; Boche, T.; et al. Incidence, Mortality, and Outcome-Predictors of Sudden Cardiac Arrest Complicating Myocardial Infarction Prior to Hospital Admission. Circ. Cardiovasc. Interv. 2019, 12, e007081. [Google Scholar] [CrossRef]
- Antman, E.M.; Cohen, M.; Bernink, P.J.L.M.; McCabe, C.H.; Horacek, T.; Papuchis, G.; Mautner, B.; Corbalan, R.; Radley, D.; Braunwald, E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000, 284, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Boersma, E.; Pieper, K.S.; Steyerberg, E.W.; Wilcox, R.G.; Chang, W.-C.; Lee, K.L.; Akkerhuis, K.M.; Harrington, R.A.; Deckers, J.W.; Armstrong, P.W.; et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000, 101, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Arisha, M.M.; Girerd, N.; Chauveau, S.; Bresson, D.; Scridon, A.; Bonnefoy, E.; Chevalier, P. In-Hospital Heart Rate Turbulence and Microvolt T-Wave Alternans Abnormalities for Prediction of Early Life-Threatening Ventricular Arrhythmia after Acute Myocardial Infarction. Ann. Noninvasive Electrocardiol. 2013, 18, 530–537. [Google Scholar] [CrossRef]
- Șerban, R.C.; Hadadi, L.; Șuș, I.; Lakatos, E.K.; Demjen, Z.; Scridon, A. Impact of chronic obstructive pulmonary disease on in-hospital morbidity and mortality in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Int. J. Cardiol. 2017, 243, 437–442. [Google Scholar] [CrossRef]
- Șerban, R.C.; Șuș, I.; Lakatos, E.K.; Demjen, Z.; Ceamburu, A.; Fișcă, P.C.; Somkereki, C.; Hadadi, L.; Scridon, A. Chronic kidney disease predicts atrial fibrillation in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Acta Cardiol. 2019, 74, 472–479. [Google Scholar] [CrossRef]
- Agarwal, M.; Agrawal, S.; Garg, L.; Garg, A.; Bhatia, N.; Kadaria, D.; Reed, G. Effect of Chronic Obstructive Pulmonary Disease on In-Hospital Mortality and Clinical Outcomes After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 119, 1555–1559. [Google Scholar] [CrossRef]
- Megaly, M.; Schmidt, C.W.; Dworak, M.W.; Garberich, R.; Stanberry, L.; Sharkey, S.; Brilakis, E.S.; Aguirre, F.V.; Pacheco, R.; Tannenbaum, M.; et al. Diabetic Patients Who Present With ST-Elevation Myocardial Infarction. Cardiovasc. Revasc. Med. 2022, 38, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Mirbolouk, F.; Gholipour, M.; Salari, A.; Shakiba, M.; Kheyrkhah, J.; Nikseresht, V.; Sotoudeh, N.; Moghadam, N.; Mirbolouk, M.J.; Far, M.M. CHA2DS2-VASc Score Predict No-Reflow Phenomenon in Primary Percutaneous Coronary Intervention in Primary Percutaneous Coronary Intervention. J. Cardiovasc. Thorac. Res. 2018, 10, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ipek, G.; Onuk, T.; Karatas, M.B.; Gungor, B.; Osken, A.; Keskin, M.; Oz, A.; Tanik, O.; Hayiroglu, M.I.; Yaka, H.Y.; et al. CHA2DS2-VASc Score is a Predictor of No-Reflow in Patients With ST-Segment Elevation Myocardial Infarction Who Underwent Primary Percutaneous Intervention. Angiology 2016, 67, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, A.; Yarlioglues, M.; Duran, M. Predictive Value of CHA2DS2-VASC Score for Contrast-Induced Nephropathy after Percutaneous Coronary Intervention for Acute Coronary Syndrome. Am. J. Cardiol. 2017, 119, 819–825. [Google Scholar] [CrossRef]
- Keskin, K.; Yıldız, S.S.; Çetinkal, G.; Aksan, G.; Kilci, H.; Çetin, Ş.; Siğirci, S.; Kılıçkesmez, K. The Value of CHA2DS2VASC Score in Predicting All-Cause Mortality in Patients with ST-Segment Elevation Myocardial Infarction Who Have Undergone Primary Percutaneous Coronary Intervention. Acta Cardiol. Sin. 2017, 33, 598–604. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Rey, P.T.; García, O.O.; Álvarez, A.B.C.; Juskova, M.; Álvarez, B.; Acuña, J.M.G.; Bermejo, R.A.; Veloso, P.R.; Otero, D.L.; Pena, J.C.S.; et al. Prognostic impact of renal function trajectories in patients with STEMI and kidney dysfunction undergoing primary percutaneous coronary intervention: An analysis of ten years all-comers registry. Hell. J. Cardiol. 2022, 66, 1–10. [Google Scholar] [CrossRef]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Hear. J. 2020, 41, 655–720. [Google Scholar] [CrossRef]
- APEX AMI Investigators; Armstrong, P.W.; Granger, C.B.; Adams, P.X.; Hamm, C.; Holmes, D., Jr.; O’Neill, W.W.; Todaro, T.G.; Vahanian, A.; Van de Werf, F. Pexelizumab for Acute ST-Elevation Myocardial Infarction in Patients Undergoing Primary Percutaneous Coronary Intervention. JAMA 2007, 297, 43–51. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Pfeffer, M.A.; Mcmurray, J.; Maggioni, A.P.; Rouleau, J.-L.; Van de Werf, F.; Kober, L.; White, H.D.; Swedberg, K.; Leimberger, J.D.; et al. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: Baseline characteristics in context. Eur. J. Heart Fail. 2003, 5, 537–544. [Google Scholar] [CrossRef]
- Grines, C.L.; Westerhausen, D.R., Jr.; Grines, L.L.; Hanlon, J.; Logemann, T.L.; Niemela, M.; Weaver, W.; Graham, M.; Boura, J.; O’Neill, W.W.; et al. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction: The air primary angioplasty in myocardial infarction study. J. Am. Coll. Cardiol. 2002, 39, 1713–1719. [Google Scholar] [CrossRef]
- Hunziker, L.; Radovanovic, D.; Jeger, R.; Pedrazzini, G.; Cuculi, F.; Urban, P.; Erne, P.; Rickli, H.; Pilgrim, T.; AMIS Plus Registry Investigators. Twenty-Year Trends in the Incidence and Outcome of Cardiogenic Shock in AMIS Plus Registry. Circ. Cardiovasc. Interv. 2019, 12, e007293. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Fach, A.; Schmucker, J.; Fiehn, E.; Garstka, D.; Stehmeier, J.; Hambrecht, R.; Wienbergen, H. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: Results from the Bremen STEMI Registry. Clin. Res. Cardiol. 2018, 107, 371–379. [Google Scholar] [CrossRef]
- Swedeheart Annual Report 2019. Available online: https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh/1-swedeheart-annual-report-2019 (accessed on 6 June 2022).
- Crenshaw, B.S.; Ward, S.R.; Granger, C.B.; Stebbins, A.L.; Topol, E.J.; Califf, R.M. Atrial Fibrillation in the Setting of Acute Myocardial Infarction: The GUSTO-I Experience. J. Am. Coll. Cardiol. 1997, 30, 406–413. [Google Scholar] [CrossRef]
- Scridon, A.; Şerban, R.C.; Chevalier, P. Atrial fibrillation: Neurogenic or myogenic? Arch. Cardiovasc. Dis. 2018, 111, 59–69. [Google Scholar] [CrossRef]
- Scridon, A.; Serban, R.C.; Balan, A.I.; Perian, M.; Pintilie, I.; Somkereki, C.; Huţanu, A. Atrial electrical remodeling induced by chronic ischemia and inflammation in patients with stable coronary artery disease. Chin. J. Physiol. 2019, 62, 11–16. [Google Scholar] [CrossRef]
- Scridon, A.; Tabib, A.; Barrès, C.; Julien, C.; Chevalier, P. Left atrial endocardial fibrosis and intra-atrial thrombosis—land-marks of left atrial remodeling in rats with spontaneous atrial tachyarrhythmias. Rom. J. Morphol. Embryol. 2013, 54, 405–411. [Google Scholar]
- Scridon, A.; Fouilloux-Meugnier, E.; Loizon, E.; Perian, M.; Rome, S.; Julien, C.; Barrès, C.; Chevalier, P. Age-dependent myocardial transcriptomic changes in the rat. Novel insights into atrial and ventricular arrhythmias pathogenesis. Rev. Romana Med. Lab. 2014, 22, 9–23. [Google Scholar] [CrossRef][Green Version]
| Parameter | Total (n = 831) | Arrhythmic Complications (n = 126) | No Arrhythmic Complications (n = 705) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age (years) | 62 (53–70) | 64 (56–74) | 61 (53–70) | 0.01 | - | - |
| Female gender (n, %) | 246 (29.6%) | 36 (28.5%) | 210 (29.7%) | 0.83 | 0.94 | 0.62–1.43 |
| Cardiovascular risk factors | ||||||
| Active smoker (n, %) | 390 (46.9%) | 59 (46.8%) | 331 (46.9%) | 0.99 | 0.99 | 0.68–1.45 |
| Arterial hypertension (n, %) | 530 (63.7%) | 81 (64.2%) | 449 (63.6%) | 0.92 | 1.02 | 0.69–1.52 |
| Diabetes mellitus (n, %) | 191 (22.9%) | 24 (19.0%) | 167 (23.6%) | 0.30 | 0.75 | 0.47–1.22 |
| Chronic kidney disease (n, %) | 76 (9.1%) | 20 (15.8%) | 56 (7.9%) | <0.01 | 2.18 | 1.26–3.79 |
| Chronic heart failure (n, %) | 109 (13.1%) | 21 (16.6%) | 88 (12.4%) | 0.19 | 1.40 | 0.83–2.35 |
| Chronic respiratory diseases (n, %) | 68 (8.1%) | 15 (11.9%) | 53 (7.5%) | 0.11 | 1.66 | 0.90–3.05 |
| Previous myocardial infarction (n, %) | 63 (7.5%) | 9 (7.1%) | 54 (7.6%) | 0.99 | 0.92 | 0.44–1.93 |
| CHA2DSS-VASc score (points) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.37 | - | - |
| Factors related to the acute phase of STEMI | ||||||
| Heart rate at admission (bpm) | 80 (67–90) | 78 (60–91) | 80 (67–90) | 0.74 | - | - |
| Systolic blood pressure (mmHg) | 130 (114–146) | 122 (103–140) | 130 (115–149) | <0.001 | - | - |
| Left ventricular ejection fraction (%) | 45 (40–45) | 42 (39–45) | 45 (40–47) | <0.001 | - | - |
| Anterior myocardial infarction (n, %) | 346 (41.6%) | 46 (36.5%) | 300 (42.5%) | 0.23 | 0.77 | 0.52–1.14 |
| Characteristics of coronary artery disease | ||||||
| Multivessel disease (n, %) | 524 (63.0%) | 75 (59.5%) | 449 (63.6%) | 0.36 | 0.83 | 0.57–1.23 |
| Culprit vessel (n, %) | ||||||
| Left anterior descending artery | 385 (46.3%) | 64 (50.7%) | 321 (45.5%) | 0.39 | - | - |
| Right coronary artery | 334 (40.1%) | 56 (44.4%) | 278 (39.4%) | |||
| Circumflex artery | 112 (13.4%) | 6 (4.7%) | 106 (15.0%) | |||
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Model 1 | |||
| Age > 51 years | 2.35 | 1.27–4.34 | <0.001 |
| Systolic blood pressure < 110 mmHg on admission | 3.75 | 2.44–5.75 | <0.001 |
| Left ventricular ejection fraction < 43% on admission | 2.15 | 1.40–3.31 | <0.001 |
| Chronic kidney disease | 1.93 | 1.07–3.50 | 0.02 |
| Anterior myocardial infarction | 0.66 | 0.42–1.04 | 0.07 |
| Model 2 | |||
| CHA2DS2-VASc score > 2 points | 1.17 | 0.46–1.35 | 0.40 |
| Chronic kidney disease | 2.18 | 1.25–3.90 | <0.001 |
| Anterior myocardial infarction | 0.76 | 0.51–1.14 | 0.17 |
| Parameter | Total (n = 839) | Hemodynamic Complications (n = 87) | No Hemodynamic Complications (n = 752) | p-Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age (years) | 62 (54–70) | 66 (56–74) | 61 (53–70) | <0.0001 | - | - |
| Female gender (n, %) | 236 (28.1%) | 26 (29.8%) | 210 (27.9%) | 0.70 | 1.10 | 0.67–1.78 |
| Cardiovascular risk factors | ||||||
| Active smoker (n, %) | 392 (46.7%) | 35 (40.2%) | 357 (47.4%) | 0.21 | 0.74 | 0.47–1.17 |
| Arterial hypertension (n, %) | 542 (64.6%) | 61 (70.1%) | 481 (63.9%) | 0.28 | 1.32 | 0.81–2.14 |
| Diabetes mellitus (n, %) | 189 (22.5%) | 24 (27.5%) | 165 (21.9%) | 0.22 | 1.35 | 0.82–2.23 |
| Chronic kidney disease (n, %) | 76 (9.1%) | 18 (20.6%) | 58 (7.7%) | <0.0001 | 3.12 | 1.74–5.59 |
| Chronic heart failure (n, %) | 100 (11.9%) | 16 (18.3%) | 84 (11.1%) | 0.05 | 1.79 | 0.99–3.22 |
| Chronic respiratory diseases (n, %) | 67 (8.1%) | 11 (12.6%) | 56 (7.4%) | 0.09 | 1.79 | 0.90–3.58 |
| Previous myocardial infarction (n, %) | 61 (7.2%) | 6 (6.8%) | 55 (7.3%) | 0.99 | 0.93 | 0.39–2.24 |
| CHA2DSS-VASc score (points) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.51 | - | - |
| Factors related to the acute phase of STEMI | ||||||
| Anterior myocardial infarction (n, %) | 352 (41.9%) | 38 (43.6%) | 314 (41.7%) | 0.73 | 1.08 | 0.69–1.69 |
| Characteristics of coronary artery disease | ||||||
| Multivessel disease (n, %) | 528 (62.9%) | 60 (68.9%) | 468 (62.2%) | 0.24 | 1.34 | 0.83–2.20 |
| Culprit vessel (n, %) | ||||||
| Anterior descending artery | 404 (48.1%) | 67 (77.0%) | 337 (44.8%) | 0.30 | - | - |
| Right coronary artery | 322 (38.3%) | 17 (19.5%) | 305 (40.5%) | |||
| Circumflex artery | 113 (13.4%) | 3 (3.4%) | 110 (14.6%) | |||
| Variable | OR | 95% | p-Value |
|---|---|---|---|
| CHA2DS2-VASc score > 2 points | 1.59 | 1.00–2.52 | 0.04 |
| Chronic kidney disease | 2.95 | 1.64–5.32 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozac, D.A.; Lakatos, E.K.; Demjen, Z.; Ceamburu, A.; Fișcă, P.C.; Șuș, I.; Hadadi, L.; Scridon, A. The CHA2DS2-VASc Score Predicts New-Onset Atrial Fibrillation and Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics 2022, 12, 2396. https://doi.org/10.3390/diagnostics12102396
Cozac DA, Lakatos EK, Demjen Z, Ceamburu A, Fișcă PC, Șuș I, Hadadi L, Scridon A. The CHA2DS2-VASc Score Predicts New-Onset Atrial Fibrillation and Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics. 2022; 12(10):2396. https://doi.org/10.3390/diagnostics12102396
Chicago/Turabian StyleCozac, Dan Alexandru, Eva Katalin Lakatos, Zoltan Demjen, Alexandru Ceamburu, Paul Ciprian Fișcă, Ioana Șuș, Laszlo Hadadi, and Alina Scridon. 2022. "The CHA2DS2-VASc Score Predicts New-Onset Atrial Fibrillation and Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention" Diagnostics 12, no. 10: 2396. https://doi.org/10.3390/diagnostics12102396
APA StyleCozac, D. A., Lakatos, E. K., Demjen, Z., Ceamburu, A., Fișcă, P. C., Șuș, I., Hadadi, L., & Scridon, A. (2022). The CHA2DS2-VASc Score Predicts New-Onset Atrial Fibrillation and Hemodynamic Complications in Patients with ST-Segment Elevation Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics, 12(10), 2396. https://doi.org/10.3390/diagnostics12102396







