Genome-Wide Cell-Free DNA Test for Fetal Chromosomal Abnormalities and Variants: Unrestricted Versus Restricted Reporting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pregnancy Outcome
2.2. Statistical Analysis
3. Results
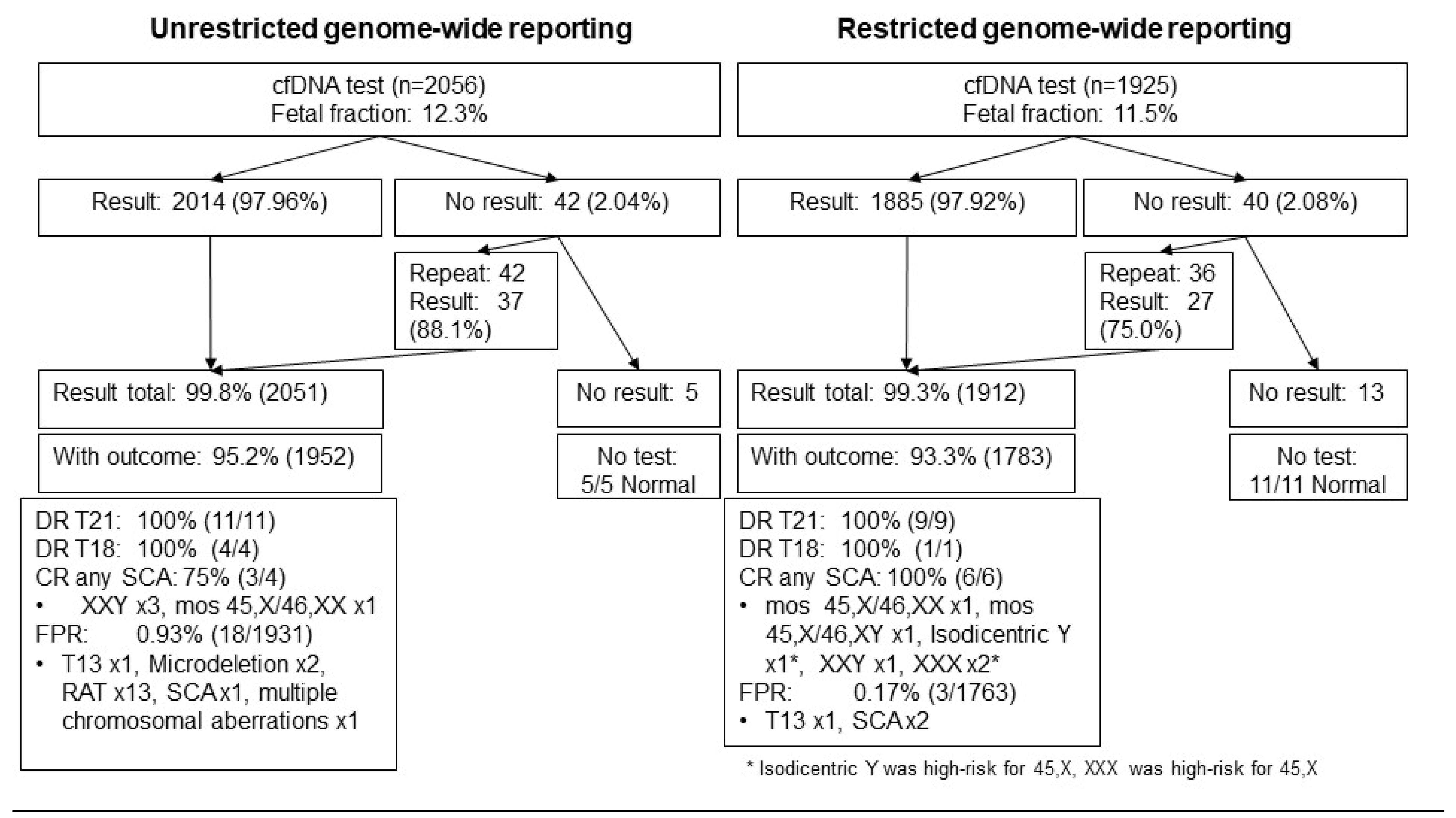
3.1. Study Population
3.2. Concordance Rate between Cell-Free DNA Test Results and Outcomes (Table 1)
| Total Study Population, n = 3735 | ||||
|---|---|---|---|---|
| Cell-Free DNA Results | Total | Outcome | n | Concordance (95% CI) |
| Low risk | 3677 (98.4%) | Normal Mos 45,X[16]/46,XX[8]) | 3676 1 | 100% (99.9–100.0) |
| High risk for trisomy 21 | 20 (0.54%) | Trisomy 21 | 20 | 100% (83.9–100.0) |
| High risk for trisomy 18 | 5 (0.13%) | Trisomy 18 | 5 | 100% (56.6–100.0) |
| High risk for trisomy 13 | 2 (0.05%) | Normal | 2 | 0% (0–65.8) |
| High risk for any SCA | 12 (0.32%) | Normal Any SCA | 3 9 | 75% (46.8–91.1) |
| • “Borderline 45,X” | 3 | Normal | 2 | 33.3% (6.2–79.2) |
| Mos X[7]/46,XX[31] | 1 | |||
| • 45,X | 3 | Normal | 2 | 0% (0–56.2) |
| Isodicentric Y | 1 | |||
| • Suspected X/XY | 1 | Normal | 0 | 0% (0–79.4) |
| • 47,XXY | 5 | 47,XXY | 4 | 100% (56.6–100.0) |
| • “Borderline 45,X” | 3 | Normal | 2 | 33.3% (6.2–79.2) |
| Mos X[7]/46,XY[25] | 1 | |||
| High risk for any RAT | 15 (0.40%) | Normal | 13 | 13.3% (3.7–37.9) |
| Abnormal | 2 | |||
| • Increased amount of chr 3 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 7 | 6 | Normal | 5 | 16.7% (3.0–56.4) |
| Mos T7; 47 XX, + 7[9]/46,XX[21] | 1 | |||
| • Increased amount of chr 8 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 9 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 10 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 11 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 16 | 2 | Normal | 1 | 50% (9.5–90.6) |
| Trisomy 16 | 1 | |||
| • Increased amount of chr 20 | 1 | Normal | 1 | 0% (0–79.4) |
| • Reduced amount of chr 16 | 1 | Normal | 1 | 0% (0–79.4) |
| • Multiple chromosomal aberrations | 1 (0.03%) | Normal | 1 | 0% (0–79.4) |
| Any microdeletion syndromes | 3 (0.08%) | Normal | 2 | 33.3% (6.2–79.2) |
| Abnormal | 1 | |||
| • Cri du Chat | 1 | Cri du Chat | 1 | 100% (20.7–100.0) |
| • 1 Mb deletion in Chr 15q11.2 | 1 | Normal | 1 | 0% (0–79.4) |
| • 19 Mb deletion in Chr 7q21.3 | 1 | Normal | 1 | 0% (0–79.4) |
| Reporting period 2015–2017, n = 1952 | ||||
| Low risk | 1914 (98.1%) | Normal | 1913 | 100% (99.8–100.0) |
| Mos 45,X[16]/46,XX[8]) | 1 | |||
| High risk for trisomy 21 | 11 (0.56%) | Trisomy 21 | 11 | 100% (74.1–100.0) |
| High risk for trisomy 18 | 4 (0.20%) | Trisomy 18 | 4 | 100% (51.0–100.0) |
| High risk for trisomy 13 | 1 (0.05%) | Normal | 1 | 0% (0–79.4) |
| High risk for any SCA | 4 (0.20%) | Any SCA | 3 | 75% (30.1–95.4) |
| • “Borderline 45,X” | 1 | Normal | 1 | 0% (0–79.4) |
| • 47,XXY | 3 | 47,XXY | 3 | 100% (43.9–100.0) |
| High risk for Any RAT | 15 (0.77%) | Normal | 13 | 13.3% (3.7–37.9) |
| Abnormal | 2 | |||
| • Increased amount of chr 3 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 7 | 6 | Normal | 5 | 16.7% (3.0–56.4) |
| Mos T7; 47 XX, + 7[9]/46,XX[21] | 1 | |||
| • Increased amount of chr 8 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 9 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 10 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 11 | 1 | Normal | 1 | 0% (0–79.4) |
| • Increased amount of chr 16 | 2 | Normal | 1 | 50% (9.5–90.6) |
| Trisomy 16 | 1 | |||
| • Increased amount of chr 20 | 1 | Normal | 1 | 0% (0–79.4) |
| • Reduced amount of chr 16 | 1 | Normal | 1 | 0% (0–79.4) |
| Multiple chromosomal aberrations | 1 (0.05%) | Normal | 1 | 0% (0–79.4) |
| Microdeletion syndromes | 2 (0.10%) | Normal | 1 | 0% (0–65.8) |
| • 1 Mb deletion in Chr 15q11.2 • 19 Mb deletion in Chr 7q21.3 | 1 1 | Normal Normal | 1 1 | 0% (0–79.4) 0% (0–79.4) |
| Reporting period 2018–2019, n = 1783 | ||||
| Low risk | 1763 (98.9%) | Normal | 1763 | 100% (99.8–100.0) |
| High risk for trisomy 21 | 9 (0.50%) | Trisomy 21 | 9 | 100% (70.1–100.0) |
| High risk for trisomy 18 | 1 (0.06%) | Trisomy 18 | 1 | 100% (20.7–100.0) |
| High risk for trisomy 13 | 1 (0.06%) | Normal | 1 | 0% (0–79.4) |
| High risk for any SCA | 8 (0.45%) | Normal | 2 | 75.0% (40.9–92.9) |
| SCA | 6 | |||
| • Borderline 45,X | 2 | Normal | 1 | 50% (9.5–90.6) |
| Mos X[7]/46,XX[31] | 1 | |||
| • 45,X | 3 | Normal | 2 | 0% (0–56.2) |
| Isodicentric Y | 1 | |||
| • Suspected X/XY | 1 | Normal | 0 | 0% (0–79.4) |
| • 47,XXY | 2 | 47,XXY | 1 | 100% (56.6–100.0) |
| Mos X[7]/46,XY[25] | 1 | 50% (9.5–90.6) | ||
| Microdeletion syndromes | 1 (0.06%) | Normal | 1 | 100% (0–20.7) |
| • Cri du Chat | 1 | Cri du Chat | 1 | 100% (0–20.7) |
| RAT | Diagnostic Testing | Testing Results | Pregnancy Outcome | Delivery at Term | Complications | Placenta |
|---|---|---|---|---|---|---|
| Increased amount of chr 3 | Amniocentesis | 46,XY | Live birth | Y | None | 46,XY |
| Increased amount of chr 7 | Amniocentesis | 47XX, + 7[9]/46,XX [21] | MTOP | N | None | Not tested |
| Amniocentesis | 46,XY | Live birth | None | Not tested | ||
| Amniocentesis | 46,XX | Live birth | Y | None | 46,XX | |
| Amniocentesis | 46,XY | Live birth | Y | None | 46,XY | |
| Amniocentesis | 46,XY | Live birth | None | Not tested | ||
| Amniocentesis | 46,XX | Live birth | Y | None | 46,XX | |
| Increased amount of chr 8 | Amniocentesis | 46,XY | Live birth | Y | Preeclampsia | 47,XY, + 8[4]/46,XY[46] (CPM) |
| Increased amount of chr 9 | Amniocentesis | 46XX | Live birth | Y | Superimposed preeclampsia on chronic renal hypertension | Not tested |
| Increased amount of chr 10 | Amniocentesis | 46,XX | Live birth | Y | Preeclampsia | Not tested |
| Increased amount of chr 11 | Amniocentesis | 46,XY | Live birth | Y | None | Not tested |
| Increased amount of chr 16 | Amniocentesis | 46,XX | Live birth | N | Fetal growth restriction | 47,XX, + 16 (CPM) |
| Not conducted | Miscarriage | N | 47,XX, + 16 | |||
| Increased amount of chr 20 | Amniocentesis | 46,XX | Live birth | Y | None | 47,XX, + 20[2]/46,XX[28] (CPM) |
| Reduced amount of chr 16 | Amniocentesis | Normal | Live birth | Y | None | 46,XY |
3.3. Test Performance of Cell-Free DNA Analysis (Table 3)
| Total Study Population, n = 3735 | ||||
|---|---|---|---|---|
| Outcome Group | Total | cfDNA Results | N, % (95% CI) | Pregnancy Outcomes, n (%) |
| Normal | 3697 (99.0%) | Low risk | 3676, 99.4 (99.1–99.6) | Live birth, 3644 (98.5) Miscarriage, 24 (0.7) Termination of pregnancy, 18 (0.5) Stillbirth, 12 (0.3) |
| High risk | 21, 0.57 (0.37–0.87) | |||
| • Trisomy 13 | 2, 0.05 (0.01–0.20) | |||
| • SCA | 3, 0.08 (0.03–0.24) | |||
| • RAT | 13, 0.35 (0.21–0.60) | |||
| • Multiple chromosomal aberrations | 1, 0.03 (0.00–0.15) | |||
| • Microdeletion syndrome | 2, 0.05 (0.01–0.20) | |||
| Trisomy 21 | 20 (0.54%) | High risk for trisomy 21 | 20, 100 (83.9–100) | Live birth, 1 (5.0) Termination of pregnancy, 19 (95.0) |
| Trisomy 18 | 5 (0.13%) | High risk for trisomy 18 | 5, 100 (56.6–100) | Termination of pregnancy, 5 (100) |
| SCA | 10 (0.27%) | Low risk | 1, 10.0 (1.8–40.4) | Live birth, 7 (70.0) Termination of pregnancy, 3 (30.0) |
| High risk for any SCA | 9, 90.0 (59.6–98.2) | |||
| • Mosaic 45,X/46,XX | 2 (0.05%) | Low risk | 1, 50.0 (9.5–90.6) | Termination of pregnancy, 2 (100) |
| High risk for “borderline 45,X” | 1, 50.0 (9.5–90.6) | |||
| • Mosaic 45,X/46,XY | 1 (0.03%) | High risk for XXY or low level of Y | 1, 100 (20.7–100) | Live birth, 1 (100) |
| • Isodicentric Y | 1 (0.03%) | High risk for 45,X | 0, 0 (0–79.4) | Live birth, 1 (100) |
| • 47,XXX | 2 (0.05%) | High risk for 45,X | 0, 0 (0–65.8) | Live birth, 2 (100) |
| • 47,XXY | 4 (0.11%) | High risk for 47,XXY | 4, 100 (51.0–100) | Live birth, 4 (100) |
| Mos T7; 47 XX, + 7 [9]/46,XX [21] | 1 (0.03%) | High risk for increased chr 7 | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
| Trisomy 16 | 1 (0.03%) | High risk for increased chr 16 | 1, 100 (20.7–100) | Miscarriage, 1 (100) |
| Cri du Chat | 1 (0.03%) | High risk for Cri du Chat | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
| Reporting period 2015–2017, n = 1952 | ||||
| Normal | 1931 (99.0%) | Low risk | 1913, 99.0 (98.5–99.4) | Live birth, 1898 (98.2) Miscarriage, 12 (0.6) Termination of pregnancy, 14 (0.7) Stillbirth, 8 (0.4) |
| High risk | 18, 0.93 (0.59–1.47) | |||
| • Trisomy 13 | 1, 0.05 (0.01–0.30) | |||
| • RAT | 13, 0.67 (0.39–1.15) | |||
| • SCA | 1, 0.05 (0.01–0.30) | |||
| • Multiple chromosomal aberrations | 1, 0.10 (0.01–0.29) | |||
| • Microdeletion syndrome | 2, 0.10 (0.03–0.38) | |||
| Trisomy 21 | 11 (0.56%) | High risk for trisomy 21 | 11, 100 (74.1–100) | Live birth, 1 (9.1) Termination of pregnancy, 10 (90.9) |
| Trisomy 18 | 4 (0.20%) | High risk for trisomy 18 | 4, 100 (51.0–100) | Termination of pregnancy, 4 (100) |
| SCA | 4 (0.20%) | Low risk | 1, 25.0 (4.6–69.9) | Live birth, 3 (75.0) Termination of pregnancy, 1 (25.0) |
| High risk for any SCA | 3, 75.0 (30.1–95.4) | |||
| • Mosaic 45,X/46,XX | 1 (0.05%) | Low risk | 0, 0 0, 0 (0–79.4) | Termination of pregnancy, 1 (100) |
| • 47,XXY | 3 (0.15%) | High risk for 47,XXY | 3, 100 (43.9–100) | Live birth, 3 (100) |
| Mos T7; 47 XX, + 7 [9]/46,XX [21] | 1 (0.05%) | High risk for increased chr 7 | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
| Trisomy 16 | 1 (0.03%) | High risk for increased chr 16 | 1, 100 (20.7–100) | Miscarriage, 1 (100) |
| Reporting period 2018–2019, n = 1783 | ||||
| Normal | 1766 (99.1%) | Low risk | 1763, 99.8 (99.5–100) | Live birth, 1746 (98.9) Miscarriage, 12 (0.7) Termination of pregnancy, 4 (0.2) Stillbirth, 4 (0.2) |
| High risk | 3, 0.17 (0.06–0.50) | |||
| • Trisomy 13 | 1, 0.06 (0.01–0.33) | |||
| • SCA | 2, 0.11 (0.03–0.41) | |||
| Trisomy 21 | 9 (0.50%) | High risk for trisomy 21 | 9, 100 (70.1–100) | Termination of pregnancy, 9 (100) |
| Trisomy 18 | 1 (0.06%) | High risk for trisomy 18 | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
| SCA | 6 (0.34%) | High risk for any SCA | 6, 100 (61.0–100) | Live birth, 4 (66.7) Termination of pregnancy, 2 (33.3) |
| • Mosaic 45,X/46,XX | 1 (0.06%) | High risk for borderline 45,X | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
| • Mosaic 45,X/46,XY | 1 (0.06%) | High risk for XXY or low level of Y | 1, 100 (20.7–100) | Live birth, 1 (100) |
| • Isodicentric Y | 1 (0.06%) | High risk for 45,X | 0, 0 (0–79.4) | Live birth, 1 (100) |
| • 47,XXX | 2 (0.11%) | High risk for 45,X | 0, 0 (0–65.8) | Live birth, 1 (100) |
| • 47,XXY | 1 (0.06%) | High risk for 47,XXY | 1, 100 (20.7–100) | Live birth, 1 (100) |
| Cri du Chat | 1 (0.06%) | Cri du Chat | 1, 100 (20.7–100) | Termination of pregnancy, 1 (100) |
3.4. Frequency of No Result
| Total Study Population, n = 82/3981 (2.06%) | |||||
|---|---|---|---|---|---|
| First Draw | Total (n = 82) | Second Draw | n | Third Draw | n |
| Pre-laboratory error | 18 (0.45%) | Low risk High risk for trisomy 18 Pre-laboratory error No redraw | 15 1 1 1 | ||
| Interference | 21 (0.53%) | Low risk Interference No redraw | 12 8 1 | High risk for SCA | 1 |
| Equivocal | 3 (0.08%) | Low risk High risk for SCA High risk for RAT | 1 1 1 | ||
| Low fetal fraction | 38 (0.95%) | Low risk Low fetal fraction High risk for trisomy 21 High risk for SCA No redraw | 27 7 1 1 2 | Low risk/low fetal fraction | 1/1 |
| Others | 2 (0.05%) | Low risk | 2 | ||
| Reporting period 2015–2017, n = 42/2056 (2.04%) | |||||
| Pre-laboratory error | 8 (0.39%) | Low risk High risk for trisomy 18 | 7 1 | ||
| Interference | 10 (0.49%) | Low risk Interference | 9 1 | ||
| Equivocal | 3 (0.15%) | Low risk High risk for SCA High risk for RAT | 1 1 1 | ||
| Low fetal fraction | 19 (0.92%) | Low risk Low fetal fraction High risk for trisomy 21 High risk for SCA | 13 4 1 1 | Low fetal fraction | 1 |
| Others | 2 (0.09%) | Low risk | 2 | ||
| Reporting period 2018–2019, n = 40/1925 (2.08%) | |||||
| Pre-laboratory error | 10 (0.52%) | Low risk Pre-laboratory error No redraw | 8 1 1 | ||
| Interference | 11 (0.57%) | Low risk Interference No redraw | 3 7 1 | High risk for SCA | 1 |
| Low fetal fraction | 19 (0.99%) | Low risk Low fetal fraction No redraw | 14 3 2 | Low risk | 1 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil, M.M.; Accurti, V.; Santacruz, B.; Plana, M.N.; Nicolaides, K.H. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 302–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaides, K.H.; Syngelaki, A.; Ashoor, G.; Birdir, C.; Touzet, G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am. J. Obstet. Gynecol. 2012, 207. [Google Scholar] [CrossRef]
- Quezada, M.S.; Gil, M.M.; Francisco, C.; Oròsz, G.; Nicolaides, K.H. Screening for trisomies 21, 18 and 13 by cell-free DNA analysis of maternal blood at 10–11 weeks’ gestation and the combined test at 11–13 weeks. Ultrasound Obstet. Gynecol. 2015, 45, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.; Syngelaki, A.; Poon, L.; Gil, M.; Wright, D. First-trimester contingent screening for trisomies 21, 18 and 13 by biomarkers and maternal blood cell-free DNA testing. Fetal Diagn. Ther. 2014, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.; Wright, D.; Poon, L.C.; Syngelaki, A.; Gil, M.M. First-trimester contingent screening for trisomy 21 by biomarkers and maternal blood cell-free DNA testing. Ultrasound Obstet. Gynecol. 2013, 42, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoor, G.; Syngelaki, A.; Wagner, M.; Birdir, C.; Nicolaides, K.H. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am. J. Obstet. Gynecol. 2012, 206, 322-e1. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, G.; Syngelaki, A.; Wang, E.; Struble, C.; Oliphant, A.; Song, K.; Nicolaides, K.H. Trisomy 13 detection in the first trimester of pregnancy using a chromosome-selective cell-free DNA analysis method. Ultrasound Obstet. Gynecol. 2013, 41, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.W.; Parker, R.L.; Wentworth, J.; Madankumar, R.; Saffer, C.; Das, A.F.; Craig, J.A.; Chudova, D.I.; Devers, P.L.; Jones, K.W.; et al. DNA sequencing versus standard prenatal aneuploidy screening. N. Engl. J. Med. 2014, 370, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, M.E.; Jacobsson, B.; Swamy, G.K.; Laurent, L.C.; Ranzini, A.C.; Brar, H.; Tomlinson, M.W.; Pereira, L.; Spitz, J.L.; Hollemon, D.; et al. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015, 372, 1589–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Liu, C.; Qi, H.; Zhang, Y.; Bian, X.; Liu, J. Noninvasive prenatal testing of fetal aneuploidies by massively parallel sequencing in a prospective Chinese population. Prenat. Diagn. 2013, 33, 700–706. [Google Scholar] [CrossRef]
- Gil, M.; Quezada, M.S.; Bregant, B.; Ferraro, M.; Nicolaides, K. Implementation of maternal blood cell-free DNA testing in early screening for aneuploidies. Ultrasound Obstet. Gynecol. 2013, 42, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Wang, W.; Dong, Y.; Zhang, M.; Wang, X.; Yin, C. Cell-free DNA screening for sex chromosome aneuploidies by non-invasive prenatal testing in maternal plasma. Mol. Cytogenet. 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhu, Q.; Liu, S.; Liu, J.; Bai, T.; Jing, X.; Xia, T.; Liu, Y.; Cheng, J.; Li, Z.; et al. Clinical application of noninvasive prenatal screening for sex chromosome aneuploidies in 50,301 pregnancies: Initial experience in a Chinese hospital. Sci. Rep. 2019, 9, 7767. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wan, S.; Dang, Y.; Song, T.; Chen, B.; Zhang, J. Non-invasive prenatal testing for detection of trisomy 13, 18, 21 and sex chromosome aneuploidies in 8594 cases. Ginekol. Pol. 2019, 90, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennuti, M.T.; Chandrasekaran, S.; Khalek, N.; Dugoff, L. Cell-free DNA screening and sex chromosome aneuploidies. Prenat. Diagn. 2015, 35, 980–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Wergifosse, S.; Bevilacqua, E.; Mezela, I.; El Haddad, S.; Gounongbe, C.; de Marchin, J.; Maggi, V.; Conotte, S.; Badr, D.A.; Fils, J.-F.; et al. Cell-free DNA analysis in maternal blood: Comparing genome-wide versus targeted approach as a first-line screening test. J. Matern. Fetal Neonatal Med. 2019, 34, 3552–3561. [Google Scholar] [CrossRef] [Green Version]
- Pescia, G.; Guex, N.; Iseli, C.; Brennan, L.; Osteras, M.; Xenarios, I.; Farinelli, L.; Conrad, B. Cell-free DNA testing of an extended range of chromosomal anomalies: Clinical experience with 6,388 consecutive cases. Genet. Med. 2017, 19, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Van Opstal, D.; Van Maarle, M.C.; Lichtenbelt, K.; Weiss, M.M.; Schuring-Blom, H.; Bhola, S.L.; Hoffer, M.J.V.; Huijsdens-van Amsterdam, K.H.; Macville, M.V.; Kooper, A.J.A.; et al. Origin and clinical relevance of chromosomal aberrations other than the common trisomies detected by genome-wide NIPS: Results of the TRIDENT study. Genet. Med. 2018, 20, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Chatron, N.; Till, M.; Abel, C.; Bardel, C.; Ramond, F.; Sanlaville, D.; Schluth-Bolard, C. Detection of rare autosomal trisomies through non-invasive prenatal testing: Benefits for pregnancy management. Ultrasound Obstet. Gynecol. 2019, 53, 129–130. [Google Scholar] [CrossRef]
- Wan, J.; Li, R.; Zhang, Y.; Jing, X.; Yu, Q.; Li, F.; Li, Y.; Zhang, L.; Yi, C.; Li, J.; et al. Pregnancy outcome of autosomal aneuploidies other than common trisomies detected by noninvasive prenatal testing in routine clinical practice. Prenat. Diagn. 2018, 38, 849–857. [Google Scholar] [CrossRef]
- Liang, D.; Lin, Y.; Qiao, F.; Li, H.; Wang, Y.; Zhang, J.; Liu, A.; Ji, X.; Ma, D.; Jiang, T.; et al. Perinatal outcomes following cell-free DNA screening in >32 000 women: Clinical follow-up data from a single tertiary center. Prenat. Diagn. 2018, 38, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hannum, G.; Geis, J.; Tynan, J.; Hogg, G.; Zhao, C.; Jensen, T.J.; Mazloom, A.R.; Oeth, P.; Ehrich, M.; et al. Determination of fetal DNA fraction from the plasma of pregnant women using sequence read counts. Prenat. Diagn. 2015, 35, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lv, W.; Wang, H.; Xu, L.; Liu, J.; Li, H.; Hu, L.; Peng, Y.; Wu, L. Non-invasive prenatal testing of fetal whole chromosome aneuploidy by massively parallel sequencing. Prenat. Diagn. 2013, 33, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Chan, K.C.A.; Sun, H.; Chen, E.Z.; Jiang, P.; Lun, F.M.F.; Zheng, Y.W.; Leung, T.Y.; Lau, T.K.; Cantor, C.R.; et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl. Med. 2010, 2, 61ra91. [Google Scholar] [CrossRef]
- Yu, S.C.Y.; Chan, K.C.A.; Zheng, Y.W.L.; Jiang, P.; Liao, G.J.W.; Sun, H.; Akolekar, R.; Leung, T.Y.; Go, A.T.J.I.; van Vugt, J.M.G.; et al. Size-based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc. Natl. Acad. Sci. USA 2014, 111, 8583–8588. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.C.; Jiang, P.; Chan, K.A.; Faas, B.H.; Choy, K.W.; Leung, W.C.; Leung, T.Y.; Lo, Y.D.; Chiu, R.W. Combined Count- and Size-Based Analysis of Maternal Plasma DNA for Noninvasive Prenatal Detection of Fetal Subchromosomal Aberrations Facilitates Elucidation of the Fetal and/or Maternal Origin of the Aberrations. Clin. Chem. 2017, 63, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Chen, M.; Wang, H.; Guo, Y.; Chau, M.H.K.; Yan, H.; Cao, Y.; Kwok, Y.K.Y.; Chen, J.; Hui, A.S.Y.; et al. Clinical utility of expanded non-invasive prenatal screening and chromosomal microarray analysis in high-risk pregnancy. Ultrasound Obstet. Gynecol. 2021, 57, 459–465. [Google Scholar] [CrossRef]
- Dharajiya, N.G.; Namba, A.; Horiuchi, I.; Miyai, S.; Farkas, D.H.; Almasri, E.; Saldivar, J.-S.; Takagi, K.; Kamei, Y. Uterine leiomyoma confounding a noninvasive prenatal test result. Prenat. Diagn. 2015, 35, 990–993. [Google Scholar] [CrossRef]
- Kornman, L.; Palma-Dias, R.; Nisbet, D.; Scott, F.; Menezes, M.; Costa, F.D.S.; McLennan, A. Non-Invasive Prenatal Testing for Sex Chromosome Aneuploidy in Routine Clinical Practice. Fetal Diagn. Ther. 2018, 44, 85–90. [Google Scholar] [CrossRef]
- Suo, F.; Wang, C.; Liu, T.; Fang, Y.; Wu, Q.; Gu, M.; Gou, L. Non-invasive prenatal testing in detecting sex chromosome aneuploidy: A large-scale study in Xuzhou area of China. Clin. Chim. Acta 2018, 481, 139–141. [Google Scholar] [CrossRef]
- Garshasbi, M.; Wang, Y.; Zadeh, S.H.; Giti, S.; Piri, S.; Hekmat, M.R. Clinical Application of Cell-Free DNA Sequencing-Based Noninvasive Prenatal Testing for Trisomies 21, 18, 13 and Sex Chromosome Aneuploidy in a Mixed-Risk Population in Iran. Fetal Diagn. Ther. 2020, 47, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, K.R.M.; Sistermans, E.A.; Macville, M.; Stevens, S.; Bax, C.J.; Bekker, M.N.; Bilardo, C.M.; Boon, E.; Boter, M.; Diderich, K.; et al. TRIDENT-2: National Implementation of Genome-wide Non-invasive Prenatal Testing as a First-Tier Screening Test in the Netherlands. Am. J. Hum. Genet. 2019, 105, 1091–1101. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, B.-Y.; Yu, B.; Zheng, F.-X.; Zhou, Q.; Chen, Y.-P.; Zhang, X.-Q. Noninvasive prenatal screening for fetal common sex chromosome aneuploidies from maternal blood. J. Int. Med. Res. 2017, 45, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Tang, Y.; Lu, Q.; Pan, A.; Shi, M. Application value of NIPT for uncommon fetal chromosomal abnormalities. Mol. Cytogenet. 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, J.; Hou, Y.; Guo, F.; Peng, H.; Wang, D.; Du, Q.; Yin, A. The significance of trisomy 7 mosaicism in noninvasive prenatal screening. Hum. Genom. 2019, 13, 18. [Google Scholar] [CrossRef]
- Sparks, T.N.; Thao, K.; Norton, M.E. Mosaic trisomy 16: What are the obstetric and long-term childhood outcomes? Genet. Med. 2017, 19, 1164–1170. [Google Scholar] [CrossRef]
- Grati, F.R.; Ferreira, J.; Benn, P.; Izzi, C.; Verdi, F.; Vercellotti, E.; Dalpiaz, C.; D’Ajello, P.; Filippi, E.; Volpe, N.; et al. Outcomes in pregnancies with a confined placental mosaicism and implications for prenatal screening using cell-free DNA. Genet. Med. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Fiorentino, F.; Bono, S.; Pizzuti, F.; Duca, S.; Polverari, A.; Faieta, M.; Baldi, M.; Diano, L.; Spinella, F. The clinical utility of genome-wide non invasive prenatal screening. Prenat. Diagn. 2017, 37, 593–601. [Google Scholar] [CrossRef]
- Yaron, Y. The implications of non-invasive prenatal testing failures: A review of an under-discussed phenomenon. Prenat. Diagn. 2016, 36, 391–396. [Google Scholar] [CrossRef]
- Galeva, S.; Gil, M.D.M.; Konstantinidou, L.; Akolekar, R.; Nicolaides, K.H. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: Factors affecting test failure. Ultrasound Obstet. Gynecol. 2019, 53, 804–809. [Google Scholar] [CrossRef]
- Pergament, E.; Cuckle, H.; Zimmermann, B.; Banjevic, M.; Sigurjonsson, S.; Ryan, A.; Hall, M.P.; Dodd, M.; Lacroute, P.; Stosic, M.; et al. Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet. Gynecol. 2014, 124, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revello, R.; Sarno, L.; Ispas, A.; Akolekar, R.; Nicolaides, K.H. Screening for trisomies by cell-free DNA testing of maternal blood: Consequences of a failed result. Ultrasound Obstet. Gynecol. 2016, 47, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet. Gynecol. 2017, 49, 714–720. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, A.H.W.; Zhu, X.; Mar Gil, M.; Kwok, Y.K.Y.; Wah, I.Y.M.; Hui, A.S.Y.; Ting, Y.-H.; Law, K.-M.; Lau, D.; Xue, S.; et al. Genome-Wide Cell-Free DNA Test for Fetal Chromosomal Abnormalities and Variants: Unrestricted Versus Restricted Reporting. Diagnostics 2022, 12, 2439. https://doi.org/10.3390/diagnostics12102439
Kwan AHW, Zhu X, Mar Gil M, Kwok YKY, Wah IYM, Hui ASY, Ting Y-H, Law K-M, Lau D, Xue S, et al. Genome-Wide Cell-Free DNA Test for Fetal Chromosomal Abnormalities and Variants: Unrestricted Versus Restricted Reporting. Diagnostics. 2022; 12(10):2439. https://doi.org/10.3390/diagnostics12102439
Chicago/Turabian StyleKwan, Angel H. W., Xiaofan Zhu, Maria Mar Gil, Yvonne K. Y. Kwok, Isabella Y. M. Wah, Annie S. Y. Hui, Yuen-Ha Ting, Kwok-Ming Law, Doris Lau, Shuwen Xue, and et al. 2022. "Genome-Wide Cell-Free DNA Test for Fetal Chromosomal Abnormalities and Variants: Unrestricted Versus Restricted Reporting" Diagnostics 12, no. 10: 2439. https://doi.org/10.3390/diagnostics12102439






