CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection and Sequencing
2.2. Power Analysis
2.3. GEPIA2
2.4. TIMER2.0
2.5. cBioPortal
2.6. Protein–Protein Interaction (PPI) Network and Functional Enrichment Analysis
2.7. Cell Lines Validation
3. Results
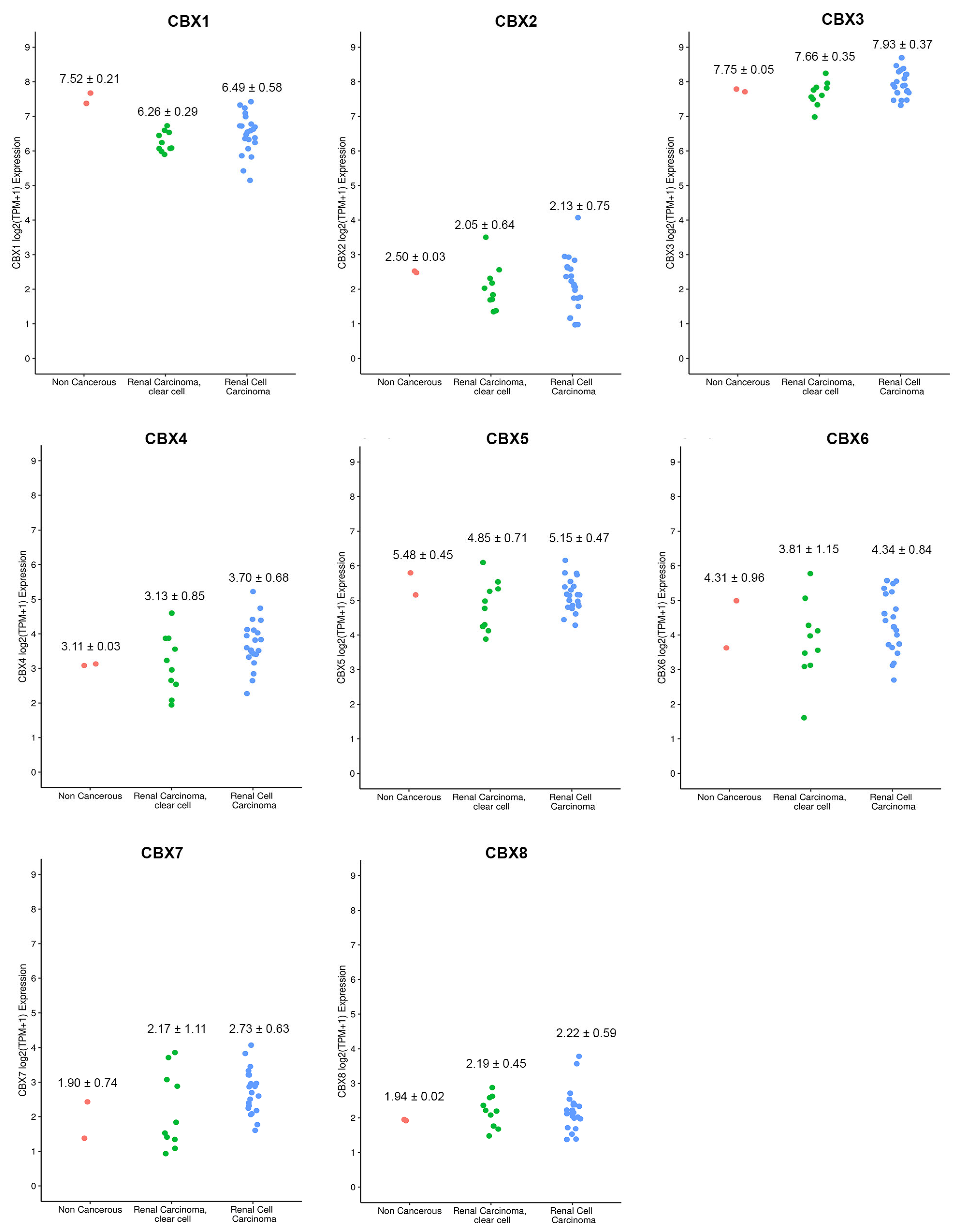
3.1. CBX Family mRNA Transcriptional Levels and Clinicopathological Parameters
3.2. Prognostic Value of CBXs Family mRNA Expression Levels in RCC Patients
3.3. Genetic Alteration and Co-Expression of CBX Family in RCC
3.4. Immune Cell Infiltration of CBXs in RCC Patients
3.5. Protein–Protein Interaction (PPI) Network and Functional Enrichment Analysis
3.6. Cell Lines Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. Jama Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Rini, B.I.; Campbell, S.C.; Escudier, B. Renal cell carcinoma. Lancet 2009, 373, 1119–1132. [Google Scholar] [CrossRef]
- Tabibu, S.; Vinod, P.K.; Jawahar, C.V. Pan-Renal Cell Carcinoma classification and survival prediction from histopathology images using deep learning. Sci. Rep. 2019, 9, 10509. [Google Scholar] [CrossRef]
- Clark, D.J.; Dhanasekaran, S.M.; Petralia, F.; Pan, J.; Song, X.; Hu, Y.; Leprevost, F.d.V.; Reva, B.; Lih, T.-S.M.; Chang, H.-Y.; et al. Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179, 964–983. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.; Pitto, M. Prognostic significance of proteomics and multi-omics studies in renal carcinoma. Expert Rev. Proteom. 2020, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Niziol, J.; Copie, V.; Tripet, B.P.; Nogueira, L.B.; Nogueira, K.O.P.C.; Ossolinski, K.; Arendowski, A.; Ruman, T. Metabolomic and elemental profiling of human tissue in kidney cancer. Metabolomics 2021, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Morgan, M.; Gunaratne, P.H.; Wheeler, D.A.; Gibbs, R.A.; Robertson, A.G.; Chu, A.; Beroukhim, R.; Cibulskis, K.; Signoretti, S.; et al. Comprehensivemolecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.-H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Osca-Verdegal, R.; Beltran-Garcia, J.; Gorriz, J.L.; Martinez Jabaloyas, J.M.; Pallardo, F.V.; Garcia-Gimenez, J.L. Use of Circular RNAs in Diagnosis, Prognosis and Therapeutics of Renal Cell Carcinoma. Front. Cell Dev. Biol. 2022, 10, 879814. [Google Scholar] [CrossRef]
- Ma, R.-g.; Zhang, Y.; Sun, T.-t.; Cheng, B. Epigenetic regulation by polycomb group complexes: Focus on roles of CBX proteins. J. Zhejiang Univ. Sci. B 2014, 15, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Wotton, D.; Merrill, J.C. Pc2 and SUMOylation. Biochem. Soc. Trans. 2007, 35, 1401–1404. [Google Scholar] [CrossRef]
- Laugesen, A.; Helin, K. Chromatin Repressive Complexes in Stem Cells, Development, and Cancer. Cell Stem Cell 2014, 14, 735–751. [Google Scholar] [CrossRef]
- Richly, H.; Aloia, L.; Di Croce, L. Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis. 2011, 2, e204. [Google Scholar] [CrossRef] [PubMed]
- van Wijnen, A.J.; Bagheri, L.; Badreldin, A.A.; Larson, A.N.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Wu, Z. Biological functions of chromobox (CBX) proteins in stem cell self-renewal, lineage-commitment, cancer and development. Bone 2021, 143, 115659. [Google Scholar] [CrossRef]
- Chan, H.L.; Morey, L. Emerging Roles for Polycomb-Group Proteins in Stem Cells and Cancer. Trends Biochem. Sci. 2019, 44, 688–700. [Google Scholar] [CrossRef]
- Pasini, D.; Di Croce, L. Emerging roles for Polycomb proteins in cancer. Curr. Opin. Genet. Dev. 2016, 36, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, N.; Di Costanzo, A.; Liu, N.Q.; Conte, L.; Dell’Aversana, C.; Bove, G.; Benedetti, R.; Montella, L.; Ciardiello, F.; Carafa, V.; et al. CBX2 shapes chromatin accessibility promoting AML via p38 MAPK signaling pathway. Mol. Cancer 2022, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-K.; Lin, H.-Y.; Chen, C.-F.; Zeng, D. Prognostic values of distinct CBX family members in breast cancer. Oncotarget 2017, 8, 92375–92387. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Spellman, P.T.; Ricketts, C.J.; Creighton, C.J.; Fei, S.S.; Davis, C.; Wheeler, D.A.; Murray, B.A.; Schmidt, L.; Vocke, C.D.; et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 135–145. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, E108–E110. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Huang, F.W.; Jane-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Joosten, S.C.; Smits, K.M.; Aarts, M.J.; Melotte, V.; Koch, A.; Tjan-Heijnen, V.C.; van Engeland, M. Epigenetics in renal cell cancer: Mechanisms and clinical applications. Nat. Rev. Urol. 2018, 15, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Rydzanicz, M.; Wrzesinski, T.; Bluyssen, H.A.R.; Wesoly, J. Genomics and epigenomics of clear cell renal cell carcinoma: Recent developments and potential applications. Cancer Lett. 2013, 341, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yan, Y.; Zhu, Z.; Liu, J.; He, X.; Dalangood, S.; Li, M.; Tan, M.; Cai, J.; Tang, P.; et al. CBX7 suppresses urinary bladder cancer progression via modulating AKR1B10-ERK signaling. Cell Death Dis. 2021, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.Q.; Tan, D.; Chen, Q.B. CASC9 Facilitates Cell Proliferation in Bladder Cancer by Regulating CBX2 Expression. Nephron 2020, 144, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Niu, G.; Pan, Y.H.; Pan, W.W.; Zhang, M.F.; Zhang, C.Z.; Shen, H.M. CBX4 transcriptionally suppresses KLF6 via interaction with HDAC1 to exert oncogenic activities in clear cell renal cell carcinoma. Ebiomedicine 2020, 53, 102692. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Pu, Z.Y.; Li, Z.F.; Lin, Y.; Li, N.; Peng, F. Comprehensive Analysis of the Expression and Prognosis Value of Chromobox Family Members in Clear Cell Renal Cell Carcinoma. Front. Oncol. 2021, 11, 700528. [Google Scholar] [CrossRef]
- Lee, Y.H.; Liu, X.Y.; Qiu, F.M.; O’Connor, T.R.; Yen, Y.; Ann, D.K. HP1 beta Is a Biomarker for Breast Cancer Prognosis and PARP Inhibitor Therapy. PLoS ONE 2015, 10, e0121207. [Google Scholar] [CrossRef]
- Li, Q.; Pan, Y.; Cao, Z.; Zhao, S. Comprehensive Analysis of Prognostic Value and Immune Infiltration of Chromobox Family Members in Colorectal Cancer. Front. Oncol. 2020, 10, 582667. [Google Scholar] [CrossRef]
- Ma, T.; Ma, N.; Chen, J.-L.; Tang, F.-X.; Zong, Z.; Yu, Z.-M.; Chen, S.; Zhou, T.-C. Expression and prognostic value of Chromobox family members in gastric cancer. J. Gastrointest. Oncol. 2020, 11, 983–998. [Google Scholar] [CrossRef]
- Yang, Y.F.; Pan, Y.H.; Tian, Q.H.; Wu, D.C.; Su, S.G. CBX1 Indicates Poor Outcomes and Exerts Oncogenic Activity in Hepatocellular Carcinoma. Transl. Oncol. 2018, 11, 1110–1118. [Google Scholar] [CrossRef]
- Zheng, S.P.; Lv, P.H.; Su, J.; Miao, K.K.; Xu, H.; Li, M.Q. Overexpression of CBX2 in breast cancer promotes tumor progression through the PI3K/AKT signaling pathway. Am. J. Transl. Res. 2019, 11, 1668–1682. [Google Scholar]
- Mao, J.K.; Tian, Y.; Wang, C.Y.; Jiang, K.Q.; Li, R.; Yao, Y.F.; Zhang, R.X.; Sun, D.G.; Liang, R.; Gao, Z.M.; et al. CBX2 Regulates Proliferation and Apoptosis via the Phosphorylation of YAP in Hepatocellular Carcinoma. J. Cancer 2019, 10, 2706–2719. [Google Scholar] [CrossRef]
- Zeng, M.; Li, B.; Yang, L.; Guan, Q. CBX2 depletion inhibits the proliferation, invasion and migration of gastric cancer cells by inactivating the YAP/beta-catenin pathway. Mol. Med. Rep. 2021, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.X.; Zhao, X.; Xia, L.; Lian, J.B.; You, J. Clinicopathological and Prognostic Significance of CBX3 Expression in Human Cancer: A Systematic Review and Meta-analysis. Dis. Markers 2020, 2020, 2412741. [Google Scholar] [CrossRef]
- Zhong, X.P.; Kan, A.; Zhang, W.C.; Zhou, J.D.; Zhang, H.Y.; Chen, J.S.; Tang, S.J. CBX3/HP1 gamma promotes tumor proliferation and predicts poor survival in hepatocellular carcinoma. Aging-Us 2019, 11, 5483–5497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Wang, F.; Yang, M.; Wang, X.Y.; Jin, C.L.; Ji, Q.K.; Li, S.; Zhao, X.L. CBX3 promotes glioma U87 cell proliferation and predicts an unfavorable prognosis. J. Neuro-Oncol. 2019, 145, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, F.F.; Zhang, D.; Ju, J.Y.; Wu, X.B.; Wang, Y.; Wang, Y.D.; Wu, Y.P.; Nie, M.; Li, Z.C.; et al. Heterochromatin Protein HP1 gamma Promotes Colorectal Cancer Progression and Is Regulated by miR-30a. Cancer Res. 2015, 75, 4593–4604. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Li, N.; Dhar, S.S.; Wu, S.J.; Lv, J.; Chen, K.F.; Flores, E.R.; Baseler, L.; Lee, M.G. HP1 gamma Promotes Lung Adenocarcinoma by Downregulating the Transcription-Repressive Regulators NCOR2 and ZBTB7A. Cancer Res. 2018, 78, 3834–3848. [Google Scholar] [CrossRef]
- Hu, K.; Yao, L.; Xu, Z.; Yan, Y.; Li, J. Prognostic Value and Therapeutic Potential of CBX Family Members in Ovarian Cancer. Front. Cell Dev. Biol. 2022, 10, 832354. [Google Scholar] [CrossRef]
- Zeng, J.-S.; Zhang, Z.-D.; Pei, L.; Bai, Z.-Z.; Yang, Y.; Yang, H.; Tian, Q.-H. CBX4 exhibits oncogenic activities in breast cancer via Notchl signaling. Int. J. Biochem. Cell Biol. 2018, 95, 1–8. [Google Scholar] [CrossRef]
- Yang, J.L.; Cheng, D.D.; Zhu, B.; Zhou, S.M.; Ying, T.; Yang, Q.C. Chromobox Homolog 4 is Positively Correlated to Tumor Growth, Survival and Activation of HIF-1 alpha Signaling in Human Osteosarcoma under Normoxic Condition. J. Cancer 2016, 7, 427–435. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Long, X.D.; Wang, W.; Jiao, H.K.; Mei, Z.; Yin, Q.Q.; Ma, L.N.; Zhou, A.W.; Wang, L.S.; et al. Cbx4 Governs HIF-1 alpha to Potentiate Angiogenesis of Hepatocellular Carcinoma by Its SUMO E3 Ligase Activity. Cancer Cell 2014, 25, 118–131. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Q.; Tang, Q.; Zhou, H.; Liu, W.; Huang, J.; Liu, Y.; Wang, Q.; Zhang, J.; Zhou, M.; et al. CBX4 promotes the proliferation and metastasis via regulating BMI-1 in lung cancer. J. Cell. Mol. Med. 2020, 24, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Chiou, G.Y.; Huang, P.I.; Lo, W.L.; Wang, C.Y.; Lu, K.H.; Yu, C.C.; Alterovitz, G.; Huang, W.C.; Lo, J.F.; et al. Network Biology of Tumor Stem-like Cells Identified a Regulatory Role of CBX5 in Lung Cancer. Sci. Rep. 2012, 2, 584. [Google Scholar] [CrossRef]
- Vad-Nielsen, J.; Nielsen, A.L. Beyond the histone tale: HP1 alpha deregulation in breast cancer epigenetics. Cancer Biol. Ther. 2015, 16, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.L.; Guan, X.W.; Gong, L.C.; Zeng, J.M.; Zhang, H.J.; Chen, M.Y.; Li, G. CBX6 is negatively regulated by EZH2 and plays a potential tumor suppressor role in breast cancer. Sci. Rep. 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jiang, W.-h.; Tian, T.; Tan, H.-s.; Chen, Y.; Qiao, G.-l.; Han, J.; Huang, S.-y.; Yang, Y.; Li, S.; et al. CBX6 overexpression contributes to tumor progression and is predictive of a poor prognosis in hepatocellular carcinoma. Oncotarget 2017, 8, 18872–18884. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.H.; Won, H.Y.; Lee, J.Y.; Kong, G. CBX7 inhibits breast tumorigenicity through DKK-1-mediated suppression of the Wnt/beta-catenin pathway. Faseb J. 2015, 29, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Forzati, F.; Federico, A.; Pallante, P.; Fedele, M.; Fusco, A. Tumor suppressor activity of CBX7 in lung carcinogenesis. Cell Cycle 2012, 11, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Pallante, P.; Terracciano, L.; Carafa, V.; Schneider, S.; Zlobec, I.; Lugli, A.; Bianco, M.; Ferraro, A.; Sacchetti, S.; Troncone, G.; et al. The loss of the CBX7 gene expression represents an adverse prognostic marker for survival of colon carcinoma patients. Eur. J. Cancer 2010, 46, 2304–2313. [Google Scholar] [CrossRef]
- Pallante, P.; Federico, A.; Berlingieri, M.T.; Bianco, M.; Ferraro, A.; Forzati, F.; Iaccarino, A.; Russo, M.; Pierantoni, G.M.; Leone, V.; et al. Loss of the CBX7 gene expression correlates with a highly malignant phenotype in thyroid cancer. Cancer Res. 2008, 68, 6770–6778. [Google Scholar] [CrossRef]
- Scott, C.L.; Gil, J.; Hernando, E.; Teruya-Feldstein, J.; Narita, M.; Martinez, D.; Visakorpi, T.; Mu, D.; Cordon-Cardo, C.; Peters, G.; et al. Role of the chromobox protein CBX7 in lymphomagenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 5389–5394. [Google Scholar] [CrossRef]
- Bernard, D.; Martinez-Leal, J.F.; Rizzo, S.; Martinez, D.; Hudson, D.; Visakorpi, T.; Peters, G.; Carnero, A.; Beach, D.; Gil, J. CBX7 controls the growth of normal and tumor-derived prostate cells by repressing the Ink4a/Arf locus. Oncogene 2005, 24, 5543–5551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.L.; Huang, J.H.; Yin, A.J.; Zhang, H. MicroRNA-18a suppresses ovarian carcinoma progression by targeting CBX7 and regulating ERK/MAPK signaling pathway and epithelial-to-mesenchymal transition. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5292–5302. [Google Scholar]
- Shinjo, K.; Yamashita, Y.; Yamamoto, E.; Akatsuka, S.; Uno, N.; Kamiya, A.; Niimi, K.; Sakaguchi, Y.; Nagasaka, T.; Takahashi, T.; et al. Expression of chromobox homolog 7 ( CBX7) is associated with poor prognosis in ovarian clear cell adenocarcinoma via TRAIL-induced apoptotic pathway regulation. Int. J. Cancer 2014, 135, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.C.; Luo, L.M.; Li, D.Y.; Guo, J.C.; Guo, M. KPNA2 interaction with CBX8 contributes to the development and progression of bladder cancer by mediating the PRDM1/c-FOS pathway. J. Transl. Med. 2021, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.J.; Chen, X.; Lu, J.; Feng, Z.H.; Chen, S.L.; Chen, R.X.; Wei, W.S.; Zhou, F.J.; Xie, D. Chromobox homolog 8 is a predictor of muscle invasive bladder cancer and promotes cell proliferation by repressing the p53 pathway. Cancer Sci. 2017, 108, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, G.; Zhang, M.; Li, F.-y.; Sang, Y.; Wang, B.; Hu, K.; Wu, Y.; Luo, R.; Liao, D.; et al. Paradoxical role of CBX8 in proliferation and metastasis of colorectal cancer. Oncotarget 2014, 5, 10778–10790. [Google Scholar] [CrossRef]
- Wang, G.; Tang, J.J.; Zhan, W.X.; Zhang, R.H.; Zhang, M.F.; Liao, D.; Wang, X.; Wu, Y.Z.; Kang, T.B. CBX8 Suppresses Tumor Metastasis via Repressing Snail in Esophageal Squamous Cell Carcinoma. Theranostics 2017, 7, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.J.; Wang, H.W.; Zhu, X.L.; Wan, C.H.; Xu, J.F.; Lu, C.; Xiao, L.; He, J.Q.; Jiang, C.Y.; Wang, W.; et al. CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer. Oncotarget 2017, 8, 8010–8021. [Google Scholar] [CrossRef]
- Ni, S.J.; Zhao, L.Q.; Wang, X.F.; Wu, Z.H.; Hua, R.X.; Wan, C.H.; Zhang, J.Y.; Zhang, X.W.; Huang, M.Z.; Gan, L.; et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-kappa B-miR-21 pathways. J. Hematol. Oncol. 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Feng, H.H.; Yang, J.J.; Yin, A.P.; Qian, A.R.; Sugiyama, H. Landscape of immune cell infiltration in clear cell renal cell carcinoma to aid immunotherapy. Cancer Sci. 2021, 112, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Yin, C.; Geng, L.L.; Cai, W.Y. Immune Infiltration Landscape in Clear Cell Renal Cell Carcinoma Implications. Front. Oncol. 2021, 10, 491621. [Google Scholar] [CrossRef]
- Zhang, S.C.; Zhang, E.D.; Long, J.H.; Hu, Z.Q.; Peng, J.; Liu, L.N.; Tang, F.Z.; Li, L.; Ouyang, Y.; Zeng, Z. Immune infiltration in renal cell carcinoma. Cancer Sci. 2019, 110, 1564–1572. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Cao, Y.Y.; Yuan, M.F.; Gao, Z.F.; Guo, X.M.; Zhu, F.H.; Wang, Y.F.; Xu, J. Polycomb chromobox (Cbx) 7 modulates activation-induced CD4(+) T cell apoptosis. Arch. Biochem. Biophys. 2014, 564, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.H.; Cao, X.T.; Wang, C.M. Polycomb chromobox Cbx2 enhances antiviral innate immunity by promoting Jmjd3-mediated demethylation of H3K27 at the Ifnb promoter. Protein Cell 2019, 10, 285–294. [Google Scholar] [CrossRef] [PubMed]


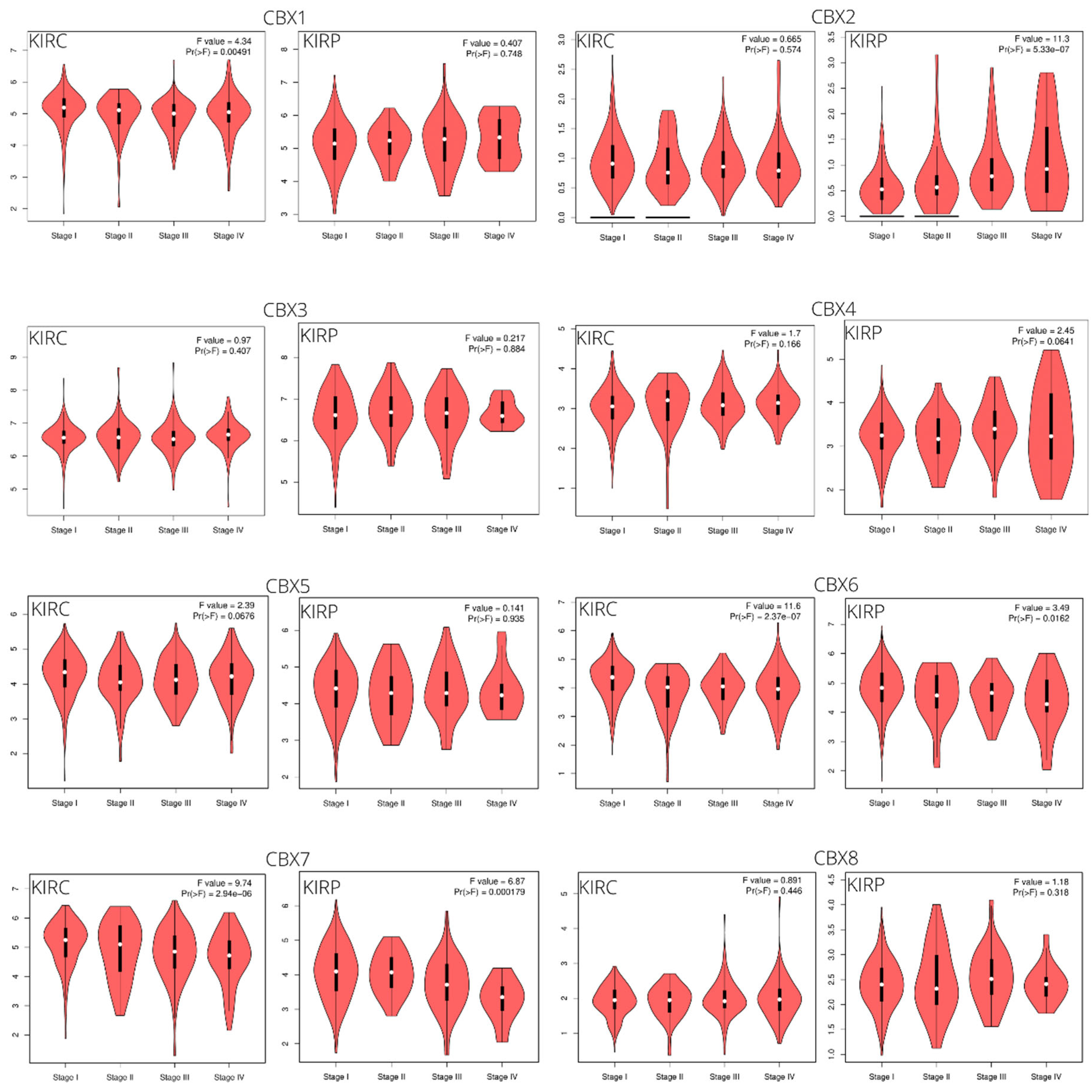

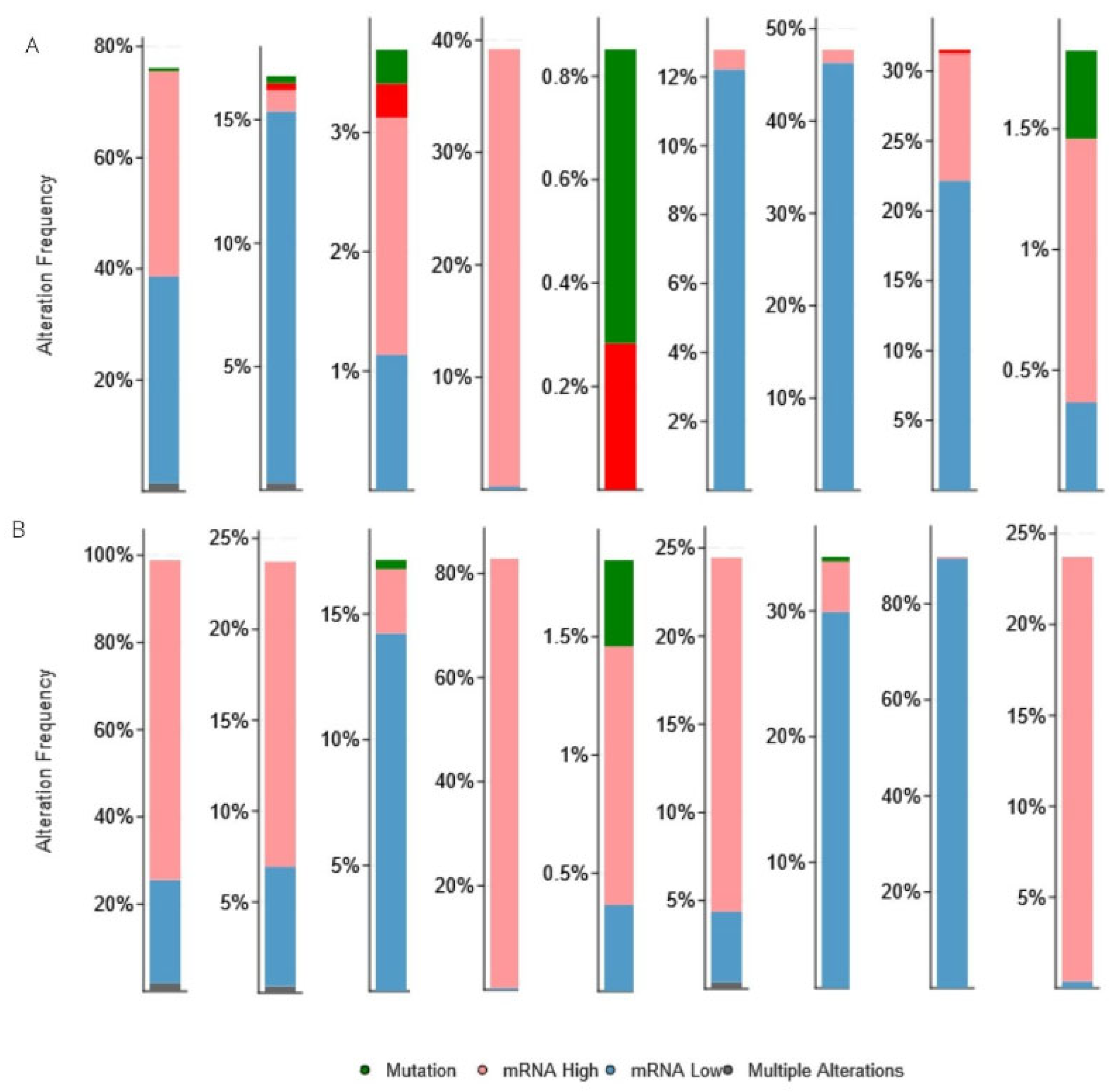
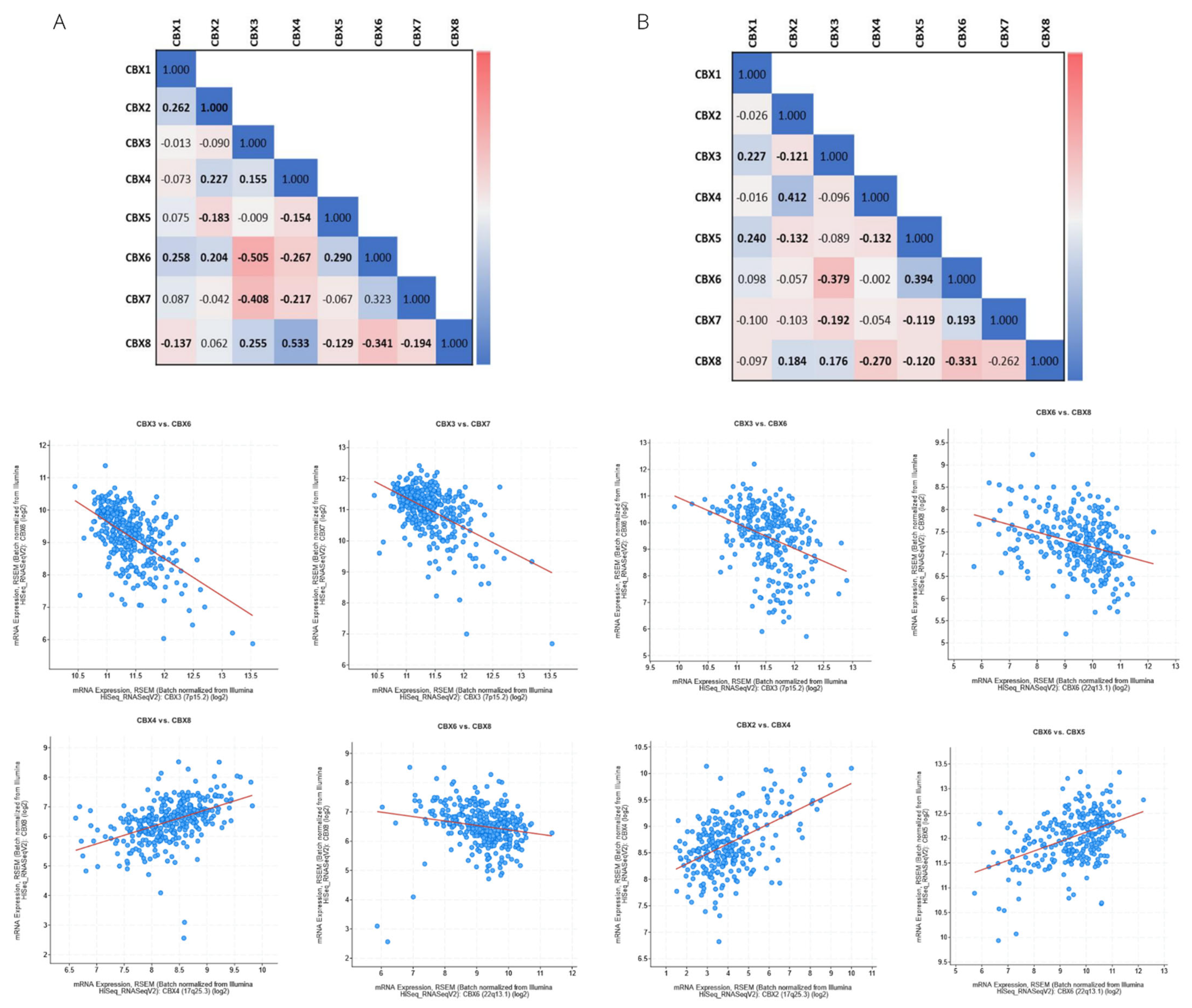
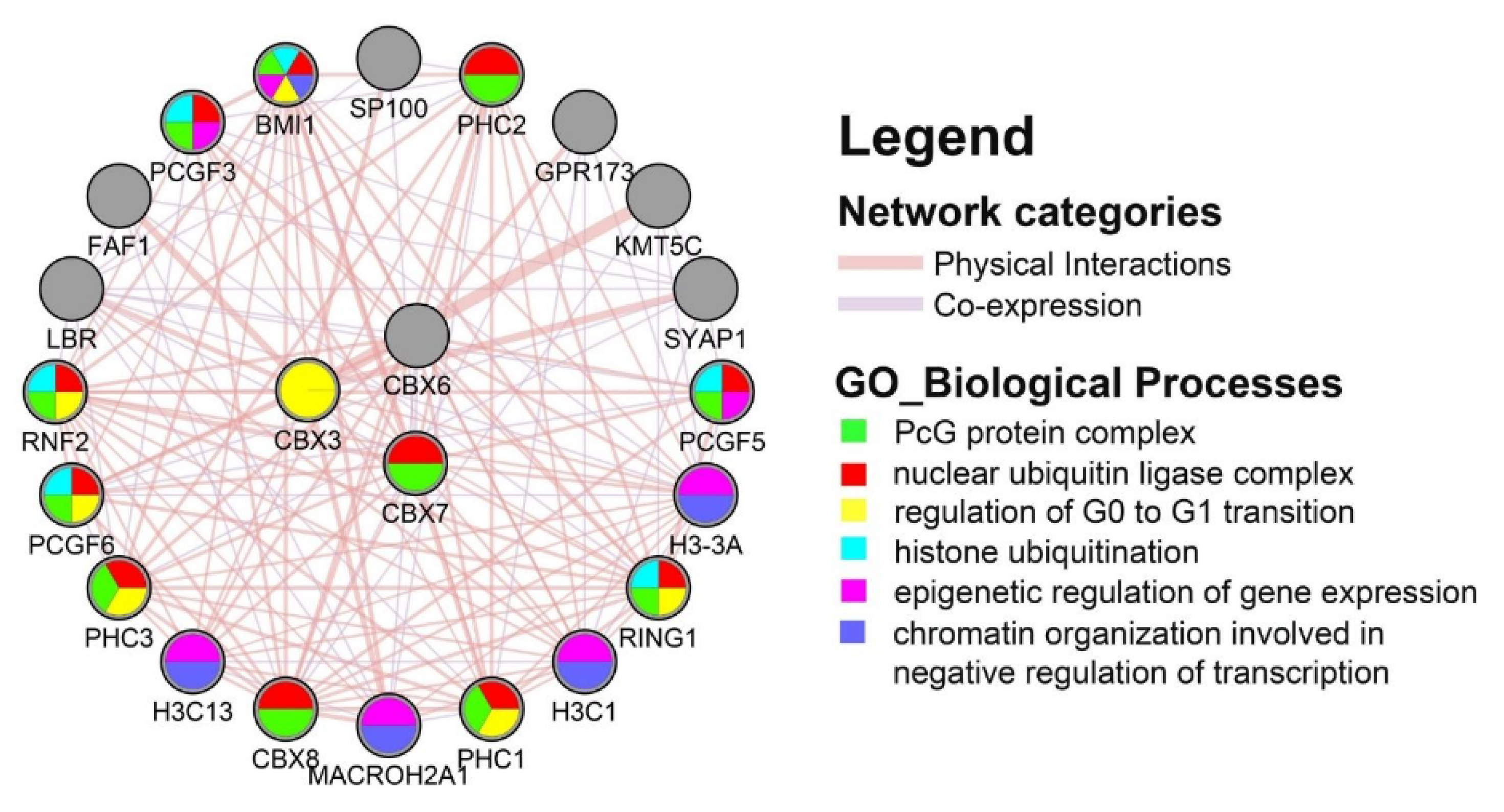
| Clinical Features | Category | KIRC, n = 533 | KIRP, n = 290 |
|---|---|---|---|
| Age | Median (range) | 61 (26–90) | 61 (28–88) |
| Gender | Female | 188 | 76 |
| Male | 345 | 214 | |
| Tumor Stage | I | 267 | 140 |
| II | 57 | 21 | |
| III | 123 | 29 | |
| IV | 84 | 11 | |
| Not Available | 2 | 89 | |
| T stage | T1 | 273 | 193 |
| T2 | 69 | 33 | |
| T3 | 180 | 60 | |
| T4 | 11 | 2 | |
| TX | 0 | 2 | |
| Not Available | 0 | 0 | |
| M stage | M0 | 422 | 95 |
| M1 | 79 | 9 | |
| MX | 30 | 171 | |
| Not Available | 2 | 15 | |
| N stage | N0 | 240 | 50 |
| N1 | 16 | 24 | |
| N2 | 0 | 4 | |
| NX | 277 | 211 | |
| Not Available | 0 | 1 | |
| Vital status | Dead | 175 | 44 |
| Alive | 358 | 246 | |
| Laterality | Bilateral | 1 | 2 |
| Left | 251 | 160 | |
| Right | 281 | 127 | |
| Not Available | 0 | 1 |
| CBX1 | CBX2 | CBX3 | CBX4 | CBX5 | CBX6 | CBX7 | CBX8 | |||||||||
| ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | ccRCC n = 533 | pRCC n = 290 | |
| B cell | −0.013 | 0.023 | −0.210 | 0.241 | −0.149 | −0.030 | 0.002 | 0.211 | −0.040 | 0.066 | −0.101 | 0.044 | 0.103 | 0.141 | 0.095 | −0.032 |
| CD4+ | 0.255 | 0.080 | 0.073 | 0.059 | 0.024 | 0.024 | 0.252 | 0.158 | 0.252 | 0.067 | 0.318 | −0.010 | 0.192 | 0.226 | 0.166 | 0.083 |
| CD8+ | 0.067 | 0.152 | 0.112 | 0.236 | 0.048 | 0.038 | 0.099 | 0.256 | −0.090 | 0.061 | −0.055 | 0.126 | 0.127 | 0.145 | −0.116 | −0.112 |
| Macrophage | 0.344 | 0.011 | −0.069 | −0.083 | 0.061 | −0.014 | 0.022 | 0.091 | 0.387 | 0.095 | 0.327 | 0.188 | 0.048 | 0.168 | −0.049 | −0.105 |
| Neutrophil | 0.295 | 0.163 | 0.213 | 0.198 | 0.328 | 0.156 | 0.288 | 0.327 | 0.364 | 0.116 | 0.295 | 0.145 | 0.181 | 0.265 | 0.028 | −0.037 |
| Dendritic cell | 0.069 | 0.208 | 0.053 | 0.184 | 0.112 | 0.063 | 0.243 | 0.328 | 0.220 | 0.162 | 0.125 | 0.220 | 0.042 | 0.309 | 0.055 | −0.134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimaldi, A.M.; Affinito, O.; Salvatore, M.; Franzese, M. CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis. Diagnostics 2022, 12, 2452. https://doi.org/10.3390/diagnostics12102452
Grimaldi AM, Affinito O, Salvatore M, Franzese M. CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis. Diagnostics. 2022; 12(10):2452. https://doi.org/10.3390/diagnostics12102452
Chicago/Turabian StyleGrimaldi, Anna Maria, Ornella Affinito, Marco Salvatore, and Monica Franzese. 2022. "CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis" Diagnostics 12, no. 10: 2452. https://doi.org/10.3390/diagnostics12102452
APA StyleGrimaldi, A. M., Affinito, O., Salvatore, M., & Franzese, M. (2022). CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis. Diagnostics, 12(10), 2452. https://doi.org/10.3390/diagnostics12102452







