SARS-CoV-2 IgG Levels Allow Predicting the Optimal Time Span of Convalescent Plasma Donor Suitability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Plasma Donor Selection
2.3. Plasma Collection
2.4. Anti-SARS-CoV-2 Serological Screening
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
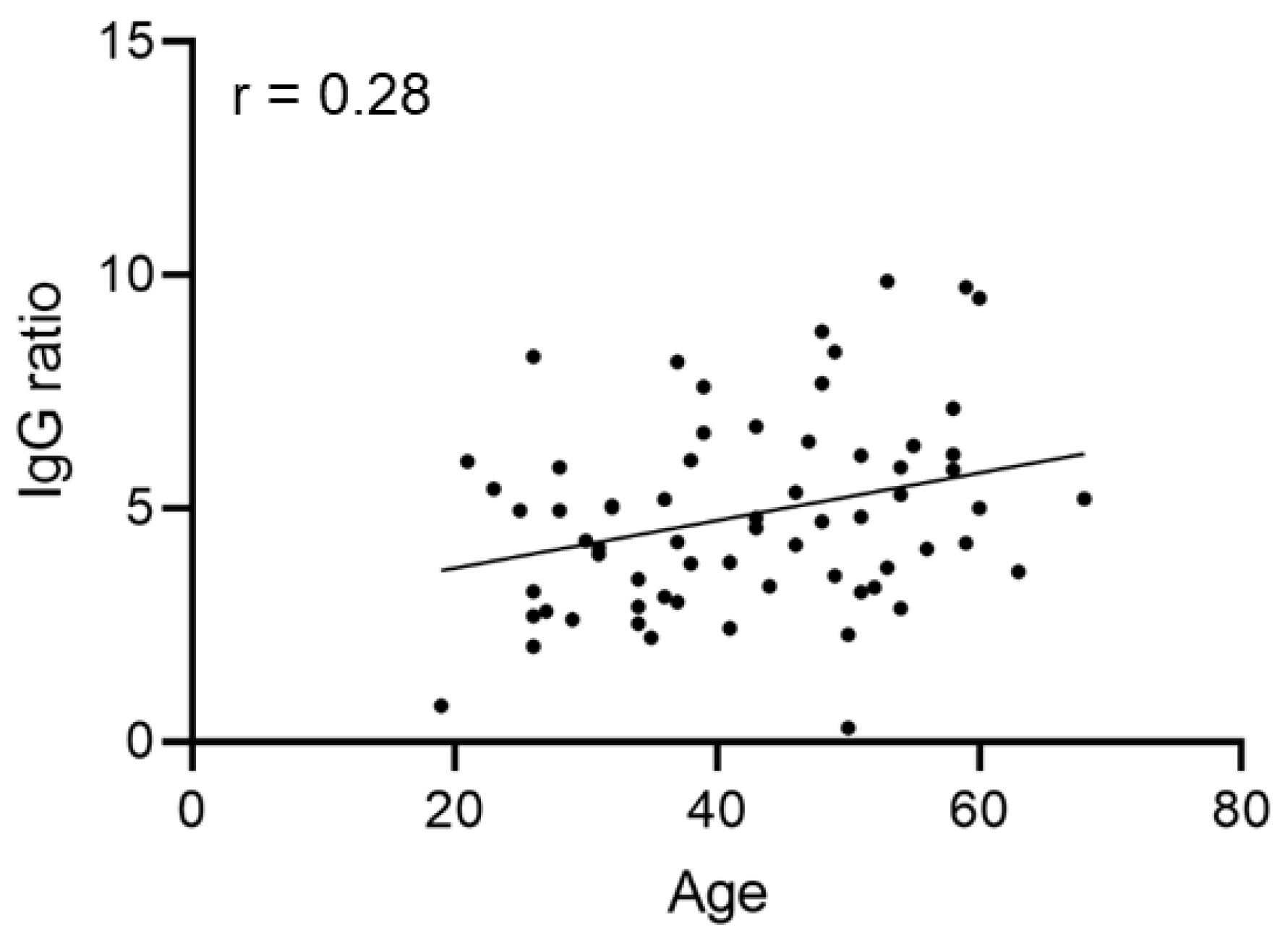
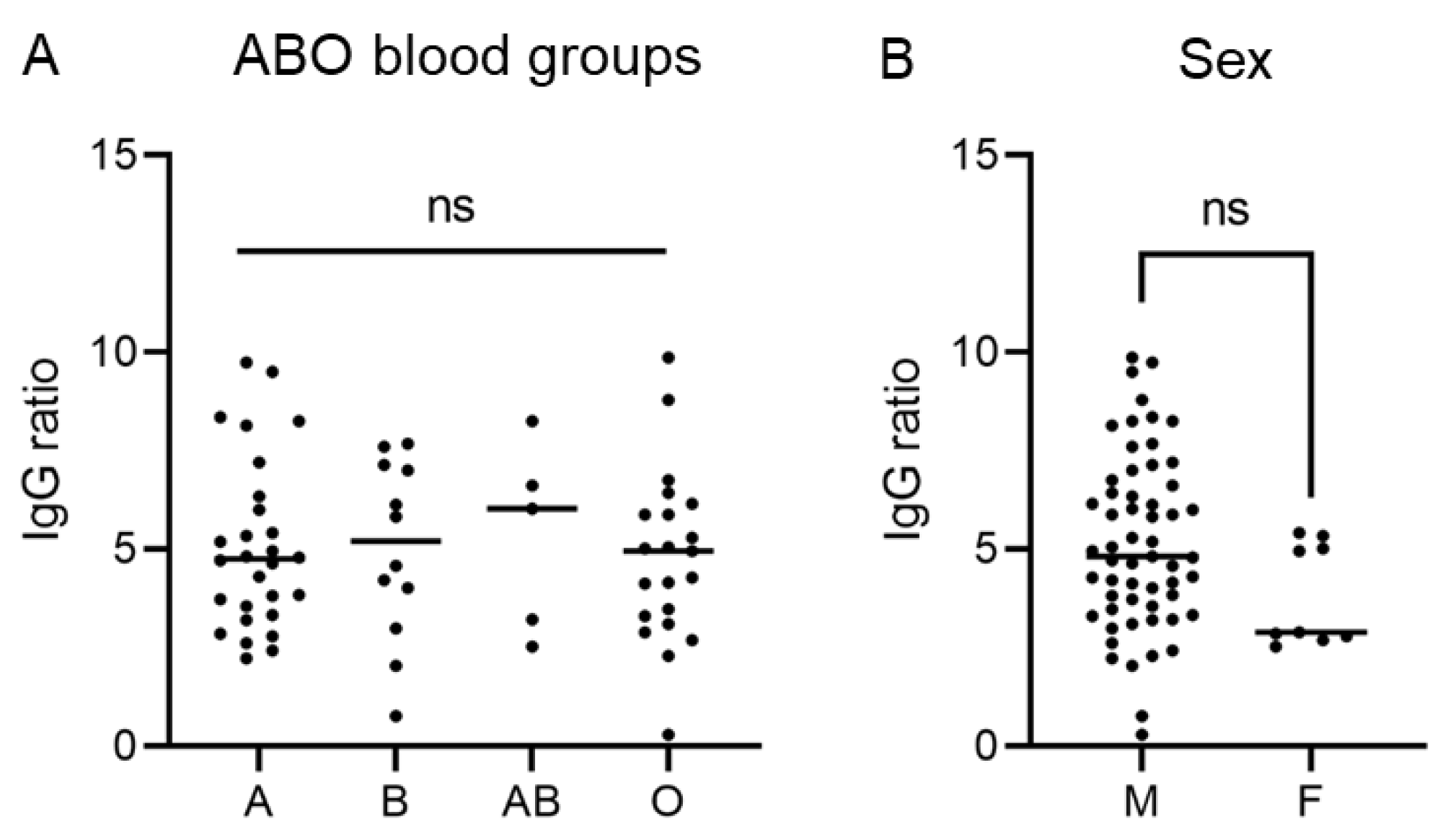
3.2. SARS-CoV-2 Anti-S Antibody Levels Are Independent of Age, Sex, ABO Blood Group and Disease Course
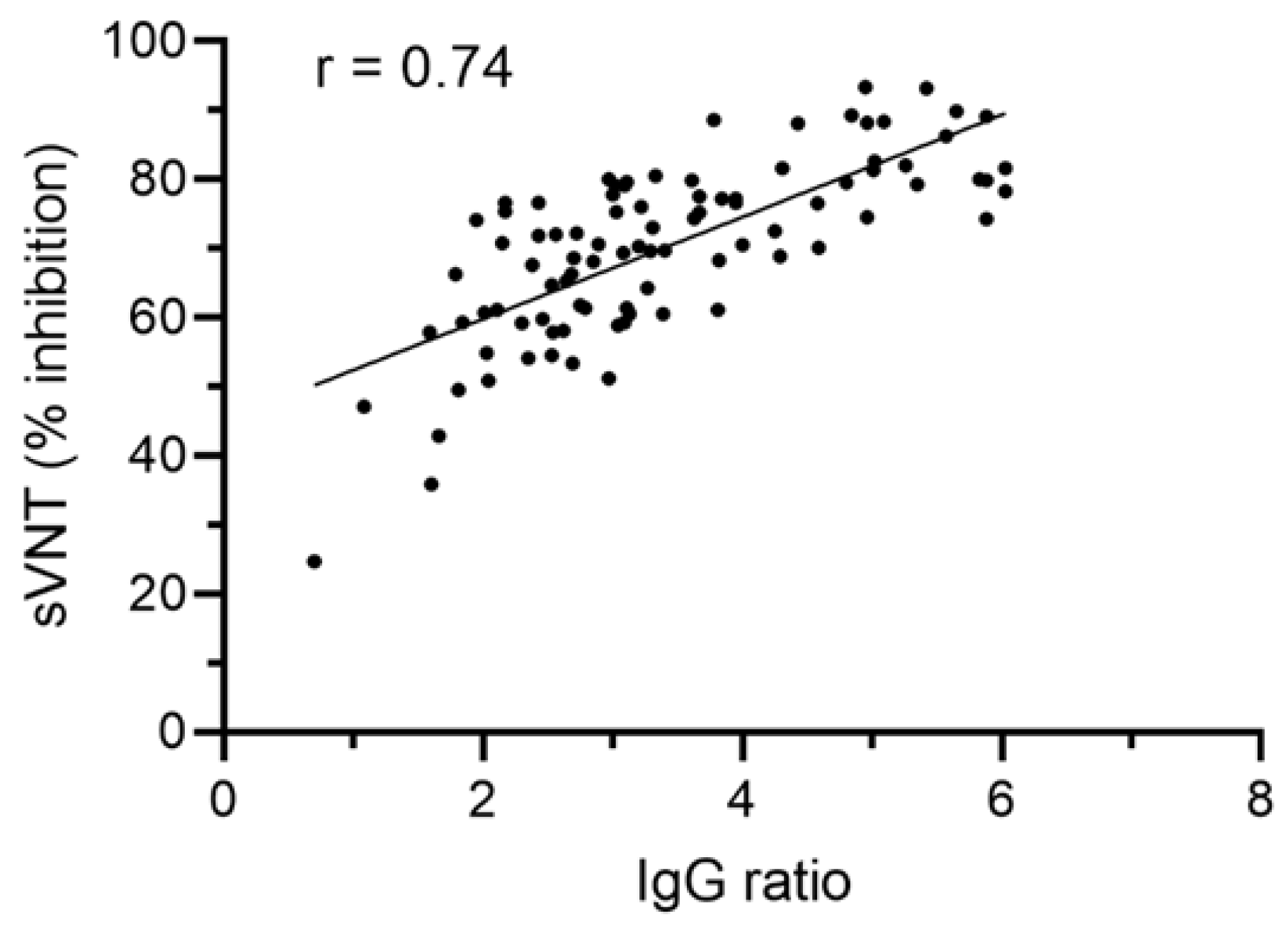
3.3. SARS-CoV-2 Anti-S IgG Ratio Values Correlate with Antibody Functionality
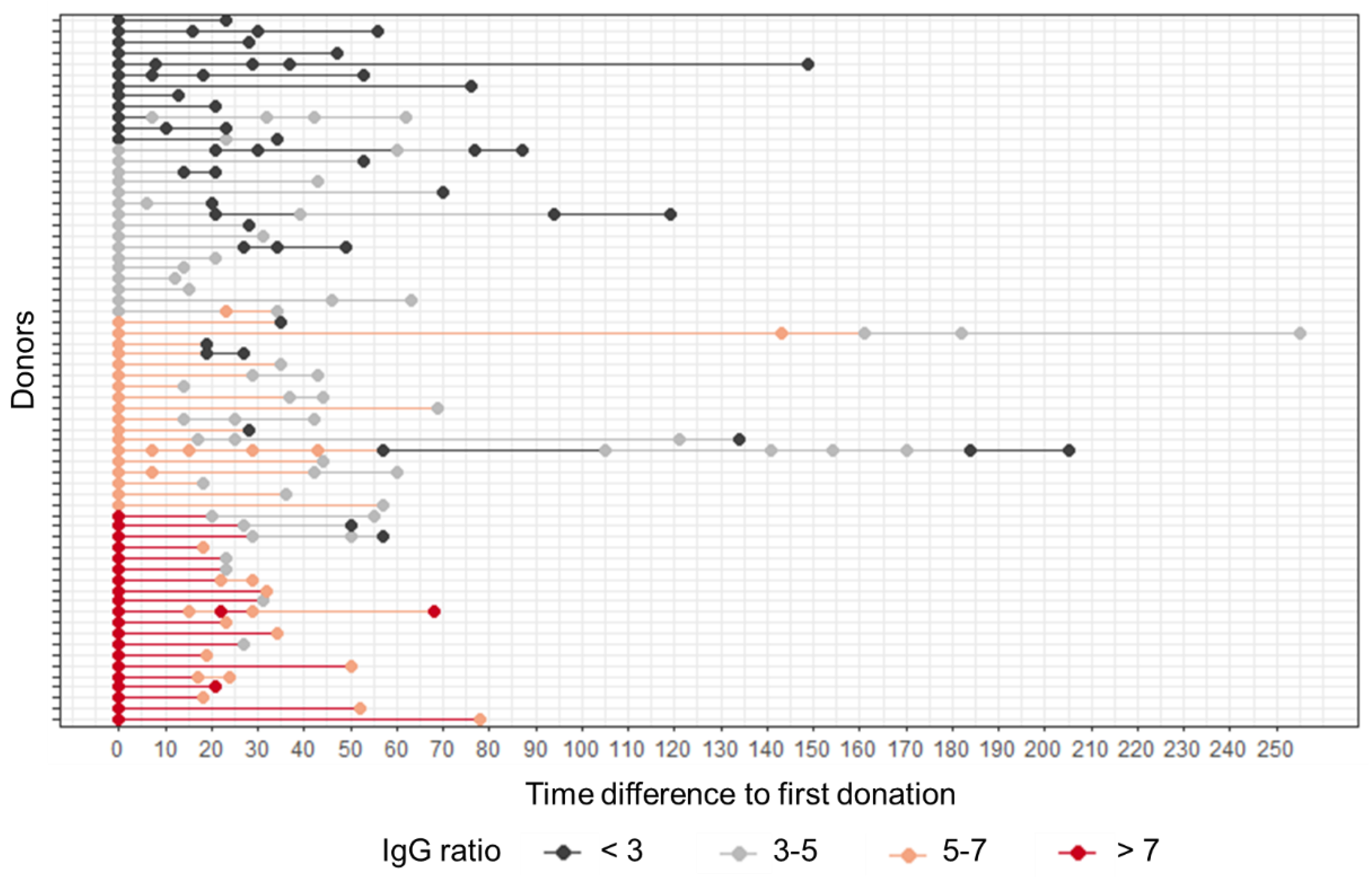
3.4. The Level of SARS-CoV-2 Anti-S Antibody Allows Determining the Time Span of CP Donor Suitability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benda, A.; Zerajic, L.; Ankita, A.; Cleary, E.; Park, Y.; Pandey, S. COVID-19 Testing and Diagnostics: A Review of Commercialized Technologies for Cost, Convenience and Quality of Tests. Sensors 2021, 21, 6581. [Google Scholar] [CrossRef] [PubMed]
- Filchakova, O.; Dossym, D.; Ilyas, A.; Kuanysheva, T.; Abdizhamil, A.; Bukasov, R. Review of COVID-19 Testing and Diagnostic Methods. Talanta 2022, 244, 123409. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y.; Pirracchio, R.; Rochwerg, B. Corticosteroids for Treating Sepsis in Children and Adults. Emergencias 2021, 33, 137–138. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Sasaki, K.; Karkar, A.; Sharif, S.; Lewis, K.; Mammen, M.J.; Alexander, P.; Ye, Z.; Lozano, L.E.C.; Munch, M.W.; et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: A Systematic Review and Meta-Analysis. Intensive Care Med. 2021, 47, 521–537. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and Safety of Three New Oral Antiviral Treatment (Molnupiravir, Fluvoxamine and Paxlovid) For COVID-19 A Meta-Analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin. Infect. Dis. 2022, 2022, ciac443. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An Oral SARS-CoV-2 M(pro) Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Alshanqeeti, S.; Bhargava, A. COVID-19 Rebound After Paxlovid Treatment: A Case Series and Review of Literature. Cureus 2022, 14, e26239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Strymish, J.; Stack, G.; Charness, M. Rapid Relapse of Symptomatic SARS-CoV-2 Infection Following Early Suppression with Nirmatrelvir/Ritonavir. Res. Sq. 2022; preprint. [Google Scholar]
- Heilmann, E.; Costacurta, F.; Volland, A.; von Laer, D. SARS-CoV-2 3CLpro Mutations Confer Resistance to Paxlovid (nirmatrelvir/ritonavir) in a VSV-based, Non-Gain-of-Function System. bioRxiv 2022. [Google Scholar]
- Service, R.F. Bad News for Paxlovid? Resistance May be Coming. Science 2022, 377, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Barbeta, E.; Millan, A.; Rello, J. Convalescent Plasma for SARS-CoV-2 Infection: Win or Learn. Eur. Respir. J. 2022, 59, 2102076. [Google Scholar] [CrossRef]
- Franchini, M. Convalescent Plasma Therapy for Managing Infectious Diseases: A Narrative Review. Ann. Blood 2021, 6, 17. [Google Scholar] [CrossRef]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent Plasma Antibody Levels and the Risk of Death from COVID-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef]
- Libster, R.; Perez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe COVID-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Ye, M.; Fu, D.; Ren, Y.; Wang, F.; Wang, D.; Zhang, F.; Xia, X.; Lv, T. Treatment with Convalescent Plasma for COVID-19 Patients in Wuhan, China. J. Med. Virol. 2020, 92, 1890–1901. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vazquez, C.; Savoy, N.; Giunta, D.H.; Perez, L.G.; Sanchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef]
- Klassen, S.A.; Senefeld, J.; Johnson, P.W.; Carter, R.E.; Wiggins, C.C.; Shoham, S.; Grossman, B.J.; Henderson, J.P.; Musser, J.M.; Salazar, E.; et al. The Effect of Convalescent Plasma Therapy on COVID-19 Patient Mortality: Systematic Review and Meta-Analysis. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.A.; Senefeld, J.W.; Senese, K.A.; Johnson, P.W.; Wiggins, C.C.; Baker, S.E.; van Helmond, N.; Bruno, K.A.; Pirofski, L.A.; Shoham, S.; et al. Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence. Front. Med. 2021, 8, 684151. [Google Scholar] [CrossRef] [PubMed]
- AABB Press. Apheresis: Principles and Practice, 3rd ed.; American Association of Blood Banks: Bethesda, MD, USA, 2010. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. Guide to the Preparation, Use and Quality Assurance of Blood Components; European Committee: Brussels, Belgium, 2017. [Google Scholar]
- Hoepler, W.P.; Weidner, L.; Traugott, M.T.; Neuhold, S.; Meyer, E.L.; Zoufaly, A.; Seitz, T.; Kitzberger, R.; Baumgartner, S.; Pawelka, E.; et al. Adjunctive Treatment with High-Titre Convalescent Plasma in Severely and Critically Ill COVID-19 Patients—A Safe but Futile Intervention. A Comparative Cohort Study. Infect. Dis. 2021, 53, 820–829. [Google Scholar] [CrossRef]
- R-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org- (accessed on 26 September 2022).
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.2-13. 2021. Available online: https://CRAN.R-project.org/package=survival (accessed on 26 September 2022).
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 26 September 2022).
- Chen, B.; Xia, R. Early Experience with Convalescent Plasma as Immunotherapy for COVID-19 in China: Knowns and Unknowns. Vox Sang. 2020, 115, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, R.; Wang, J.; Lv, Q.; Ren, M.; Zhao, L.; Chen, H.; Xu, H.; Xie, S.; Xie, J.; et al. Feasibility of a Pilot Program for COVID-19 Convalescent Plasma Collection in Wuhan, China. Transfusion 2020, 60, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.L.; Yu, Z.J.; Gou, J.J.; Li, G.M.; Ma, S.H.; Zhang, G.F.; Xu, J.H.; Lin, W.B.; Cui, G.L.; Zhang, M.M.; et al. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in Patients with Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 38–43. [Google Scholar] [CrossRef]
- Jorda, A.; Kussmann, M.; Kolenchery, N.; Siller-Matula, J.M.; Zeitlinger, M.; Jilma, B.; Gelbenegger, G. Convalescent Plasma Treatment in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 817829. [Google Scholar] [CrossRef]
- Thompson, M.A.; Henderson, J.P.; Shah, P.K.; Rubinstein, S.M.; Joyner, M.J.; Choueiri, T.K.; Flora, D.B.; Griffiths, E.A.; Gulati, A.P.; Hwang, C.; et al. Association of Convalescent Plasma Therapy with Survival in Patients with Hematologic Cancers and COVID-19. JAMA Oncol. 2021, 7, 1167–1175. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M.; Pirofski, L.A.; Burnouf, T.; Paneth, N.; Joyner, M.J.; Casadevall, A. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin. Microbiol. Rev. 2022, 35, e0020021. [Google Scholar] [CrossRef]
- Vaselli, N.M.; Hungerford, D.; Shenton, B.; Khashkhusha, A.; Cunliffe, N.A.; French, N. The Seroprevalence of SARS-CoV-2 during the First Wave in Europe 2020: A Systematic Review. PLoS ONE 2021, 16, e0250541. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, J.H.; Hsueh, P.R. Population-based Seroprevalence Surveys of Anti-SARS-CoV-2 Antibody: An Up-to-Date Review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Liang, B.; Fang, Y.; Lu, S.; Li, S.; Wang, H.; Li, H.; Yang, X.; Shen, S.; Zhu, B.; et al. Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset. Front. Immunol. 2021, 12, 708523. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.B.; Yavuzer, S.; Islamoglu, M.S.; Cengiz, M. Measurement of Antibody Levels in Patients with COVID-19 over Time by Immunofluorescence Assay: A Longitudinal Observational Study. J. Int. Med. Res. 2022, 50, 3000605211069279. [Google Scholar] [CrossRef]
- Nunhofer, V.; Weidner, L.; Hoeggerl, A.D.; Zimmermann, G.; Badstuber, N.; Grabmer, C.; Jungbauer, C.; Lindlbauer, N.; Held, N.; Pascariuc, M.; et al. Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection. Viruses 2022, 14, 637. [Google Scholar] [CrossRef]
- Weidner, L.; Nunhofer, V.; Jungbauer, C.; Hoeggerl, A.D.; Gruner, L.; Grabmer, C.; Zimmermann, G.; Rohde, E.; Laner-Plamberger, S. Seroprevalence of Anti-SARS-CoV-2 Total Antibody is Higher in Younger Austrian Blood Donors. Infection 2021, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Ziberna, K.; Jez, M.; Jazbec, K.; Mali, P.; Potokar, U.R.; Rozman, P. ABO Blood Group Does Not Influence the Level of Anti-SARS-CoV-2 Antibodies in Convalescent Plasma Donors. Transfusion 2022, 62, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of Age with SARS-CoV-2 Antibody Response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef]
- Klein, S.L.; Pekosz, A.; Park, H.S.; Ursin, R.L.; Shapiro, J.R.; Benner, S.E.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.J.; et al. Sex, Age, and Hospitalization Drive Antibody Responses in a COVID-19 Convalescent Plasma Donor Population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease Severity Dictates SARS-CoV-2-Specific Neutralizing Antibody Responses in COVID-19. Signal Transduct. Target. Ther. 2020, 5, 180. [Google Scholar] [CrossRef]
- Roltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, abe0240. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate. An EU Programme of COVID-19 Convalescent Plasma Collection and Transfusion, Guidance on collection, Testing, Processing, Storage, Distribution and Monitored Use; European Commission: Brussels, Belgium, 2021. [Google Scholar]


| Total Number of Participating Donors/Donations | 66/189 |
| Sex | |
| male | 57 |
| female | 9 |
| Age | |
| 19–25 years | 4 |
| 26–35 years | 17 |
| 36–45 years | 15 |
| 46–55 years | 20 |
| 56+ years | 10 |
| Median age: 43 years | |
| ABO blood group | |
| A | 28 |
| B | 12 |
| AB | 5 |
| 0 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laner-Plamberger, S.; Lindlbauer, N.; Weidner, L.; Gänsdorfer, S.; Weseslindtner, L.; Held, N.; Lauth, W.; Zimmermann, G.; Kern, J.M.; Föttinger, F.; et al. SARS-CoV-2 IgG Levels Allow Predicting the Optimal Time Span of Convalescent Plasma Donor Suitability. Diagnostics 2022, 12, 2567. https://doi.org/10.3390/diagnostics12112567
Laner-Plamberger S, Lindlbauer N, Weidner L, Gänsdorfer S, Weseslindtner L, Held N, Lauth W, Zimmermann G, Kern JM, Föttinger F, et al. SARS-CoV-2 IgG Levels Allow Predicting the Optimal Time Span of Convalescent Plasma Donor Suitability. Diagnostics. 2022; 12(11):2567. https://doi.org/10.3390/diagnostics12112567
Chicago/Turabian StyleLaner-Plamberger, Sandra, Nadja Lindlbauer, Lisa Weidner, Simon Gänsdorfer, Lukas Weseslindtner, Nina Held, Wanda Lauth, Georg Zimmermann, Jan Marco Kern, Fabian Föttinger, and et al. 2022. "SARS-CoV-2 IgG Levels Allow Predicting the Optimal Time Span of Convalescent Plasma Donor Suitability" Diagnostics 12, no. 11: 2567. https://doi.org/10.3390/diagnostics12112567
APA StyleLaner-Plamberger, S., Lindlbauer, N., Weidner, L., Gänsdorfer, S., Weseslindtner, L., Held, N., Lauth, W., Zimmermann, G., Kern, J. M., Föttinger, F., Ombres, L., Jungbauer, C., Rohde, E., & Grabmer, C. (2022). SARS-CoV-2 IgG Levels Allow Predicting the Optimal Time Span of Convalescent Plasma Donor Suitability. Diagnostics, 12(11), 2567. https://doi.org/10.3390/diagnostics12112567







