The Relationship between Visceral Adiposity and Nonalcoholic Fatty Liver Disease Diagnosed by Controlled Attenuation Parameter in People with HIV: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical and Biological Parameters
2.3. Statistical Analysis
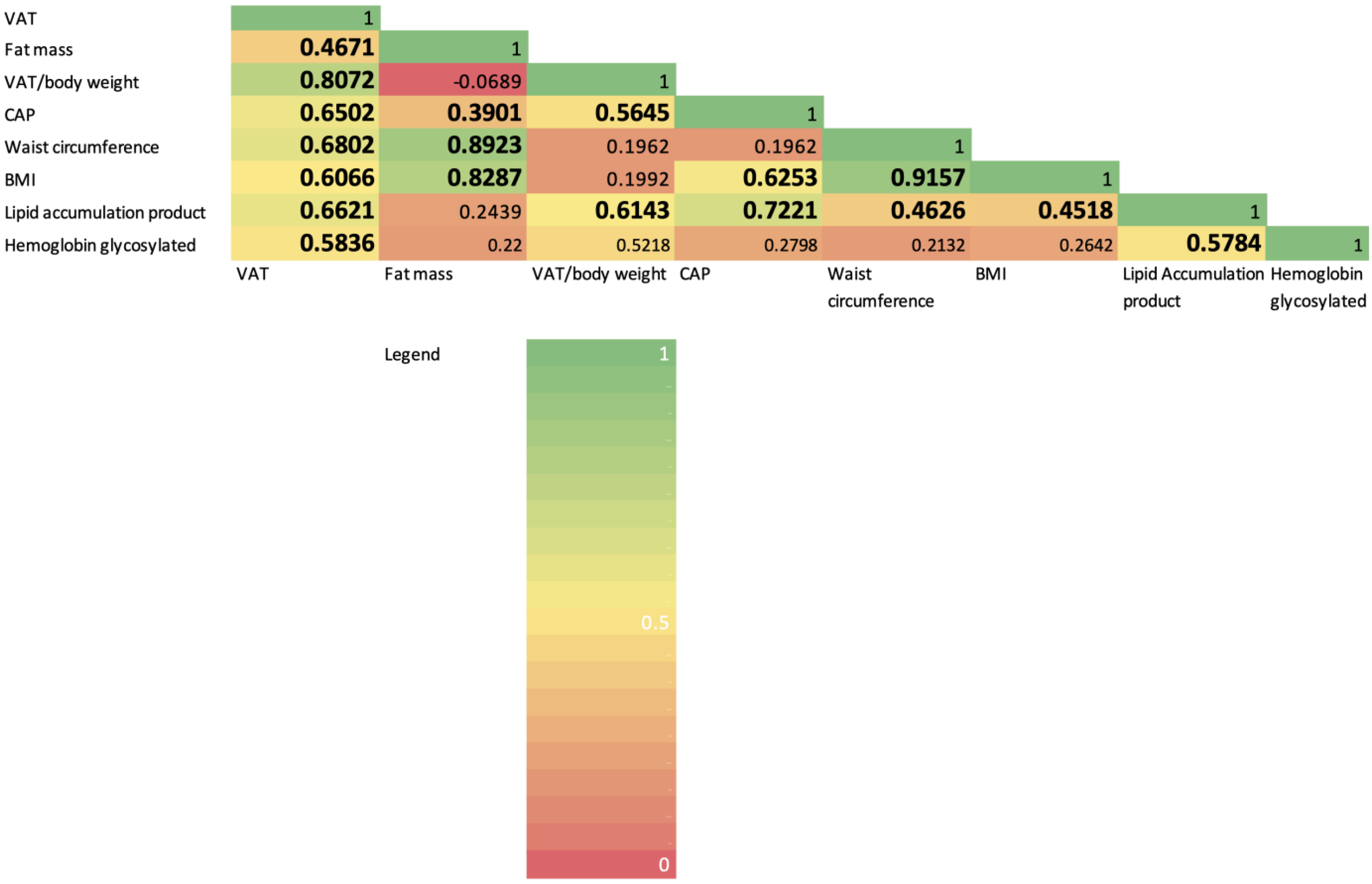
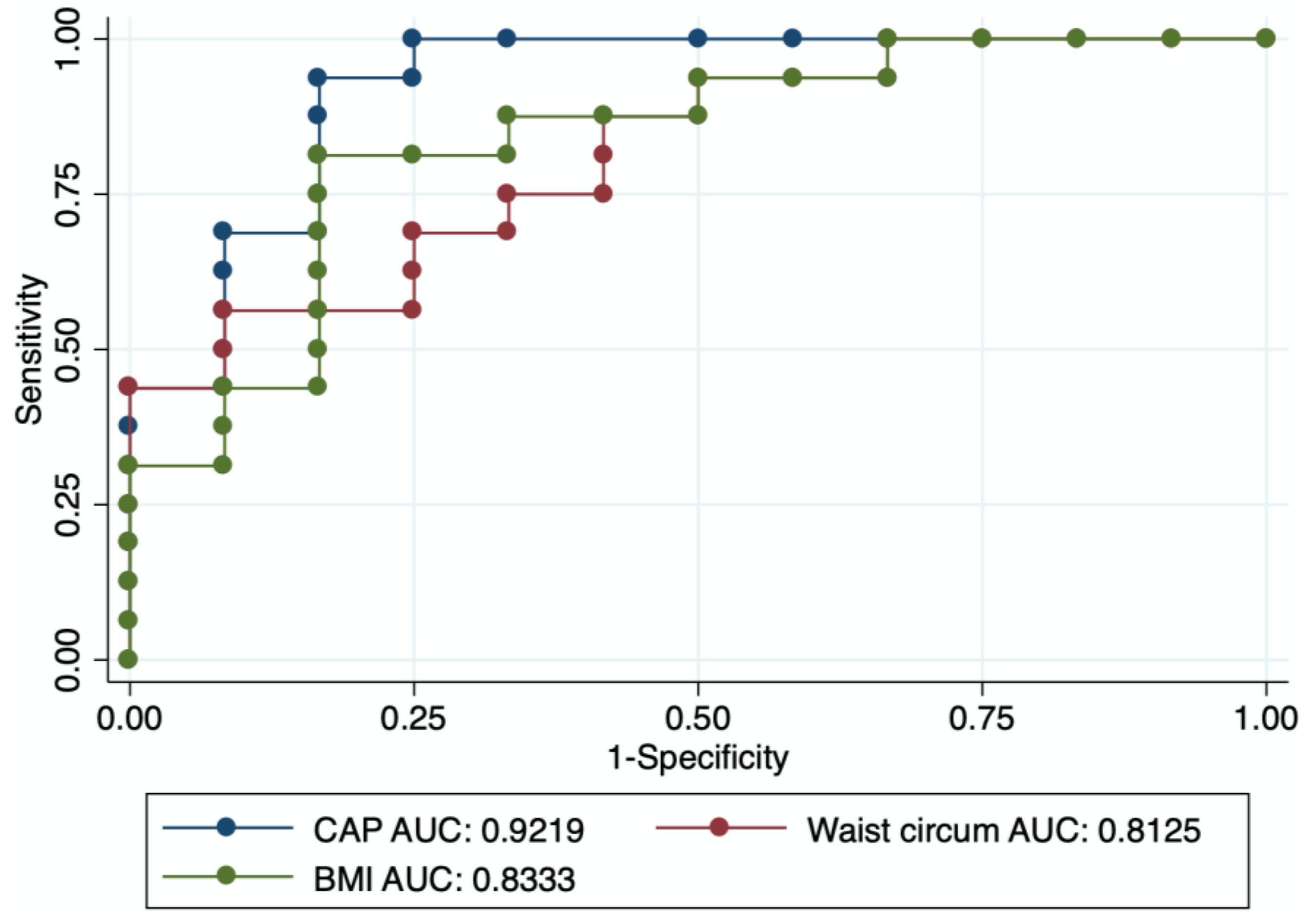
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, C.J.; Ryom, L.; Weber, R.; Morlat, P.; Pradier, C.; Reiss, P.; Kowalska, J.D.; de Wit, S.; Law, M.; el Sadr, W.; et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet 2014, 384, 241–248. [Google Scholar] [CrossRef]
- Guaraldi, G.; Lonardo, A.; Maia, L.; Palella, F.J., Jr. Metabolic concerns in aging HIV-infected persons: From serum lipid phenotype to fatty liver. AIDS 2017, 31, S147–S156. [Google Scholar] [CrossRef]
- Bischoff, J.; Gu, W.; Schwarze-Zander, C.; Boesecke, C.; Wasmuth, J.C.; van Bremen, K.; Dold, L.; Rockstroh, J.K.; Trebicka, J. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). eClinicalMedicine 2021, 40, 101116. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.B.; Patel, A.; Scott, A.J.; Patel, K.; Thursz, M.; Lemoine, M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS 2017, 31, 1621–1632. [Google Scholar] [CrossRef]
- Vuille-Lessard, E.; Lebouche, B.; Lennox, L.; Routy, J.P.; Costiniuk, C.T.; Pexos, C.; Giannakis, A.; Szabo, J.; Klein, M.B.; Sebastiani, G. Nonalcoholic fatty liver disease diagnosed by transient elastography with controlled attenuation parameter in unselected HIV monoinfected patients. AIDS 2016, 30, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Lake, J.E.; Overton, T.; Naggie, S.; Sulkowski, M.; Loomba, R.; Kleiner, D.E.; Price, J.C.; Chew, K.W.; Chung, R.T.; Corey, K.E. Expert Panel Review on Nonalcoholic Fatty Liver Disease in Persons With Human Immunodeficiency Virus. Clin. Gastroenterol. Hepatol. 2022, 20, 256–268. [Google Scholar] [CrossRef]
- Liu, D.; Shen, Y.; Zhang, R.; Xun, J.; Wang, J.; Liu, L.; Steinhart, C.; Chen, J.; Lu, H. Prevalence and risk factors of metabolic associated fatty liver disease among people living with HIV in China. J. Gastroenterol. Hepatol. 2021, 36, 1670–1678. [Google Scholar] [CrossRef]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef]
- Lake, J.E. The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Curr. HIV/AIDS Rep. 2017, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.L.; Yuan, L. Nonalcoholic Fatty Liver Disease: The Role of Visceral Adipose Tissue. Clin. Liver Dis. 2022, 19, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.; Assoumou, L.; De Wit, S.; Girard, P.M.; Valantin, M.A.; Katlama, C.; Necsoi, C.; Campa, P.; Huefner, A.D.; Schulze Zur Wiesch, J.; et al. Diagnostic Accuracy of Noninvasive Markers of Steatosis, NASH, and Liver Fibrosis in HIV-Monoinfected Individuals at Risk of Nonalcoholic Fatty Liver Disease (NAFLD): Results From the ECHAM Study. J. Acquir. Immune Defic. Syndr. 2019, 80, e86–e94. [Google Scholar] [CrossRef]
- Ajmera, V.H.; Cachay, E.R.; Ramers, C.B.; Bassirian, S.; Singh, S.; Bettencourt, R.; Richards, L.; Hamilton, G.; Middleton, M.; Fowler, K.; et al. Optimal Threshold of Controlled Attenuation Parameter for Detection of HIV-Associated NAFLD With Magnetic Resonance Imaging as the Reference Standard. Clin. Infect. Dis. 2021, 72, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.G.; McLaughlin, M.; Proschan, M.; Koh, C.; Kleiner, D.E.; Heller, T.; Kovacs, J.A. Transient elastography for the detection of hepatic fibrosis in HIV-monoinfected adults with elevated aminotransferases on antiretroviral therapy. AIDS 2015, 29, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Cervo, A.; Shengir, M.; Patel, K.; Sebastiani, G. NASH in HIV. Curr. HIV/AIDS Rep. 2020, 17, 601–614. [Google Scholar] [CrossRef]
- Pembroke, T.; Deschenes, M.; Lebouche, B.; Benmassaoud, A.; Sewitch, M.; Ghali, P.; Wong, P.; Halme, A.; Vuille-Lessard, E.; Pexos, C.; et al. Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J. Hepatol. 2017, 67, 801–808. [Google Scholar] [CrossRef]
- Cervo, A.; Milic, J.; Mazzola, G.; Schepis, F.; Petta, S.; Krahn, T.; Lebouche, B.; Deschenes, M.; Cascio, A.; Guaraldi, G.; et al. Prevalence, Predictors, and Severity of Lean Nonalcoholic Fatty Liver Disease in Patients Living with Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 71, e694–e701. [Google Scholar] [CrossRef]
- Reinert, D.F.; Allen, J.P. The Alcohol Use Disorders Identification Test (AUDIT): A review of recent research. Alcohol. Clin. Exp. Res. 2002, 26, 272–279. [Google Scholar] [CrossRef]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef]
- Mazidi, M.; Gao, H.K.; Kengne, A.P. Lipid accumulation product and visceral adiposity index are associated with dietary patterns in adult Americans. Medicine 2018, 97, e0322. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Billingham, S.A.; Whitehead, A.L.; Julious, S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.H. On the use of a pilot sample for sample size determination. Stat. Med. 1995, 14, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Jongraksak, T.; Sobhonslidsuk, A.; Jatchavala, J.; Warodomwichit, D.; Kaewduang, P.; Sungkanuparph, S. Prevalence and predicting factors of metabolic-associated fatty liver disease diagnosed by transient elastography with controlled attenuation parameters in HIV-positive people. Int. J. STD AIDS 2021, 32, 266–275. [Google Scholar] [CrossRef]
- Michel, M.; Labenz, C.; Wahl, A.; Anders, M.; Armandi, A.; Huber, Y.; Galle, P.R.; Sprinzl, M.; Schattenberg, J.M. Prevalence and risk factors of nonalcoholic steatohepatitis with significant fibrosis in people with HIV. AIDS 2022, 36, 1665–1674. [Google Scholar] [CrossRef]
- Lemoine, M.; Serfaty, L.; Capeau, J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: Diagnosis and management. Curr. Opin. Infect. Dis. 2012, 25, 10–16. [Google Scholar] [CrossRef]
- van Welzen, B.J.; Mudrikova, T.; El Idrissi, A.; Hoepelman, A.I.M.; Arends, J.E. A Review of Non-Alcoholic Fatty Liver Disease in HIV-Infected Patients: The Next Big Thing? Infect. Dis. Ther. 2019, 8, 33–50. [Google Scholar] [CrossRef]
- Caussy, C.; Alquiraish, M.H.; Nguyen, P.; Hernandez, C.; Cepin, S.; Fortney, L.E.; Ajmera, V.; Bettencourt, R.; Collier, S.; Hooker, J.; et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018, 67, 1348–1359. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Lemoine, M.; Assoumou, L.; Girard, P.M.; Valantin, M.A.; Katlama, C.; De Wit, S.; Campa, P.; Rougier, H.; Meynard, J.L.; Necsoi, C.; et al. Screening HIV patients at risk for NAFLD using MRI-PDFF and transient elastography: A European multicenter prospective study. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ryom, L.; De Miguel, R.; Cotter, A.G.; Podlekareva, D.; Beguelin, C.; Waalewijn, H.; Arribas, J.R.; Mallon, P.W.G.; Marzolini, C.; Kirk, O.; et al. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022, 23, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Conde, M.; Llop, E.; Carrillo, C.F.; Tormo, B.; Abad, J.; Rodriguez, L.; Perello, C.; Gomez, M.L.; Martinez-Porras, J.L.; Puga, N.F.; et al. Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 6658–6668. [Google Scholar] [CrossRef] [PubMed]
| Excess VAT (n = 17) | No Excess VAT (n = 13) | p-Value | |
|---|---|---|---|
| Age (years) | 52.9 (12.4) | 42.8 (12.5) | 0.035 |
| Male sex (%) | 16 (94.2) | 11 (84.6) | 0.390 |
| Ethnicity (%) | |||
| White/Caucasian | 7 (41.2) | 3 (23.1) | 0.503 |
| Black non-Hispanic | 3 (16.7) | 4 (30.8) | |
| Hispanic | 4 (23.6) | 3 (23.1) | |
| Hypertension (%) | 7 (41.2) | 4 (30.8) | 0.558 |
| Diabetes (%) | 6 (35.3) | 2 (15.4) | 0.222 |
| History of cardiovascular event (%) | 5 (29.4) | 0 | 0.032 |
| Waist circumference (cm) | 106.6 (10.8) | 93.3 (11.8) | 0.004 |
| BMI (Kg/m2) | 32.1 (4.3) | 26.9 (3.8) | 0.002 |
| Time since HIV diagnosis (years) | 20.0 (13.4) | 9.4 (9.0) | 0.021 |
| CD4 cell count (cells/μL) | 679.4 (396.6) | 595.5 (315.6) | 0.357 |
| Nadir CD4 cell count (cells/μL) | 254.2 (151.9) | 214.6 (206.4) | 0.684 |
| Current ART regimen (%) | |||
| NRTIs | 14 (82.4) | 13 (100) | 0.110 |
| NNRTIs | 2 (11.8) | 0 | 0.201 |
| PIs | 2 (11.8) | 1 (7.7) | 0.713 |
| Integrase inhibitors | 17 (100) | 12 (92.3) | 0.245 |
| Past exposure to d-drugs (%) | 4 (25.0) | 1 (8.3) | 0.254 |
| ALT (IU/L) | 51.9 (43.9) | 26.2 (21.4) | 0.118 |
| AST (IU/L) | 34.4 (26.4) | 24.3 (7.3) | 0.210 |
| Platelets (109/L) | 242.4 (178.4) | 195.5 (46.9) | 0.366 |
| Triglycerides (mmol/L) | 2.92 (2.04) | 1.04 (0.56) | 0.047 |
| Total cholesterol (mmol/L) | 4.51 (1.12) | 4.14 (0.95) | 0.490 |
| HDL cholesterol (mmol/L) | 1.03 (0.26) | 1.37 (0.28) | 0.025 |
| LDL cholesterol (mmol/L) | 2.48 (1.11) | 2.17 (0.83) | 0.573 |
| Lipid accumulation product | 111.5 (53.3) | 38.1 (36.8) | <0.001 |
| Fasting glucose (mmol/L) | 6.14 (1.75) | 5.33 (1.24) | 0.253 |
| Hemoglobin glycosylated (%) | 6.23 (1.32) | 5.91 (1.14) | 0.394 |
| LSM (kPa) | 7.4 (2.5) | 4.8 (1.5) | 0.002 |
| CAP (dB/m) | 319.2 (51.6) | 213.1 (52.4) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastiani, G.; Paisible, N.; Costiniuk, C.; Cox, J.; Kablawi, D.; Klein, M.B.; Kronfli, N.; Routy, J.-P.; Falutz, J.; Lebouché, B.; et al. The Relationship between Visceral Adiposity and Nonalcoholic Fatty Liver Disease Diagnosed by Controlled Attenuation Parameter in People with HIV: A Pilot Study. Diagnostics 2022, 12, 2590. https://doi.org/10.3390/diagnostics12112590
Sebastiani G, Paisible N, Costiniuk C, Cox J, Kablawi D, Klein MB, Kronfli N, Routy J-P, Falutz J, Lebouché B, et al. The Relationship between Visceral Adiposity and Nonalcoholic Fatty Liver Disease Diagnosed by Controlled Attenuation Parameter in People with HIV: A Pilot Study. Diagnostics. 2022; 12(11):2590. https://doi.org/10.3390/diagnostics12112590
Chicago/Turabian StyleSebastiani, Giada, Nathalie Paisible, Cecilia Costiniuk, Joseph Cox, Dana Kablawi, Marina B. Klein, Nadine Kronfli, Jean-Pierre Routy, Julian Falutz, Bertrand Lebouché, and et al. 2022. "The Relationship between Visceral Adiposity and Nonalcoholic Fatty Liver Disease Diagnosed by Controlled Attenuation Parameter in People with HIV: A Pilot Study" Diagnostics 12, no. 11: 2590. https://doi.org/10.3390/diagnostics12112590
APA StyleSebastiani, G., Paisible, N., Costiniuk, C., Cox, J., Kablawi, D., Klein, M. B., Kronfli, N., Routy, J.-P., Falutz, J., Lebouché, B., & Guaraldi, G. (2022). The Relationship between Visceral Adiposity and Nonalcoholic Fatty Liver Disease Diagnosed by Controlled Attenuation Parameter in People with HIV: A Pilot Study. Diagnostics, 12(11), 2590. https://doi.org/10.3390/diagnostics12112590









