Left Ventricular Function and Iron Loading Status in a Tertiary Center Hemochromatosis Cohort—A Cardiac Magnetic Resonance Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Image Acquisition
2.3. CMR Image Analysis
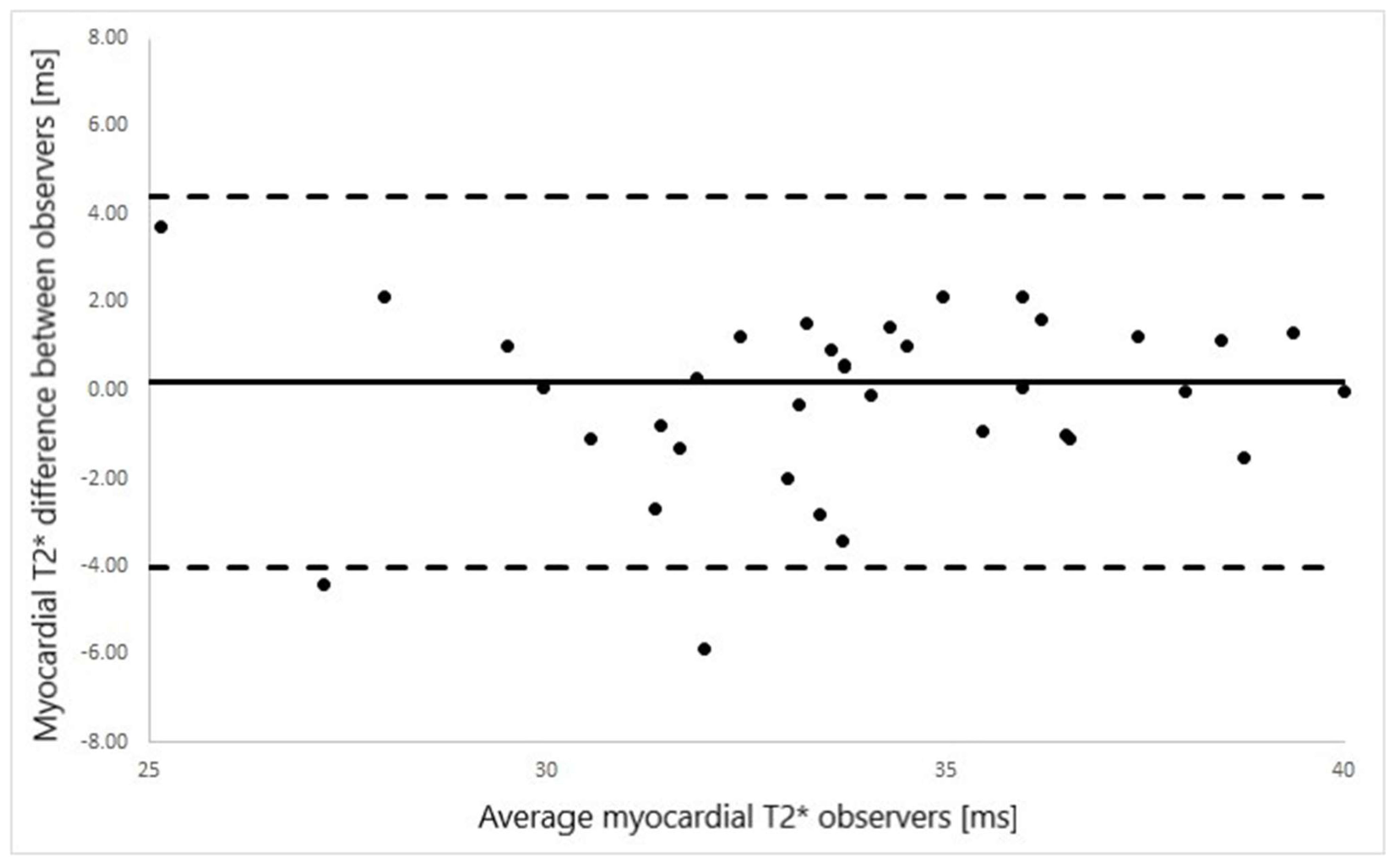
2.4. Statistics
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulati, V.; Harikrishnan, P.; Palaniswamy, C.; Aronow, W.S.; Jain, D.; Frishman, W.H. Cardiac involvement in hemochromatosis. Cardiol. Rev. 2014, 22, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.Q.; Griffen, L.M.; Goldgar, D.; Drummond, C.; Skolnick, M.H.; Kushner, J.P. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N. Eng. J. Med. 1988, 318, 1355–1362. [Google Scholar] [CrossRef]
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R., Jr.; Ellis, M.C.; Fullan, A.; et al. A novel MHC class I-likegene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 1996, 13, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Merryweather-Clarke, A.T.; Pointon, J.J.; Shearman, J.D.; Robson, K.J. Global prevalence of putative haemochromatosis mutations. J. Med. Genet. 1997, 34, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardou-Jacquet, E.; Ben Ali, Z.; Beaumont-Epinette, M.P.; Loreal, O.; Jouanolle, A.M.; Brissot, P. Non-HFE hemochromatosis: Pathophysiological and diagnostic aspects. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, E.P.; Garlitz, B.A.; Harris, E.L.; Beil, T.L.; Smith, P.R. Screening for hereditary hemochromatosis, a systematic review for the U.S. Preventive Services Task Force. Ann. Intern Med. 2006, 145, 209–223. [Google Scholar] [CrossRef]
- Olynyk, J.K.; Cullen, D.J.; Aquilla, S.; Rossi, E.; Summerville, L.; Powell, L.W. A population-based study of the clinical expression of the hemochromatosis gene. N. Eng. J. Med. 1999, 341, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Grosse, S.D.; Gurrin, L.C.; Bertalli, N.A.; Allen, K.J.; Disabilities, D.; Epidemiology, A. Clinical penetrance in hereditary hemochromatosis: Estimates of the cumulative incidence of severe liver disease among HFE C282Y homozygotes. Genet. Med. 2018, 20, 383–389. [Google Scholar] [CrossRef]
- Allen, K.J.; Gurrin, L.C.; Constantine, C.C.; Osborne, N.J.; Delatycki, M.B.; Nicoll, A.J.; McLaren, C.E.; Bahlo, M.; Nisselle, A.E.; Vulpe, C.D.; et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med. 2008, 358, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.J.; Powell, L.W.; Ramm, G.A. Environmental and genetic modifiers of the progression to fibrosis and cirrhosis in hemochromatosis. Blood 2008, 111, 4456–4462. [Google Scholar] [CrossRef]
- Weiss, G. Genetic mechanisms and modifying factors in hereditary hemochromatosis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sticker, F.; Osterreicher, C.H.; Datz, C.; Ferenci, P.; Wölfel, M.; Norgauer, W.; Kraus, M.R.; Wrba, F.; Hellerbrand, C.; Schuppan, D. Prediction of progression to cirrhosis by a glutathione S-transferase P1 polymorphism in subjects with hereditary hemochromatosis. Arch. Intern. Med. 2005, 165, 1835–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, G.J.; Evans, T.W.; Gutteridge, M.C. Iron and the redox of the lungs. Free Radic. Biol. Med. 2002, 33, 1306–1313. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines for HFE hemochromatosis. J. Hepatol. 2010, 53, 3–22. [Google Scholar] [CrossRef]
- Strohmeyer, G.; Niederau, C.; Stremmel, W. Survival and causes of death in hemochromatosis. Observations in 163 patients. Ann. N. Y. Acad. Sci. 1988, 526, 245–257. [Google Scholar] [CrossRef]
- Niederau, C.; Fischer, R.; Sonnenberg, A.; Stremmel, W.; Trampisch, H.J.; Strohmeyer, G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985, 313, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, K.; Sikorska, K.; Panas, D.; Sworczak, K. The Role of the Trabecular Bone Score in the Assessment of Osteoarticular Disorders in Patients with HFE-Hemochromatosis, A Single-Center Study from Poland. Genes 2021, 25, 1304. [Google Scholar] [CrossRef]
- Kiely, P.D. Haemochromatosis arthropathy—A conundrum of the Celtic curse. J. R. Coll. Physicians Edinb. 2018, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, R.G.; Wickenden, A.D.; Bouchard, R.A.; Oudit, G.Y.; Liu, P.P.; Backx, P.H. Modulation of iron uptake in heart by L- type Ca2+ channel modifiers, possible implications in iron overload. Circ. Res. 1999, 84, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Tauchenová, L.; Křížová, B.; Kubánek, M.; Fraňková, S.; Melenovský, V.; Tintěra, J.; Kautznerová, D.; Malušková, J.; Jirsa, M.; Kautzner, J. Successful treatment of iron- overload cardiomyopathy in hereditary hemochromatosis with deferoxamine and deferiprone: A case report. Can. J. Cardiol. 2016, 32, 1574.e1–1574.e3. [Google Scholar] [CrossRef]
- Rivers, J.; Garrahy, P.; Robinson, W.; Murphy, A. Reversible cardiac dysfunction in hemochromatosis. Am. Heart. J. 1987, 113, 216–217. [Google Scholar] [CrossRef]
- Rahko, P.S.; Salerni, R.; Uretsky, B.F. Successful reversal by chelation therapy of congestive cardiomyopathy due to iron overload. J. Am. Coll. Cardiol. 1986, 146, 1910–1911. [Google Scholar] [CrossRef]
- Daniłowicz-Szymanowicz, L.; Świątczak, M.; Sikorska, K.; Starzyński, R.R.; Raczak, A.; Lipiński, P. Pathogenesis, Diagnosis, and Clinical Implications of Hereditary Hemochromatosis-The Cardiological Point of View. Diagnostics 2021, 16, 1279. [Google Scholar] [CrossRef]
- Rozwadowska, K.; Daniłowicz-Szymanowicz, L.; Fijałkowski, M.; Sikorska, K.; Gałąska, R.; Kozłowski, D.; Gruchała, M.; Raczak, G. Can two-dimensional speckle tracking echocardiography be useful for left ventricular assessment in the early stages of hereditary haemochromatosis? Echocardiography 2018, 35, 1772–1781. [Google Scholar] [CrossRef]
- Świątczak, M.; Sikorska, K.; Raczak, G.; Daniłowicz-Szymanowicz, L. Nonroutine use of 2-dimensional speckle tracking echocardiography and fatigue assessment to monitor the effects of therapeutic venesections in a patient with newly diagnosed hereditary hemochromatosis. Kardiol. Pol. 2020, 78, 786–787. [Google Scholar] [CrossRef]
- Golfeyz, S.; Lewis, S.; Weisberg, I.S. Hemochromatosis: Pathophysiology, evaluation, and management of hepatic iron overload with a focus on MRI. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 767–778. [Google Scholar] [CrossRef]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.J. Assessment of Iron Overload with T2 * Magnetic Resonance Imaging. Prog. Cardiovasc. Dis. 2011, 54, 287–294. [Google Scholar] [CrossRef]
- Triadyaksa, P.; Oudkerk, M.; Sijens, P.E. Cardiac T2* mapping: Techniques and clinical applications. J. Magn. Reson. Imaging 2019, 52, 1340–1351. [Google Scholar] [CrossRef] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imagin. J. Cardiovasc. Magn. Reson. 2017, 19, 1–24. [Google Scholar] [CrossRef]
- Carpenter, J.-P.; He, T.; Kirk, P.; Roughton, M.; Anderson, L.J.; De Noronha, S.V.; Sheppard, M.N.; Porter, J.B.; Walker, J.M.; Wood, J.C.; et al. On T2* magnetic resonance and cardiac iron. Circulation 2011, 123, 1519–1528. [Google Scholar] [PubMed] [Green Version]
- Carpenter, J.P.; Grasso, A.E.; Porter, J.B.; Shah, F.; Dooley, J.; Pennell, D.J. On myocardial siderosis and left ventricular dysfunction in hemochromatosis. J. Cardiovasc. Magn. Reson. 2013, 15, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2 * mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Kirk, P.; Firmin, D.N.; Lam, W.M.; Chu, W.C.; Au, W.-Y.; Chan, G.C.; Tan, R.S.; Ng, I.; Biceroglu, S.; et al. Multi-center transferability of a breath-hold T2 technique for myocardial iron assessment. J. Cardiovasc. Magn. Reson. 2008, 10, 11. [Google Scholar] [CrossRef]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.; Anderson, L.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [Green Version]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Roughton, M.; Assomull, R.; Nair, S.V.; Walker, J.M.; et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007, 115, 1876–1884. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.-R.; El-Beshlawy, A.; Elalfy, M.; Yesilipek, A.; Kilinç, Y.; Habr, D.; Musallam, K.M.; et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am. J. Hematol. 2015, 90, 91–96. [Google Scholar] [CrossRef]
- Feng, Y.; He, T.; Carpenter, J.-P.; Jabbour, A.; Alam, M.H.; Gatehouse, P.D.; Greiser, A.; Messroghli, D.; Firmin, D.; Pennell, D. In vivo comparison of myocardial T1 with T2 and T2* in thalassaemia major. J. Magn. Reson. Imaging 2013, 38, 588–593. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, A.; Leal, J.; McMillan, F.; Francis, J.; Greiser, A.; Rider, O.J.; Myerson, S.; Neubauer, S.; Ferreira, V.M.; et al. Measurement of myocardial native T1 in cardiovascular diseases and norm in 1291 subjects. J. Cardiovasc. Magn. Reson. 2017, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sado, D.M.; Maestrini, V.; Piechnik, S.K.; Banypersad, S.M.; White, S.K.; Flett, A.S.; Robson, M.D.; Neubauer, S.; Ariti, C.; Arai, A.; et al. Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J. Magn. Reson. Imaging. 2015, 41, 1505–1511. [Google Scholar] [CrossRef]
- Positano, V.; Meloni, A.; Santarelli, M.F.; Gerardi, C.; Bitti, P.P.; Cirotto, C.; De Marchi, D.; Salvatori, C.; Landini, L.; Pepe, A. Fast generation of T2* maps in the entire range of clinical interest: Application to thalassemia major patients. Comput. Biol. Med. 2015, 56, 200–210. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef]
- Kritsaneepaiboon, S.; Ina, N.; Chotsampancharoen, T.; Roymanee, S.; Cheewatanakornkul, S. The relationship between myocardial and hepatic T2 and T2* at 1.5T and 3T MRI in normal and iron-overloaded patients. Acta Radiol. 2017, 59, 355–362. [Google Scholar] [CrossRef]
- Zoller, H.; Schaefer, B.; Vanclooster, A.; Griffiths, B.; Bardou-Jacquet, E.; Corradini, E.; Porto, G.; Ryan, J.; Cornberg, M. EASL Clinical Practice Guidelines on haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: Board of trustees task force on standardized protocols. J. Cardiovasc. Magn. Reason. 2008, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Adeyanju, O.; Heiberg, E.; Sjogren, J. Automated calculation of T2*mapping for MR images with application of certainty criterion for enhanced display. In Proceedings of the ISMRM 18th Scientific Sessions, Stockholm, Sweden, 1–7 May 2010. [Google Scholar]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update, Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference Ranges (“Normal Values”) for Cardiovascular Magnetic Resonance (CMR) in Adults and Children: 2020 Update. J. Cardiovasc. Magn. Reson. 2020, 22, 1–63. [Google Scholar] [CrossRef]
- Liguori, C.; Pitocco, F.; Di Giampietro, I.; de Vivo, A.E.; Schena, E.; Cianciulli, P.; Zobel, B.B. Relationship between myo- cardial T2* values and cardiac volumetric and functional parameters in β-thalassemia patients evaluated by cardiac magnetic resonance in association with serum ferritin levels. Eur. J. Radiol. 2013, 82, e441–e447. [Google Scholar]
- Rochitte, C.E.; Sara, L.; Shiozaki, A.A.; Szarf, G.; Blasbalg, R.; Jasinowodolinski, D. Iron overload measurements by CMR in patients with suspected hemochromatosis—Comparison of methods for T2* calculation in myocardium and liver. J. Cardiovasc. Magn. Reson. 2010, 12, P285. [Google Scholar] [CrossRef] [Green Version]
- Torlasco, C.; Cassinerio, E.; Roghi, A.; Faini, A.; Capecchi, M.; Abdel-Gadir, A.; Giannattasio, C.; Parati, G.; Moon, J.C.; Cappellini, M.D.; et al. Role of T1 mapping as a complementary tool to T2* for non-invasive cardiac iron overload assessment. PLoS ONE 2018, 13, e0192890. [Google Scholar]
- Krittayaphong, R.; Zhang, S.; Saiviroonporn, P.; Viprakasit, V.; Tanapibunpon, P.; Komoltri, C.; Wangworatrakul, W. Detection of cardiac iron overload with native magnetic resonance T1 and T2 mapping in patients with thalassemia. Int. J. Cardiol. 2017, 248, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Adams Pc Reboussin, D.M.; Barton, J.C.; McLaren, C.E.; Eckfeldt, J.H.; McLaren, G.D.; Dawkins, F.W.; Acton, R.T.; Harris, E.L.; Gordeuk, V.R.; Leiendecker-Foster, C.; et al. Hemochromatosis and iron Overload Screening (HeiRS) Study Research investigators. Hemochromatosis and iron-overload screening in a racially diverse population. N. Engl. J Med. 2005, 352, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Bidhult, S.; Xanthis, C.G.; Liljekvist, L.L.; Greil, G.; Nagel, E.; Aletras, A.H.; Heiberg, E.; Hedström, E. Validation of a New T2 * Algorithm and Its Uncertainty Value for Cardiac and Liver Iron Load Determination from MRI Magnitude Images. Magn. Reson. Med. 2015, 75, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
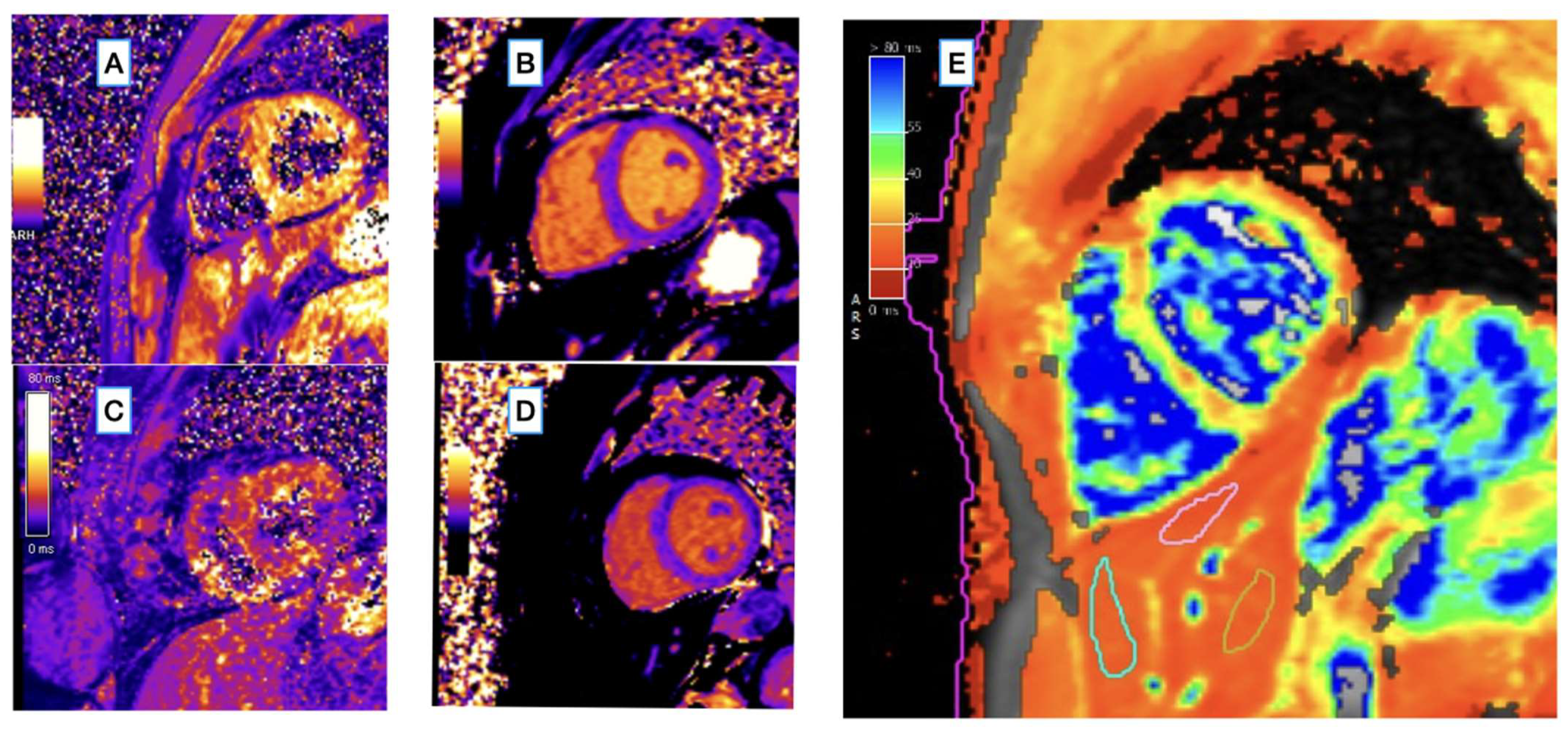

| Patients (N = 42) | Controls (N = 36) | p Value | |
|---|---|---|---|
| Age [years] median (Q1–Q3) | 47 (36–59) | 42 (29–55) | 0.140 |
| Sex (F) | 13 | 16 | 0.219 |
| LVEF median (Q1–Q3) | 81 (70–92) | 70 (58–82) | 0.211 |
| LVEDVI median (Q1–Q3) | 32 (25–39) | 31 (23–39) | 0.691 |
| LVESVI median (Q1–Q3) | 67 (58–76) | 62 (53–71) | 0.551 |
| LVMI median (Q1–Q3) | 60 (56–64) | 61 (57–65) | 0.148 |
| Parameter | Median (Q1–Q3) | Range (Min–Max) |
|---|---|---|
| Iron (μg/dL) | 204 (173–235) | 62–283 |
| Ferritin (ng/mL) | 625 (238–1012) | 100–3550 |
| TSAT [%] | 82 (67–97) | 48–100 |
| Haemoglobin (mg/dL) | 15 (14.1–15.9) | 12.0–17.8 |
| Glycemia (mg %) | 96 (85–107) | 75–139 |
| AsT | 26 (20–38.3) | 13–76 |
| AlaT | 35 (23.5–46.5) | 11–150 |
| Parameter | Patients | Controls | p |
|---|---|---|---|
| myoT2* [ms] (MyoMaps) median (Q1–Q3) | 34 (32–36) | 36 (34–38) | p = 0.004 |
| myoT1 [ms] (MyoMaps) median (Q1–Q3) | 962 (947–988) | 981 (961–998) | p = 0.028 |
| livT2* [ms] (MyoMaps) median (Q1–Q3) | 23 (17–30) | 31 (28–38) | p = 0.004 |
| livT2* [ms] (multiecho GRE) median (Q1–Q3) | 25 (17–30) | 27 (23–34) | p = 0.041 |
| Relaxometry Parameter below the Reference Range | All N (%) | Treatment Naive N (%) | On Treatment N (%) |
|---|---|---|---|
| Single parameter | 15 (35.7) | 11 (73) | 4 (27) |
| Low livT2* | 12 (28.6) | 9 (75) | 3 (25) |
| Low myoT2* | 1 (2.4) | 0 | 1 (100) |
| Low myoT1 | 2 (4.7) | 2 (100) | 0 |
| Multiple parameters | 7 (16.7) | 4 (57) | 3 (43) |
| Low myoT2* and myoT1 + normal livT2* | 1 (2.4) | 1 (100) | 0 |
| Low myoT2* and myoT1 + low livT2* | 3 (7.1) | 2 (66.7) | 1 (33.3) |
| Low myoT2* or low myoT1 + low livT2* | 3 (7.1) | 1 (33.3) | 2 (66.7) |
| Total | 22 (52.4) | 15 (68.2) | 7 (31.8) |
| Treatment Status | On Treatment n = 24 (57%) | Treatment Naive n = 18 (43%) | p |
|---|---|---|---|
| Age [years] mean ± SD (range) | 42 ± 15 (range 18–77) | 51 ± 14 (range 20–68) | 0.055 |
| Gender, N | (F = 9, M = 15) | (F = 4, M = 14) | 0.289 |
| myoT2* [ms] median (Q1–Q3) | 34 (32–37) | 33 (31–35) | 0.180 |
| myoT1 [ms] median (Q1–Q3) | 970 (946–988) | 956 (951–973) | 0.570 |
| livT2* [ms] median (Q1–Q3) | 20 (2–44) | 22 (3–44) | 0.434 |
| Iron [mcg/dL] median (Q1–Q3) | 196 (165–225) | 206 (179–252) | 0.286 |
| Ferritin [mcg/dL] median (Q1–Q3) | 421 (266–936) | 714 (368–1037) | 0.315 |
| TSAT [mcg/dL] median (Q1–Q3) | 81 (65–92) | 84 (66–95) | 0.447 |
| Haemoglobin [mcg/dL] median (Q1–Q3) | 15 (14.0–16.4) | 15.3 (14.7–16.0) | 0.716 |
| Glucose [mcg/dL] median (Q1–Q3) | 93 (85–102) | 97 (89–111) | 0.332 |
| ASPAT [mcg/dL] median (Q1–Q3) | 30 (20–39) | 34 (24–53) | 0.253 |
| ALAT [mcg/dL] median (Q1–Q3) | 39 (24–67) | 55 (34–95) | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorniak, K.; Daniłowicz-Szymanowicz, L.; Sikorska, K.; Rozwadowska, K.; Fijałkowska, J.; Glińska, A.; Tuzimek, M.; Sabisz, A.; Żarczyńska-Buchowiecka, M.; Świątczak, M.; et al. Left Ventricular Function and Iron Loading Status in a Tertiary Center Hemochromatosis Cohort—A Cardiac Magnetic Resonance Study. Diagnostics 2022, 12, 2620. https://doi.org/10.3390/diagnostics12112620
Dorniak K, Daniłowicz-Szymanowicz L, Sikorska K, Rozwadowska K, Fijałkowska J, Glińska A, Tuzimek M, Sabisz A, Żarczyńska-Buchowiecka M, Świątczak M, et al. Left Ventricular Function and Iron Loading Status in a Tertiary Center Hemochromatosis Cohort—A Cardiac Magnetic Resonance Study. Diagnostics. 2022; 12(11):2620. https://doi.org/10.3390/diagnostics12112620
Chicago/Turabian StyleDorniak, Karolina, Ludmiła Daniłowicz-Szymanowicz, Katarzyna Sikorska, Katarzyna Rozwadowska, Jadwiga Fijałkowska, Anna Glińska, Magdalena Tuzimek, Agnieszka Sabisz, Marta Żarczyńska-Buchowiecka, Michał Świątczak, and et al. 2022. "Left Ventricular Function and Iron Loading Status in a Tertiary Center Hemochromatosis Cohort—A Cardiac Magnetic Resonance Study" Diagnostics 12, no. 11: 2620. https://doi.org/10.3390/diagnostics12112620
APA StyleDorniak, K., Daniłowicz-Szymanowicz, L., Sikorska, K., Rozwadowska, K., Fijałkowska, J., Glińska, A., Tuzimek, M., Sabisz, A., Żarczyńska-Buchowiecka, M., Świątczak, M., Dudziak, M., & Szurowska, E. (2022). Left Ventricular Function and Iron Loading Status in a Tertiary Center Hemochromatosis Cohort—A Cardiac Magnetic Resonance Study. Diagnostics, 12(11), 2620. https://doi.org/10.3390/diagnostics12112620







