Abstract
The purpose of this study was to search for correlations between contrast-enhanced ultrasound (CEUS) imaging and histopathological results in salivary gland lesions and to determine the accuracy of CEUS in the preoperative differentiation of salivary gland tumours according to postoperative histopathological results. The study included 54 consecutive patients with 63 salivary gland lesions who underwent CEUS examination prior to surgical treatment at the Department of Otolaryngology, Medical University of Łódź (Poland) in 2019–2022. The accuracy of CEUS in differential diagnostics of salivary gland lesions was later verified against final histological diagnosis. Among 63 salivary gland lesions, 26 were categorized as malignant or with malignant potential, and 37 were benign. There was a correlation between professional photographs of CEUS imaging and microscope slides containing postoperative specimens. A strong heterogeneous enhancement was observed mainly in benign lesions, with while weak heterogeneity mostly among the malignant or with malignant potential lesions. A pattern of contrast enhancement in specific structures reflected histopathological images. These results suggest that contrast-enhanced ultrasonography is a promising tool for the preoperative diagnostics of salivary gland lesions.
1. Introduction
There are many types of salivary gland tumours, including those caused by inflammatory or autoimmunologic processes. “Up to 80% of salivary gland neoplasms occur in the parotid gland” [1]. “The most common histologic types of benign parotid tumours are pleomorphic adenoma and Warthin tumour, while the most often diagnosed malignancies of the parotid are mucoepidermoid carcinoma and adenoid cystic carcinoma” [2]. While Warthin tumour is untroublesome to differ from parotid malignancies, differential diagnostics between malignant tumour and pleomorphic adenoma in some cases remains a challenge. Preoperative diagnostics should provide crucial information on salivary gland lesions, among which the type of pathology is determinant as far as resection method is concerned. Other features, such as tumour’s size, topography, infiltration of other structures and vascularisation of both adjacent structures and the tumour itself may well facilitate planning the surgery. Fine-needle biopsy is frequently used for the preoperative differentiation of salivary gland tumours, but owing to sampling difficulties and the great heterogeneity of these neoplasms (especially pleomorphic adenoma), the results are not always conclusive. Moreover, there might always be a risk of spreading neoplasmatic cells during the procedure. Therefore, in the cases of patients with concomitant diseases, for whom a surgery may be hazardous, it is crucial that decisions of watchful waiting be based on safe and reliable diagnostics techniques. “Fine needle biopsy should not be relied upon as the sole determinant of a surgeon’s management plan due to its limited sensitivity” [3]. Methods currently used for differential diagnostics of salivary gland tumours include conventional ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). A contrast-enhanced USG might be more accurate than conventional USG. It might also pose a cheaper, faster and of comparable accuracy alternative to CT or MRI. “CEUS has the potential to differentiate salivary gland lesions preoperatively” [4]. It appears to be a promising diagnostic tool, especially in combination with clinical complaint assessment [4,5]. Guiban et al. in 2021 suggested that “the combination of B-mode US and CEUS greatly improved the sensitivity of the CEUS performed individually and presented remarkable accuracy, with the potential to reduce the number of invasive procedures” [6]. Saito et al. in 2020 reported that “CEUS is a simple, safe, and noninvasive examination for patients that can be performed conveniently in real-time and could help differentiate PMA from WT in salivary glands” [7]. Papers present in the literature suggest that there is a constant need for further studies with large patient groups [4,5,6,7]. The authors had access to a relatively large group of patients who underwent preoperative contrast-enhanced ultrasound (CEUS) examination. The aim of this study was to search for correlations between CEUS imaging and the results of postoperative histopathological examinations of surgical specimens and to establish the accuracy of CEUS in differential diagnostics of salivary gland lesions.
2. Materials and Methods
A prospective study was conducted in the Otolaryngology Department and Radiology Department, Medical University of Łódź. The study included 54 consecutive patients with 63 salivary gland lesions treated surgically at the department in 2019–2022. The group included 21 men and 33 women 21 to 82 years old. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee at the Medical University of Łódź, and all patients gave their informed consent to participate in the project. Before surgery, conventional USG and CEUS of salivary glands were performed in every patient. The time between the CEUS examination and the surgery did not exceed 10 days, and most often it was less than 48 h. Surgical specimens were subjected to routine histologic examination at the Department of Pathomorphology, Medical University of Łódź. Based on histopathological examinations, the lesions were classified into two groups. The first group involved benign lesions: monomorphic adenomas, including Warthin tumours, an oncocytic adenoma, myoepithelial adenomas, a lymphoepithelial cyst, sarcoidosis, lymph nodes, a fibrolipoma and inflammatory lesions. The second group involved malignant or of malignant potential lesions: a lymphoepithelial carcinoma, an adenoid cystic carcinoma, pleomorphic adenomas and basal cell adenomas.
2.1. Image Acquisition and Processing
GE logiq 7 Espert system with linear probe 9 L was used. CEUS was performed according to literature guidelines for CEUS in parotid glands [8,9]. Examination included standard grey scale (B-mode) ultrasound. Size, localization and quantity of lesions were noted. Next, colour Doppler was performed. In the last step, CEUS was performed starting from the injection of 4.8 mL of contrast agent (SonoVue, Amsterdam, Netherlands) into the medial cubital vein. CEUS was performed with low mechanical index (<0.1) to avoid the destruction of contrast agent bubbles and the disruption of signal caused by it [10,11]. Three main phases of acquisition were noticed. The acquisition of each phase was intervallic: arterial phase (10–45 s), portal venous phase (30–120 s), late venous phase (120–640 s) [10,12]. During examination, enhancement of tumour in arterial phase was compared with salivary gland parenchyma.
SonoVue is a contrast agent containing sulphur hexafluoride (SF6) microbubbles for CEUS imaging. “It is used to enhance the echogenicity of the blood, which can improve the signal-to-noise ratio in US. (…) Low solubility gas contrast agents such as SonoVue allow imaging at low mechanical index, which in turn leads to effective tissue signal suppression” [13].
2.2. Image Analysis
The following factors were taken into consideration: shape (round, multiple curved, blurred), echogenicity (hypoechogenic, isoechogenic, hyperechogenic) and specific pattern of contrast enhancement (strong homogeneous, weak homogeneous, strong heterogeneous, weak heterogeneous). The enhancement level referred to the enhancement of an intact salivary gland tissue (Scheme 1, Scheme 2 and Scheme 3). The additional variable was patient’s age.
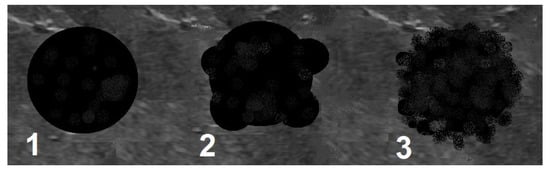
Scheme 1.
Shape of a lesion. 1—round, 2—multiple curved, 3—blurred.
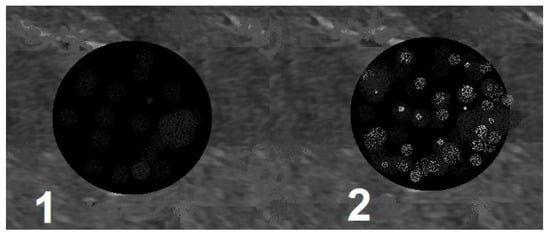
Scheme 2.
Echogenicity. 1—hypoechogenic, 2—hyperechogenic.
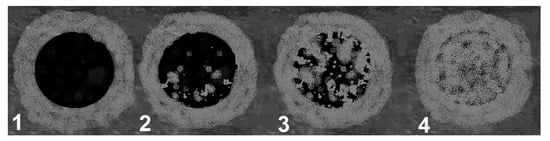
Scheme 3.
Pattern of contrast enhancement. 1—weak homogeneous, 2—weak heterogeneous, 3—strong heterogeneous, 4—strong homogeneous.
Two models were analysed. Only the first one included contrast enhancement. The CEUS images were in the following step compared with professional photographs of microscope slides containing postoperative specimens after preparation and proper staining at the Department of Pathomorphology, Medical University of Łódź. The authors focused on specific contrast enhancement areas and structures revealed on photographs that overlapped.
2.3. Statistical Analysis
Statistical analysis was performed in TIBCO Statistica 13.3 Software. (TIBCO, Palo Alto, CA, USA).
Categorical variables were reported in 2 × 2 contingency tables, and chi-squared tests with Yates correction for continuity were applied where total number of cases was smaller than 100 and greater than 5 cases in any analysed cell. Fisher’s exact tests were applied when any cell count was lower than 5, and Haldane-Anscome correction was applied to cell counts lower than 1. For 2 × 3 and 2 × 4 tables, the Freeman-Halton extension was applied. Where applicable, Odds Ratio and 95% Confidence Interval was calculated. All calculations were carried out with the threshold of statistical significance set at a p value no greater than 0.05, unless otherwise mentioned.
Multivariate Analysis
To perform logistic regression analysis of the probability of correct assignment of a salivary gland lesion to one of the abovementioned groups (benign and malignant/with malignant potential), the following characteristics were analysed: age, shape, echogenicity and pattern of contrast enhancement. As the first step in building a logistic regression model, a univariate analysis was performed using the likelihood ratio (LR) test.
Each variable with p value from the LR test no greater than 0.35 was included in the multivariate analysis using mixed-effects logistic regression. Continuous variables were checked for linearity. There were no statistically significant interactions between the variables.
To assess the influence of contrast enhancement on diagnostic accuracy, two logistic regression models were constructed. Age, shape and contrast enhancement were included in model, while model 2 included only age and shape. The cut-off values for both regression models were established based on receiver operating characteristic (ROC) curve analysis, and the highest Youden’s index determined the proposed cut-off value. To assess the diagnostic performance of the CEUS, the measures of occurrence (sensitivity, specificity and accuracy) and the possibility of discriminating (positive and negative predictive values) were calculated per the determined cut-off values.
3. Results
Among the 63 salivary gland lesions, 26 (41.27%) were categorized as malignant or with malignant potential and 37 (58.73%) as benign. Mean age among the whole group was 60.30 years with SD = 12.77 and OR = 0.972 (0.934; 1.013).
In the first group, strong homogeneous enhancement was found for 1 (3.85%) lesion; weak homogeneous, 1 (3.85%); strong heterogeneous, 10 (38.46%) and weak heterogeneous, 14 (53.85%). In the second group, strong homogeneous enhancement was found for 2 (5.41%) lesions; weak homogeneous, 2 (5.41%); strong heterogeneous, 26 (70.27%) and weak heterogeneous, 7 (18.92%). Within the first group, 9 (34.62%) lesions were round, 16 (61.54%) were multicurved and 1 (3.85%) was blurred. Within the second group, 7 (18.92%) lesions were round, 27 (72.97%) were multicurved and 3 (8.11%) were blurred.
A correlation between professional photographs of CEUS imaging and those of microscope slides containing postoperative specimens was observed. It appears that the strong heterogeneous enhancement pattern is more likely to occur in benign lesions, while malignant, and malignant potential lesions tend to present weak heterogeneous enhancement. The foundation of such tendencies is revealed in pathomorphological specimens. Various tissues such as fluid and cartilage commonly present in salivary gland tumours, are generally poorly enhanced. The enhancement of such areas in CEUS is poor if any. The cross-section of Warthin tumour, for example, is presented by one or more often numerous minor cysts containing mucus.
Those not-enhanced areas and structures revealed on histopathological photographs overlapped (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
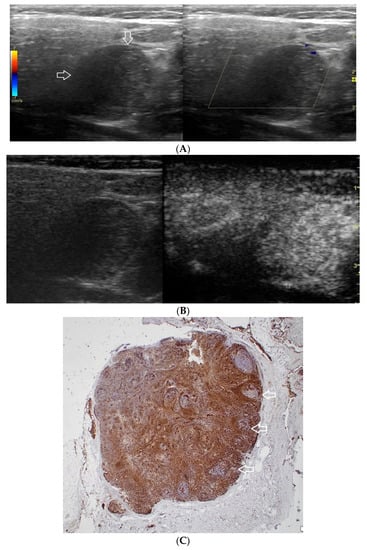
Figure 1.
(A−C) Lymphoepithelial carcinoma. (A) Routine ultrasonographic examination in B presentation and colour Doppler (CD): a hypoechogenic focal lesion of a heterogeneous echostructure (white arrows). On CD, low blood flow is observed. (B) Contrast-enhanced imaging in arterial phase: a strong heterogeneous enhancement of a lesion containing minor oval areas with no enhancement (white arrows). (C) Pathomorphological image, immunohistochemistry for CD3+. Aggregations of carcinomatous cells (white arrows) surrounded by scattered lymphoid tissue cells.
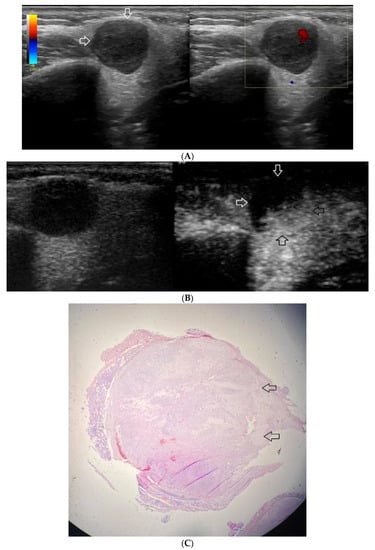
Figure 2.
(A−C) Pleomorphic adenoma. (A) Routine ultrasonographic examination in B presentation and colour Doppler: a well-demarcated focal lesion of a homogeneous echostructure (white arrows). On CD, low blood flow is observed. (B) Contrast-enhanced imaging in arterial phase: a strong homogeneous enhancement of a deeply located part of a lesion (black arrows). A superficial part is not enhanced (white arrows). (C) Pathomorphological image, H & E stain. A large area containing chondroid tissue (black arrows) with glandular tissue located beneath the lesion.
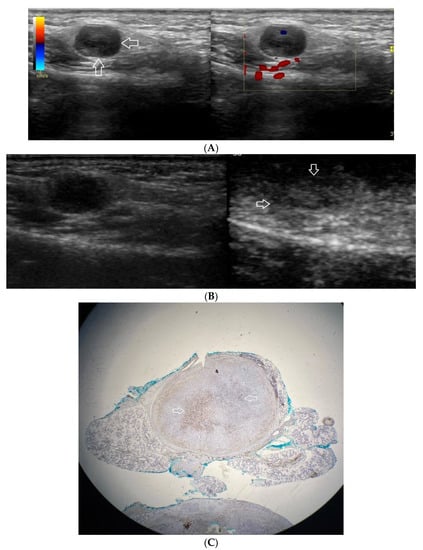
Figure 3.
(A−C) Pleomorphic adenoma. (A) Routine ultrasonographic examination in B presentation and colour Doppler: a small, well-demarcated focal lesion of a homogeneous echostructure. (white arrows). On CD, low blood flow is observed. (B) Contrast-enhanced imaging in arterial phase: a strong homogeneous enhancement of a central part of the lesion. A weakly enhanced peripheral part. (C) Pathomorphological image, immunohistochemistry for CD34. An aggregation of small vessels in central part of the lesion (white arrows).
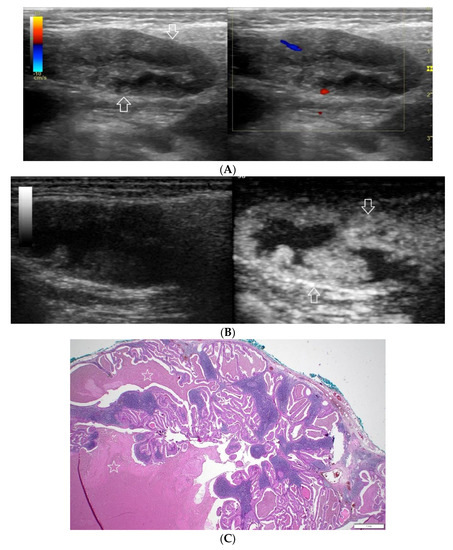
Figure 4.
(A−C) Warthin’s tumour. (A) Routine ultrasonographic examination in B presentation and colour Doppler: a big hypoechogenic well-demarcated focal lesion of a heterogeneous echostructure (white arrows). On CD, a low blood flow is observed. (B) Contrast-enhanced imaging in arterial phase: a strong homogeneous enhancement of a peripheral part of the lesion (white arrows). In the central part of the tumour, irregular areas without enhancement. (C) Pathomorphological image, H & E stain. Large areas of irregular shape containing fluid (white stars). A part containing glandular tissue located peripherally.
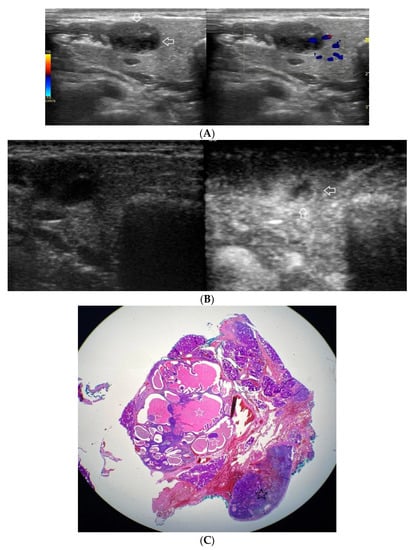
Figure 5.
(A−C) Warthin’s tumour. (A) Routine ultrasonographic examination in B presentation and colour Doppler: a small multi-arched hypoechogenic, well-demarcated focal lesion of a heterogeneous echostructure. On CD, low blood flow is observed. (B) Contrast-enhanced imaging in arterial phase: a strong homogeneous enhancement of a peripheral part of the lesion (white arrows). A weakly enhanced central part. (C) Pathomorphological image, H & E stain. Large areas of irregular shape containing fluid (white stars). A part containing glandular tissue located peripherally. Black star indicates a lymph node.
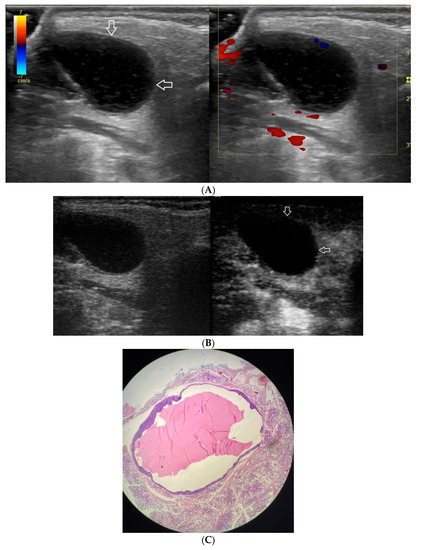
Figure 6.
(A−C) Lymphoepithelial cyst. (A) Routine ultrasonographic examination in B presentation and colour Doppler: an oval hypoechogenic well-demarcated focal lesion of a homogeneous echostructure (white arrows). On CD, no blood flow within the lesion is observed. (B) Contrast-enhanced imaging in arterial phase: no contrast enhancement of the lesion is observed (white arrows). (C) Pathomorphological image, H & E stain. A large area containing fluid with no signs of neoplastic growth.
Univariate analysis revealed that all lesions except for one were hypoechogenic on conventional US; therefore, echogenicity was not included in the regression model (Table 1). The ultrasound images were assessed by two people according to a scheme by consensus.

Table 1.
Summary of univariate analysis conducted as the first step towards formulating a logistic regression model.
In the multivariate analysis, only contrast enhancement was statistically significant. Weak heterogeneous contrast enhancement was 5.2 times more likely to be a malignant or of malignant potential lesion. (OR 5.2 95% CI 1.623; 16.656, p = 0.006).
Area under the curve (AUC) was compared between ROC curves prepared for each regression model. The AUC in model 1 with CEUS (Figure 7) was 0.770 (SE 0.062), while in model 2 without CEUS (Figure 8) it was 0.683 (SE 0.0687), a difference of 0.087. The addition of contrast enhancement to the regression model improved specificity by 16.2% while lowering the sensitivity by only 3.9% (Table 2).
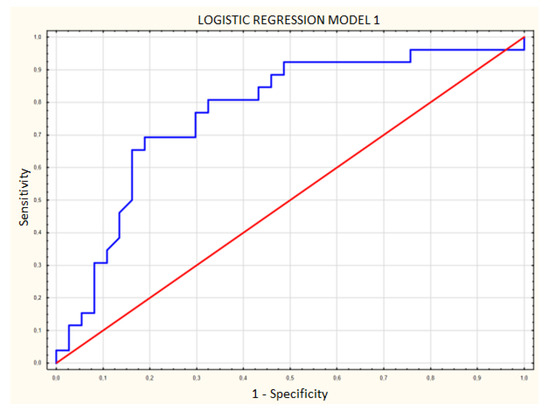
Figure 7.
Logistic regression model including age, shape and contrast enhancement. Blue line—receiver operating characteristic (ROC) curve for model 1; red line—random classifier.
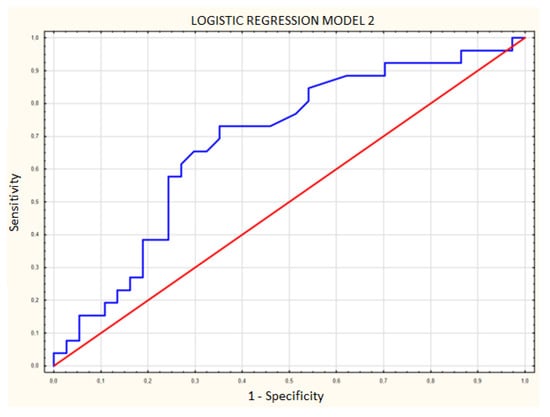
Figure 8.
Logistic regression model including age and shape without contrast enhancement. Blue line—receiver operating characteristic (ROC) curve for model 2; red line—random classifier.

Table 2.
Effectiveness of ultrasonographic assessment after inclusion of contrast pattern analysis.
4. Discussion
This study shows that preoperative contrast-enhanced ultrasonography is a promising tool for the differential diagnostics of salivary gland lesions. The authors searched for factors that might contribute to higher diagnostic accuracy of ultrasound imaging. Analysis showed that there were characteristic features in CEUS imaging and specific patterns of contrast enhancement in tumours’ structures that reflected photographs of pathomorphological preparations to a certain extent. Not only does the study show the vascularisation of a lesion and adjacent structures, but it also presents that specific structures within salivary gland and the tumour itself, such as cicatricial tissue, connective tissue, fluids or necrosis, have individual patterns of size and shape of enhancement after contrast injection. Due to numerous disadvantages of fine-needle biopsy, there is a constant need to develop radiological, noninvasive methods of preoperative differentiation among salivary gland pathologies. The role of MRI in this field is growing significantly. Currently, MRI plays a key role in the preoperative diagnosis of tumours. Should a surgeon consider a watchful waiting attitude in patients suffering from numerous diseases, there is a need for a sensitive, specific, repeatable and noninvasive procedure that could help qualify a lesion as either malignant or prone to malignant transformation. A crucial thing would be to capture the moment of possible turning a benign tumour into a malignant one. In such cases, when a surgery and anaesthesia pose a major risk for the patient, avoiding biopsy with potential neoplasmatic cells spreading around healthy tissue should be taken into consideration. “Carcinoma Ex-Pleomorphic Adenoma (CExPA) accounts for 5% to 15% of all salivary gland malignancies and can arise in up to 25% of untreated pleomorphic adenoma. Malignant transformation is often seen in recurrent pleomorphic adenoma (PA) with the risk of transformation ranging from 5% to 10% for untreated pleomorphic adenomas over a 15-year period” [14]. “Basal cell adenomas follow PA and WT as the third most common benign parotid tumour. Their distinctive membranous subtype (…) has the highest risk of malignant transformation (up to 28%)” [15]. “It is noteworthy that in most cases, in which the biopsy results were falsely negative, the parotid malignancies were misdiagnosed preoperatively as pleomorphic adenomas. According to the literature, because of their heterogeneous histologic structure, distinguishing pleomorphic adenomas from other tumour types, including malignant ones, can be challenging even for an experienced pathologist” [2]. The study findings imply that contrast-enhanced USG would be able to provide additional information helpful for establishing the differential diagnostics of salivary gland tumours. The ultrasound technique provides more detailed information, enabling the diagnosis to be made before the operation. Promising observations concern elastography. The authors are aware of the potential limitations of this study. The present findings suggest that future research should focus on the role of contrast-enhanced USG in distinguishing salivary gland malignancies from specific histologic types of benign lesions, particularly as far as pleomorphic adenoma is concerned. In CEUS imaging, the experience of a radiologist has a major impact on the results and their repeatability. Moreover, the still limited availability of this method might be an obstacle in making CEUS a standard procedure. On the other hand, as far as both patient’s reconvalescence and cost-effectiveness are concerned, a routinely performed preoperative CEUS, even combined with a fine-needle biopsy, might be a cheaper alternative to repeated surgery after the suboptimal resection of a malignant tumour initially misdiagnosed as a benign one. Even considering all potential limitations, the present results suggest that contrast-enhanced USG is a promising tool in the preoperative diagnostics of salivary gland lesions.
Author Contributions
Conceptualization, L.S., K.K. and W.P.; methodology, L.S., K.K., P.W.; validation, O.S., L.S. and K.K.; formal analysis, K.K., L.S., P.W. and O.S.; investigation, L.S., K.K., Z.K., A.P., P.W. and O.S.; resources, K.K., O.S., L.S., Z.K., A.P. and W.P.; data curation, K.K., O.S., L.S. and A.P.; writing—original draft preparation, K.K.; writing—review and editing, K.K., A.P., W.P. and L.S.; visualization, K.K. and A.P.; supervision, L.S., W.P. and Z.K.; project administration, L.S. and W.P.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Lodz, Poland, grant number 503/1-136-01/503-11-001.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee at the Medical University of Łódź (protocol code RNN/267/20/KE; date of approval: 17 November 2020).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
All of the authors would like to thank Oskar Rosiak, (Balance Disorders Unit, Department of Otolaryngology, Medical University of Lodz, the Norbert Barlicki Memorial Teaching Hospital, Lodz, Poland), whose contribution to statistical analysis in this study was invaluable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aghaghazvini, L.; Salahshour, F.; Yazdani, N.; Sharifian, H.; Kooraki, S.; Pakravan, M.; Shakiba, M. Dynamic contrast-enhanced MRI for differentiation of major salivary glands neoplasms, a 3-T MRI study. Dentomaxillofacial Radiol. 2015, 44, 20140166. [Google Scholar] [CrossRef] [PubMed]
- Mikaszewski, B.; Markiet, K.; Smugała, A.; Stodulski, D.; Szurowska, E.; Stankiewicz, C. Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging—An Alternative to Fine Needle Biopsy or Only an Adjunct Test in Preoperative Differential Diagnostics of Malignant and Benign Parotid Tumors? J. Oral Maxillofac. Surg. 2017, 75, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Hamour, A.F.; O’Connell, D.; Biron, V.L.; Allegretto, M.; Seemann, R.; Harris, J.R.; Seikaly, H.; Côté, D.W.J. Clinical diagnostic utility of ultrasound-guided fine needle aspiration biopsy in parotid masses. Ear Nose Throat J. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.v.; Gürkov, R.; Eichhorn, M.E.; Siedek, V.; Krause, E.; Jauch, K.W.; Reiser, M.F.; Clevert, D.A. Perfusion characteristics of parotid gland tumors evaluated bycontrast-enhanced ultrasound. Eur. J. Radiol. 2013, 82, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Strieth, S.; Siedek, V.; Rytvina, M.; Gürkov, R.; Berghaus, A.; Clevert, D.A. Dynamic contrast-enhanced ultrasound for differential diagnosis of submandibular gland disease. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Guiban, O.; Rubini, A.; Fresilli, D.; Lucarelli, G.T.; Ralli, M.; Cassoni, A.; Bezzi, M.; Radzina, M.; Greco, A.; de Vincentiis, M.; et al. Preoperative Multiparametric Ultrasound and Fine Needle Aspiration Cytology evaluation of parotid gland tumors: Which is the best technique? Med. Ultrason. 2021, 23, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.; Shiga, K.; Katagiri, K.; Oikawa, S.I.; Ikeda, A.; Tsuchida, K.; Miyaguchi, J.; Kusaka, T.; Kuroda, H.; Takahashi, F. Contrast-enhanced ultrasonography for the differential diagnosis of pleomorphic adenomas and Warthin tumors in salivary glands. Laryngoscope Investig. Otolaryngol. 2021, 6, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Stoia, S.; Băciuț, G.; Lenghel, M.; Badea, R.; Băciuț, M.; Bran, S.; Dinu, C. Ultrasonography techniques in the preoperative diagnosis of parotid gland tumors—An updated review of the literature. Med. Ultrason. 2021, 23, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.S.; Dai, Y.-L. Routine and Advanced Ultrasound of Major Salivary Glands. Neuroimaging Clin. N. Am. 2018, 28, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Cosgrove, D.; Albrecht, T.; Bolondi, L.; Bosio, M.; Calliada, F.; Correas, J.M.; Darge, K.; Dietrich, C.; D’Onofrio, M.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—Update 2008. Ultraschall der Med. Eur. J. Ultrasound 2008, 29, 28–44. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Cantisani, V.; De Vincentiis, M.; Sidhu, P.; Greco, A.; Tombolini, M.; Drudi, F.M.; Messineo, D.; Gigli, S.; Rubini, A.; et al. Contrast-enhanced ultrasound in the evaluation of parotid gland lesions: An update of the literature. Ultrasound 2016, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.; Joore, M.; Grutters, J.; Redekop, K.; Armstrong, N.; Lee, K.; Gloy, V.; Raatz, H.; Misso, K.; Severens, J.; et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast- enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2013, 17, 7–243. [Google Scholar] [CrossRef]
- Young, A.; Okuyemi, O.T. Malignant Salivary Gland Tumors. [Updated February 16 2022]; StatPearls Publishing: St. Petersburg, FL, USA, January 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563022/ (accessed on 24 July 2022).
- Zhan, K.Y.; Khaja, S.F.; Flack, A.B.; Day, T.A. Benign Parotid Tumors. Otolaryngol. Clin. N. Am. 2016, 49, 327–342. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).