Recent HIV Infection: Diagnosis and Public Health Implications
Abstract
:1. Introduction
2. What Is Recent HIV Infection and Why It Is Important
3. Identification and Diagnosis of Acute/Recent HIV Infection
4. Use of Recency Assays in HIV Incidence Estimations
5. Interventions Based on Assays to Detect Recent HIV Infection
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2021; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2021. [Google Scholar]
- Nikolopoulos, G.; Bonovas, S.; Tsantes, A.; Sitaras, N.M. HIV/AIDS: Recent Advances in Antiretroviral Agents. Mini-Rev. Med. Chem. 2009, 9, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.N.; Adhikari, R.; Karki, S.; Koirala, A.K.; Wasti, S.P. Life Expectancy and Disparities in Survival among HIV-Infected People Receiving Antiretroviral Therapy: An Observational Cohort Study in Kathmandu, Nepal. BMJ Glob. Health 2019, 4, e001319. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.L.; Rebeiro, P.F.; Cesar, C.; Belaunzaran-Zamudio, P.F.; Crabtree-Ramirez, B.; Padgett, D.; Gotuzzo, E.; Cortes, C.P.; Pape, J.; Veloso, V.G.; et al. Estimated Life Expectancy Gains with Antiretroviral Therapy among Adults with HIV in Latin America and the Caribbean: A Multisite Retrospective Cohort Study. Lancet HIV 2021, 8, e266–e273. [Google Scholar] [CrossRef]
- Trickey, A.; May, M.T.; Vehreschild, J.-J.; Obel, N.; Gill, M.J.; Crane, H.M.; Boesecke, C.; Patterson, S.; Grabar, S.; Cazanave, C.; et al. Survival of HIV-Positive Patients Starting Antiretroviral Therapy between 1996 and 2013: A Collaborative Analysis of Cohort Studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef] [Green Version]
- Giannou, F.K.; Tsiara, C.G.; Nikolopoulos, G.K.; Talias, M.; Benetou, V.; Kantzanou, M.; Bonovas, S.; Hatzakis, A. Condom Effectiveness in Reducing Heterosexual HIV Transmission: A Systematic Review and Meta-Analysis of Studies on HIV Serodiscordant Couples. Expert Rev. Pharm. Outcomes Res. 2016, 16, 489–499. [Google Scholar] [CrossRef]
- Nikolopoulos, G.; Tsiodras, S.; Bonovas, S.; Hatzakis, A. Antiretrovirals for HIV Exposure Prophylaxis. Curr. Med. Chem. 2012, 19, 5924–5939. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Christaki, E.; Paraskevis, D.; Bonovas, S. Pre-Exposure Prophylaxis for HIV: Evidence and Perspectives. Curr. Pharm. Des. 2017, 23, 2579–2591. [Google Scholar] [CrossRef]
- Molina, J.M.; Charreau, I.; Spire, B.; Cotte, L.; Chas, J.; Capitant, C.; Tremblay, C.; Rojas-Castro, D.; Cua, E.; Pasquet, A.; et al. Efficacy, Safety, and Effect on Sexual Behaviour of on-Demand Pre-Exposure Prophylaxis for HIV in Men Who Have Sex with Men: An Observational Cohort Study. Lancet HIV 2017, 4, e402–e410. [Google Scholar] [CrossRef] [Green Version]
- Antoni, G.; Tremblay, C.; Delaugerre, C.; Charreau, I.; Cua, E.; Rojas Castro, D.; Raffi, F.; Chas, J.; Huleux, T.; Spire, B.; et al. On-Demand Pre-Exposure Prophylaxis with Tenofovir Disoproxil Fumarate plus Emtricitabine among Men Who Have Sex with Men with Less Frequent Sexual Intercourse: A Post-Hoc Analysis of the ANRS IPERGAY Trial. Lancet HIV 2020, 7, e113–e120. [Google Scholar] [CrossRef]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.S.; et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; van Lunzen, J.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Sexual Activity without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016, 316, 171–181. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Phillips, A.N.; Bruun, T.; Raben, D.; Lundgren, J.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; et al. Risk of HIV Transmission through Condomless Sex in Serodifferent Gay Couples with the HIV-Positive Partner Taking Suppressive Antiretroviral Therapy (PARTNER): Final Results of a Multicentre, Prospective, Observational Study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef] [Green Version]
- Bavinton, B.R.; Pinto, A.N.; Phanuphak, N.; Grinsztejn, B.; Prestage, G.P.; Zablotska-Manos, I.B.; Jin, F.; Fairley, C.K.; Moore, R.D.; Roth, N.; et al. Viral Suppression and HIV Transmission in Serodiscordant Male Couples: An International, Prospective, Observational, Cohort Study. Lancet HIV 2018, 5, e438–e447. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Sypsa, V.; Bonovas, S.; Paraskevis, D.; Malliori-Minerva, M.; Hatzakis, A.; Friedman, S.R. Big Events in Greece and HIV Infection among People Who Inject Drugs. Subst. Use Misuse 2015, 50, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.R.; Mateu-Gelabert, P.; Nikolopoulos, G.K.; Cerdá, M.; Rossi, D.; Jordan, A.E.; Townsend, T.; Khan, M.R.; Perlman, D.C. Big Events Theory and Measures May Help Explain Emerging Long-Term Effects of Current Crises. Glob. Public Health 2021, 16, 1167–1186. [Google Scholar] [CrossRef]
- Friedman, S.R.; Jordan, A.E.; Perlman, D.C.; Nikolopoulos, G.K.; Mateu-Gelabert, P. Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective. Int. J. Environ. Res. Public Health 2022, 19, 995. [Google Scholar] [CrossRef]
- Kim, J.; Vasan, S.; Kim, J.H.; Ake, J.A. Current Approaches to HIV Vaccine Development: A Narrative Review. J. Int. AIDS Soc. 2021, 24, e25793. [Google Scholar] [CrossRef]
- Cohen, J. A Campaign to End AIDS by 2030 Is Faltering Worldwide. Science 2018. [Google Scholar] [CrossRef]
- Fauci, A.S.; Redfield, R.R.; Sigounas, G.; Weahkee, M.D.; Giroir, B.P. Ending the HIV Epidemic. JAMA 2019, 321, 844. [Google Scholar] [CrossRef]
- Fisher, M.; Pao, D.; Brown, A.E.; Sudarshi, D.; Gill, O.N.; Cane, P.; Buckton, A.J.; Parry, J.V.; Johnson, A.M.; Sabin, C.; et al. Determinants of HIV-1 Transmission in Men Who Have Sex with Men: A Combined Clinical, Epidemiological and Phylogenetic Approach. AIDS 2010, 24, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Pilcher, C.D.; Keating, S.M.; Kassanjee, R.; Facente, S.N.; Welte, A.; Grebe, E.; Marson, K.; Busch, M.P.; Dailey, P.; et al. Moving towards a Reliable HIV Incidence Test-Current Status, Resources Available, Future Directions and Challenges Ahead. Epidemiol. Infect. 2017, 145, 925–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO/UNAIDS. When and How to Use Assays for Recent Infection to Estimate HIV Incidence at Population Level; WHO/UNAIDS: Geneva, Switzerland, 2011. [Google Scholar]
- Miller, W.C.; Rosenberg, N.E.; Rutstein, S.E.; Powers, K.A. Role of Acute and Early HIV Infection in the Sexual Transmission of HIV. Curr. Opin. HIV AIDS 2010, 5, 277–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, G.D.; Haynes, B.F. HIV-1-Specific Antibody Responses during Acute and Chronic HIV-1 Infection. Curr. Opin. HIV AIDS 2009, 4, 373–379. [Google Scholar] [CrossRef] [Green Version]
- US Centers for Disease Control and Prevention; Association of Public Health Laboratories. Laboratory Testing for the Diagnosis of HIV Infection. Updated Recommendations; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014.
- Cohen, M.S.; Shaw, G.M.; McMichael, A.J.; Haynes, B.F. Acute HIV-1 Infection. N. Engl. J. Med. 2011, 364, 1943–1954. [Google Scholar] [CrossRef] [Green Version]
- Peters, P.J.; Westheimer, E.; Cohen, S.; Hightow-Weidman, L.B.; Moss, N.; Tsoi, B.; Hall, L.; Fann, C.; Daskalakis, D.C.; Beagle, S.; et al. Screening Yield of HIV Antigen/Antibody Combination and Pooled HIV RNA Testing for Acute HIV Infection in a High-Prevalence Population. JAMA J. Am. Med. Assoc. 2016, 315, 682–690. [Google Scholar] [CrossRef]
- Parekh, B.S.; Ou, C.Y.; Fonjungo, P.N.; Kalou, M.B.; Rottinghaus, E.; Puren, A.; Alexander, H.; Cox, M.H.; Nkengasong, J.N. Diagnosis of Human Immunodeficiency Virus Infection. Clin. Microbiol. Rev. 2019, 32, e00064-18. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.R.; Bain, R.; Varsaneux, O.; Peeling, R.W.; Stevens, M.M.; McKendry, R.A. P24 Revisited: A Landscape Review of Antigen Detection for Early HIV Diagnosis. AIDS 2018, 32, 2089–2102. [Google Scholar] [CrossRef] [Green Version]
- Murphy, G.; Parry, J. V Assays for the Detection of Recent Infections with Human Immunodeficiency Virus Type 1. Eurosurveillance 2008, 13, 18966. [Google Scholar] [CrossRef]
- Eshleman, S.H.; Laeyendecker, O.; Kammers, K.; Chen, A.; Sivay, M.V.; Kottapalli, S.; Sie, B.M.; Yuan, T.; Monaco, D.R.; Mohan, D.; et al. Comprehensive Profiling of HIV Antibody Evolution. Cell Rep. 2019, 27, 1422–1433. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Decker, J.M.; Wang, S.; Hui, H.; Kappes, J.C.; Wu, X.; Salazar-Gonzalez, J.F.; Salazar, M.G.; Kilby, J.M.; Saag, M.S.; et al. Antibody Neutralization and Escape by HIV-1. Nature 2003, 422, 307–312. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Yates, N.L.; Liu, P.; Qin, L.; Fouda, G.G.; Chavez, L.L.; Decamp, A.C.; Parks, R.J.; Ashley, V.C.; Lucas, J.T.; et al. Initial B-Cell Responses to Transmitted Human Immunodeficiency Virus Type 1: Virion-Binding Immunoglobulin M (IgM) and IgG Antibodies Followed by Plasma Anti-Gp41 Antibodies with Ineffective Control of Initial Viremia. J. Virol. 2008, 82, 12449–12463. [Google Scholar] [CrossRef] [Green Version]
- Colfax, G.N.; Buchbinder, S.P.; Cornelisse, P.G.A.; Vittinghoff, E.; Mayer, K.; Celum, C. Sexual Risk Behaviors and Implications for Secondary HIV Transmission during and after HIV Seroconversion. AIDS 2002, 16, 1529–1535. [Google Scholar] [CrossRef]
- Fox, J.; White, P.J.; Macdonald, N.; Weber, J.; McClure, M.; Fidler, S.; Ward, H. Reductions in HIV Transmission Risk Behaviour Following Diagnosis of Primary HIV Infection: A Cohort of High-Risk Men Who Have Sex with Men. HIV Med. 2009, 10, 432–438. [Google Scholar] [CrossRef]
- Eaton, J.W.; Hallett, T.B.; Garnett, G.P. Concurrent Sexual Partnerships and Primary HIV Infection: A Critical Interaction. AIDS Behav. 2011, 15, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhong, L.; Romero-Severson, E.; Alam, S.J.; Henry, C.J.; Volz, E.M.; Koopman, J.S. Episodic HIV Risk Behavior Can Greatly Amplify HIV Prevalence and the Fraction of Transmissions from Acute HIV Infection. Stat. Commun. Infect. Dis. 2012, 4, 1041. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.S.; Demers, K.; Kwok, C.; Bulime, S.; Rinaldi, A.; Munjoma, M.; Dunbar, M.; Chipato, T.; Byamugisha, J.; Van Der Pol, B.; et al. Plasma and Cervical Viral Loads among Ugandan and Zimbabwean Women during Acute and Early HIV-1 Infection. AIDS 2010, 24, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, C.D.; Joaki, G.; Hoffman, I.F.; Martinson, F.E.A.; Mapanje, C.; Stewart, P.W.; Powers, K.A.; Galvin, S.; Chilongozi, D.; Gama, S.; et al. Amplified Transmission of HIV-1: Comparison of HIV-1 Concentrations in Semen and Blood during Acute and Chronic Infection. AIDS 2007, 21, 1723–1730. [Google Scholar] [CrossRef] [Green Version]
- Quinn, T.C.; Wawer, M.J.; Sewankambo, N.; Serwadda, D.; Li, C.; Wabwire-Mangen, F.; Meehan, M.O.; Lutalo, T.; Gray, R.H. Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1. Rakai Project Study Group. N. Engl. J. Med. 2000, 342, 921–929. [Google Scholar] [CrossRef]
- Hollingsworth, T.D.; Anderson, R.M.; Fraser, C. HIV-1 Transmission, by Stage of Infection. J. Infect. Dis. 2008, 198, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellan, S.E.; Dushoff, J.; Galvani, A.P.; Meyers, L.A. Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts. PLoS Med. 2015, 12, e1001801. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.G.; Roger, M.; Routy, J.-P.; Moisi, D.; Ntemgwa, M.; Matte, C.; Baril, J.-G.; Thomas, R.; Rouleau, D.; Bruneau, J.; et al. High Rates of Forward Transmission Events after Acute/Early HIV-1 Infection. J. Infect. Dis. 2007, 195, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Marzel, A.; Shilaih, M.; Yang, W.-L.; Böni, J.; Yerly, S.; Klimkait, T.; Aubert, V.; Braun, D.L.; Calmy, A.; Furrer, H.; et al. HIV-1 Transmission During Recent Infection and During Treatment Interruptions as Major Drivers of New Infections in the Swiss HIV Cohort Study. Clin. Infect. Dis. 2016, 62, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiridou, M.; Geskus, R.; de Wit, J.; Coutinho, R.; Kretzschmar, M. Primary HIV Infection as Source of HIV Transmission within Steady and Casual Partnerships among Homosexual Men. AIDS 2004, 18, 1311–1320. [Google Scholar] [CrossRef] [Green Version]
- Powers, K.A.; Ghani, A.C.; Miller, W.C.; Hoffman, I.F.; Pettifor, A.E.; Kamanga, G.; Martinson, F.E.; Cohen, M.S. The Role of Acute and Early HIV Infection in the Spread of HIV and Implications for Transmission Prevention Strategies in Lilongwe, Malawi: A Modelling Study. Lancet 2011, 378, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Eaton, J.W.; Hallett, T.B. Why the Proportion of Transmission during Early-Stage HIV Infection Does Not Predict the Long-Term Impact of Treatment on HIV Incidence. Proc. Natl. Acad. Sci. USA 2014, 111, 16202–16207. [Google Scholar] [CrossRef] [Green Version]
- Powers, K.A.; Kretzschmar, M.E.; Miller, W.C.; Cohen, M.S. Impact of Early-Stage HIV Transmission on Treatment as Prevention. Proc. Natl. Acad. Sci. USA 2014, 111, 15867–15868. [Google Scholar] [CrossRef] [Green Version]
- Escudero, D.J.; Lurie, M.N.; Mayer, K.H.; Weinreb, C.; King, M.; Galea, S.; Friedman, S.R.; Marshall, B.D.L. Acute HIV Infection Transmission among People Who Inject Drugs in a Mature Epidemic Setting. AIDS 2016, 30, 2537–2544. [Google Scholar] [CrossRef]
- UNAIDS/WHO. Guidelines on Surveillance among Populations Most at Risk for HIV; UNAIDS/WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Buthelezi, U.E.; Davidson, C.L.; Kharsany, A.B.M. Strengthening HIV Surveillance: Measurements to Track the Epidemic in Real Time. African J. AIDS Res. 2016, 15, 89–98. [Google Scholar] [CrossRef]
- Facente, S.N.; Grebe, E.; Maher, A.D.; Fox, D.; Scheer, S.; Mahy, M.; Dalal, S.; Lowrance, D.; Marsh, K. Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review. JMIR Public Health Surveill. 2022, 8, e34410. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Liu, J.; Duan, X.; Chen, M.; Yang, J.; Yang, T.; Yang, S.; Guan, P.; Jiang, Y.; et al. Identifying Major Drivers of Incident HIV Infection Using Recent Infection Testing Algorithms (RITAs) to Precisely Inform Targeted Prevention. Int. J. Infect. Dis. 2020, 101, 131–137. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; Ozer, E.A.; Achenbach, C.J.; D’Aquila, R.T.; Hultquist, J.F. Molecular Epidemiology in the HIV and SARS-CoV-2 Pandemics. Curr. Opin. HIV AIDS 2021, 16, 11–24. [Google Scholar] [CrossRef]
- Janssen, R.S.; Satten, G.A.; Stramer, S.L.; Rawal, B.D.; O’Brien, T.R.; Weiblen, B.J.; Hecht, F.M.; Jack, N.; Cleghorn, F.R.; Kahn, J.O.; et al. New Testing Strategy to Detect Early HIV-1 Infection for Use in Incidence Estimates and for Clinical and Prevention Purposes. J. Am. Med. Assoc. 1998, 280, 42–48. [Google Scholar] [CrossRef]
- Parekh, B.S.; Hu, D.J.; Vanichseni, S.; Satten, G.A.; Candal, D.; Young, N.L.; Kitayaporn, D.; Srisuwanvilai, L.O.; Rakhtam, S.; Janssen, R.; et al. Evaluation of a Sensitive/Less-Sensitive Testing Algorithm Using the 3A11-LS Assay for Detecting Recent HIV Seroconversion among Individuals with HIV-1 Subtype B or E Infection in Thailand. AIDS Res. Hum. Retrovir. 2001, 17, 453–458. [Google Scholar] [CrossRef]
- Young, C.L.; Hu, D.J.; Byers, R.; Vanichseni, S.; Young, N.L.; Nelson, R.; Mock, P.A.; Choopanya, K.; Janssen, R.; Mastro, T.D.; et al. Evaluation of a Sensitive/Less Sensitive Testing Algorithm Using the BioMérieux Vironostika-LS Assay for Detecting Recent HIV-1 Subtype B’ or E Infection in Thailand. AIDS Res. Hum. Retrovir. 2003, 19, 481–486. [Google Scholar] [CrossRef]
- Rawal, B.D.; Degula, A.; Lebedeva, L.; Janssen, R.S.; Hecht, F.M.; Sheppard, H.W.; Busch, M.P. Development of a New Less-Sensitive Enzyme Immunoassay for Detection of Early HIV-1 Infection. J. Acquir. Immune Defic. Syndr. 2003, 33, 349–355. [Google Scholar] [CrossRef]
- Smoleń-Dzirba, J.; Wasik, T.J. Current and Future Assays for Identifying Recent HIV Infections at the Population Level. Med. Sci. Monit. 2011, 17, RA124–RA133. [Google Scholar] [CrossRef] [Green Version]
- Dobbs, T.; Kennedy, S.; Pau, C.-P.; McDougal, J.S.; Parekh, B.S. Performance Characteristics of the Immunoglobulin G-Capture BED-Enzyme Immunoassay, an Assay to Detect Recent Human Immunodeficiency Virus Type 1 Seroconversion. J. Clin. Microbiol. 2004, 42, 2623–2628. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Liu, X.; Dobbs, T.; Kuehl, D.; Nkengasong, J.N.; Hu, D.J.; Parekh, B.S. Development of Two Avidity-Based Assays to Detect Recent HIV Type 1 Seroconversion Using a Multisubtype Gp41 Recombinant Protein. AIDS Res. Hum. Retrovir. 2010, 26, 61–71. [Google Scholar] [CrossRef]
- Duong, Y.T.; Qiu, M.; De, A.K.; Jackson, K.; Dobbs, T.; Kim, A.A.; Nkengasong, J.N.; Parekh, B.S. Detection of Recent HIV-1 Infection Using a New Limiting-Antigen Avidity Assay: Potential for HIV-1 Incidence Estimates and Avidity Maturation Studies. PLoS ONE 2012, 7, e33328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, Y.T.; Kassanjee, R.; Welte, A.; Morgan, M.; De, A.; Dobbs, T.; Rottinghaus, E.; Nkengasong, J.; Curlin, M.E.; Kittinunvorakoon, C.; et al. Recalibration of the Limiting Antigen Avidity EIA to Determine Mean Duration of Recent Infection in Divergent HIV-1 Subtypes. PLoS ONE 2015, 10, e0114947. [Google Scholar] [CrossRef] [Green Version]
- Cousins, M.M.; Laeyendecker, O.; Beauchamp, G.; Brookmeyer, R.; Towler, W.I.; Hudelson, S.E.; Khaki, L.; Koblin, B.; Chesney, M.; Moore, R.D.; et al. Use of a High Resolution Melting (HRM) Assay to Compare Gag, Pol, and Env Diversity in Adults with Different Stages of HIV Infection. PLoS ONE 2011, 6, e27211. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, X.Y.; He, X.; Yang, S.L.; Ruan, Y.H.; Zhao, Q.B.; Wang, Z.X.; Shao, Y.M.; Pan, X.M. A New Pattern-Based Method for Identifying Recent HIV-1 Infections from the Viral Env Sequence. Sci. China Life Sci. 2012, 55, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostaki, E.G.; Limnaios, S.; Roussos, S.; Psichogiou, M.; Nikolopoulos, G.K.; Friedman, S.R.; Antoniadou, A.; Chini, M.; Hatzakis, A.; Sypsa, V.; et al. Validation of Molecular Clock Inferred HIV Infection Ages: Evidence for Accurate Estimation of Infection Dates. Infect. Genet. Evol. 2021, 91, 104799. [Google Scholar] [CrossRef]
- Kurdekar, A.D.; Chunduri, L.A.A.; Manohar, C.S.; Haleyurgirisetty, M.K.; Hewlett, I.K.; Venkataramaniah, K. Streptavidin-Conjugated Gold Nanoclusters as Ultrasensitive Fluorescent Sensors for Early Diagnosis of HIV Infection. Sci. Adv. 2018, 4, eaar6280. [Google Scholar] [CrossRef] [Green Version]
- Nikolopoulos, G.K.; Kostaki, E.-G.; Paraskevis, D. Overview of HIV Molecular Epidemiology among People Who Inject Drugs in Europe and Asia. Infect. Genet. Evol. 2016, 46, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Esbjörnsson, J.; Månsson, F.; Kvist, A.; da Silva, Z.J.; Andersson, S.; Fenyö, E.M.; Isberg, P.E.; Biague, A.J.; Lindman, J.; Palm, A.A.; et al. Long-Term Follow-up of HIV-2-Related AIDS and Mortality in Guinea-Bissau: A Prospective Open Cohort Study. Lancet HIV 2019, 6, e25–e31. [Google Scholar] [CrossRef]
- Malm, K.; Von Sydow, M.; Andersson, S. Performance of Three Automated Fourth-Generation Combined HIV Antigen/Antibody Assays in Large-Scale Screening of Blood Donors and Clinical Samples. Transfus. Med. 2009, 19, 78–88. [Google Scholar] [CrossRef]
- Salmona, M.; Delarue, S.; Delaugerre, C.; Simon, F.; Maylin, S. Clinical Evaluation of BioPlex 2200 HIV Ag-Ab, an Automated Screening Method Providing Discrete Detection of HIV-1 P24 Antigen, HIV-1 Antibody, and HIV-2 Antibody. J. Clin. Microbiol. 2014, 52, 103–107. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; Department of Health and Human Services: Washington, DC, USA, 2022.
- Ekouévi, D.K.; Avettand-Fènoël, V.; Tchounga, B.K.; Coffie, P.A.; Sawadogo, A.; Minta, D.; Minga, A.; Eholie, S.P.; Plantier, J.C.; Damond, F.; et al. Plasma HIV-2 RNA According to CD4 Count Strata among HIV-2-Infected Adults in the IeDEA West Africa Collaboration. PLoS ONE 2015, 10, e0129886. [Google Scholar] [CrossRef] [Green Version]
- Murugavel, K.; Thakar, M.; Mehendale, S. Recent HIV Infection Testing Algorithms. Indian J. Med. Res. 2020, 152, 181–183. [Google Scholar]
- Parekh, B.S.; Kennedy, M.S.; Dobbs, T.; Pau, C.-P.; Byers, R.; Green, T.; Hu, D.J.; Vanichseni, S.; Young, N.L.; Choopanya, K.; et al. Quantitative Detection of Increasing HIV Type 1 Antibodies after Seroconversion: A Simple Assay for Detecting Recent HIV Infection and Estimating Incidence. AIDS Res. Hum. Retrovir. 2002, 18, 295–307. [Google Scholar] [CrossRef]
- Kassanjee, R.; Pilcher, C.D.; Keating, S.M.; Facente, S.N.; McKinney, E.; Price, M.A.; Martin, J.N.; Little, S.; Hecht, F.M.; Kallas, E.G.; et al. Independent Assessment of Candidate HIV Incidence Assays on Specimens in the CEPHIA Repository. AIDS 2014, 28, 2439–2449. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, M.; Ni, M.; Duan, S.; Wang, Y.; Feng, J.; Xiao, Y.; Dong, Y.; Wang, D.; Han, M.; et al. HIV-1 Incidence Estimates Using IgG-Capture BED-Enzyme Immunoassay from Surveillance Sites of Injection Drug Users in Three Cities of China. AIDS 2007, 21, S47–S51. [Google Scholar] [CrossRef]
- Hargrove, J.; van Schalkwyk, C.; Eastwood, H. Bed Estimates of HIV Incidence: Resolving the Differences, Making Things Simpler. PLoS ONE 2012, 7, e29736. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.S.; Duong, Y.T.; Le, L.V.; Tuan, N.A.; Parekh, B.S.; Ha, H.T.T.; Pham, Q.D.; Cuc, C.T.T.; Dobbs, T.; Tram, T.H.; et al. Estimating False-Recent Classification for the Limiting-Antigen Avidity EIA and BED-Capture Enzyme Immunoassay in Vietnam: Implications for HIV-1 Incidence Estimates. AIDS Res. Hum. Retrovir. 2017, 33, 546–554. [Google Scholar] [CrossRef]
- WHO/UNAIDS. Technical Update on HIV Incidence Assays for Surveillance and Epidemic Monitoring; WHO/UNAIDS: Geneva, Switzerland, 2013. [Google Scholar]
- Nesheim, S.; Parekh, B.; Sullivan, K.; Bulterys, M.; Dobbs, T.; Lindsay, M.; Cashat-Cruz, M.; Byers, B.; Lee, F. Temporal Trends in HIV Type 1 Incidence among Inner-City Childbearing Women in Atlanta: Use of the IgG-Capture BED-Enzyme Immunoassay. AIDS Res. Hum. Retrovir. 2005, 21, 537–544. [Google Scholar] [CrossRef]
- Lakshmi, V.; Sudha, T.; Dandona, R.; Teja, V.D.; Kumar, G.A.; Dandona, L. Application of Human Immunodeficiency Virus Type 1 BED Enzyme Immunoassay on Dried Blood Spots in India. J. Med. Microbiol. 2009, 58, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Bärnighausen, T.; McWalter, T.A.; Rosner, Z.; Newell, M.L.; Welte, A. HIV Incidence Estimation Using the BED Capture Enzyme Immunoassay: Systematic Review and Sensitivity Analysis. Epidemiology 2010, 21, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Hallett, T.B.; Ghys, P.; Bärnighausen, T.; Yan, P.; Garnett, G.P. Errors in ‘BED’-Derived Estimates of HIV Incidence Will Vary by Place, Time and Age. PLoS ONE 2009, 4, e5720. [Google Scholar] [CrossRef]
- Hayashida, T.; Gatanaga, H.; Tanuma, J.; Oka, S. Effects of Low HIV Type 1 Load and Antiretroviral Treatment on IgG-Capture BED-Enzyme Immunoassay. AIDS Res. Hum. Retrovir. 2008, 24, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Laeyendecker, O.; Brookmeyer, R.; Oliver, A.E.; Mullis, C.E.; Eaton, K.P.; Mueller, A.C.; Jacobson, L.P.; Margolick, J.B.; Brown, J.; Rinaldo, C.R.; et al. Factors Associated with Incorrect Identification of Recent HIV Infection Using the BED Capture Immunoassay. AIDS Res. Hum. Retrovir. 2012, 28, 816–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, B.S.; Hanson, D.L.; Hargrove, J.; Branson, B.; Green, T.; Dobbs, T.; Constantine, N.; Overbaugh, J.; McDougal, J.S. Determination of Mean Recency Period for Estimation of HIV Type 1 Incidence with the BED-Capture EIA in Persons Infected with Diverse Subtypes. AIDS Res. Hum. Retrovir. 2011, 27, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Konikoff, J.; Laeyendecker, O.; Eshleman, S.H. Estimation of HIV Incidence Using Multiple Biomarkers. Am. J. Epidemiol. 2013, 177, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Kassanjee, R.; Pilcher, C.D.; Busch, M.P.; Murphy, G.; Facente, S.N.; Keating, S.M.; McKinney, E.; Marson, K.; Price, M.A.; Martin, J.N.; et al. Viral Load Criteria and Threshold Optimization to Improve HIV Incidence Assay Characteristics. AIDS 2016, 30, 2361–2371. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.M.; Hanson, D.; Lebedeva, M.; Laeyendecker, O.; Ali-Napo, N.L.; Owen, S.M.; Stramer, S.L.; Moore, R.D.; Norris, P.J.; Busch, M.P. Lower-Sensitivity and Avidity Modifications of the Vitros Anti-HIV 1+2 Assay for Detection of Recent HIV Infections and Incidence Estimation. J. Clin. Microbiol. 2012, 50, 3968–3976. [Google Scholar] [CrossRef] [Green Version]
- Keating, S.M.; Kassanjee, R.; Lebedeva, M.; Facente, S.N.; Macarthur, J.C.; Grebe, E.; Murphy, G.; Welte, A.; Martin, J.N.; Little, S.; et al. Performance of the Bio-Rad Geenius HIV1/2 Supplemental Assay in Detecting “Recent” HIV Infection and Calculating Population Incidence. J. Acquir. Immune Defic. Syndr. 2016, 73, 581–588. [Google Scholar] [CrossRef]
- Konikoff, J.; Brookmeyer, R.; Longosz, A.F.; Cousins, M.M.; Celum, C.; Buchbinder, S.P.; Seage, G.R.; Kirk, G.D.; Moore, R.D.; Mehta, S.H.; et al. Performance of a Limiting-Antigen Avidity Enzyme Immunoassay for Cross-Sectional Estimation of HIV Incidence in the United States. PLoS ONE 2013, 8, e82772. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.A.; Behel, S.; Northbrook, S.; Parekh, B.S. Tracking with Recency Assays to Control the Epidemic: Real-Time HIV Surveillance and Public Health Response. AIDS 2019, 33, 1527–1529. [Google Scholar] [CrossRef]
- Granade, T.C.; Nguyen, S.; Kuehl, D.S.; Parekh, B.S. Development of a Novel Rapid HIV Test for Simultaneous Detection of Recent or Long-Term HIV Type 1 Infection Using a Single Testing Device. AIDS Res. Hum. Retrovir. 2013, 29, 61–67. [Google Scholar] [CrossRef]
- Yufenyuy, E.L.; Detorio, M.; Dobbs, T.; Patel, H.K.; Jackson, K.; Vedapuri, S.; Parekh, B.S. Performance Evaluation of the Asante Rapid Recency Assay for Verification of HIV Diagnosis and Detection of Recent HIV-1 Infections: Implications for Epidemic Control. PLOS Glob. Public Health 2022, 2, e0000316. [Google Scholar] [CrossRef]
- Chaillon, A.; Le Vu, S.; Brunet, S.; Gras, G.; Bastides, F.; Bernard, L.; Meyer, L.; Barin, F. Decreased Specificity of an Assay for Recent Infection in HIV-1-Infected Patients on Highly Active Antiretroviral Treatment: Implications for Incidence Estimates. Clin. Vaccine Immunol. 2012, 19, 1248–1253. [Google Scholar] [CrossRef] [Green Version]
- Fogel, J.M.; Piwowar-Manning, E.; Debevec, B.; Walsky, T.; Schlusser, K.; Laeyendecker, O.; Wilson, E.A.; McCauley, M.; Gamble, T.; Tegha, G.; et al. Brief Report: Impact of Early Antiretroviral Therapy on the Performance of HIV Rapid Tests and HIV Incidence Assays. J. Acquir. Immune Defic. Syndr. 2017, 75, 426–430. [Google Scholar] [CrossRef]
- Klock, E.; Mwinnya, G.; Eller, L.A.; Fernandez, R.E.; Kibuuka, H.; Nitayaphan, S.; Kosgei, J.; Moore, R.D.; Robb, M.; Eshleman, S.H.; et al. Impact of Early Antiretroviral Treatment Initiation on Performance of Cross-Sectional Incidence Assays. AIDS Res. Hum. Retrovir. 2020, 36, 583–589. [Google Scholar] [CrossRef]
- Wendel, S.K.; Mullis, C.E.; Eshleman, S.H.; Blankson, J.N.; Moore, R.D.; Keruly, J.C.; Brookmeyer, R.; Quinn, T.C.; Laeyendecker, O. Effect of Natural and ARV-Induced Viral Suppression and Viral Breakthrough on Anti-HIV Antibody Proportion and Avidity in Patients with HIV-1 Subtype B Infection. PLoS ONE 2013, 8, e55525. [Google Scholar] [CrossRef] [Green Version]
- Longosz, A.F.; Serwadda, D.; Nalugoda, F.; Kigozi, G.; Franco, V.; Gray, R.H.; Quinn, T.C.; Eshleman, S.H.; Laeyendecker, O. The Impact of HIV Subtype on Specificity of Cross-Sectional HIV Incidence Assays in Rakai, Uganda. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 3–6 March 2014; Topics in Antiviral Medicine. Volume 22, p. 532. [Google Scholar]
- Longosz, A.F.; Morrison, C.S.; Chen, P.L.; Arts, E.; Nankya, I.; Salata, R.A.; Franco, V.; Quinn, T.C.; Eshleman, S.H.; Laeyendecker, O. Immune Responses in Ugandan Women Infected with Subtypes a Anddhiv Using the BED Capture Immunoassay and an Antibody Avidity Assay. J. Acquir. Immune Defic. Syndr. 2014, 65, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Kassanjee, R.; McWalter, T.A.; Bärnighausen, T.; Welte, A. A New General Biomarker-Based Incidence Estimator. Epidemiology 2012, 23, 721–728. [Google Scholar] [CrossRef] [Green Version]
- WHO/UNAIDS. Technical Update on HIV Incidence Assays for Surveillance and Monitoring Purposes; WHO/UNAIDS: Geneva, Switzerland, 2015. [Google Scholar]
- de Wit, M.M.; Rice, B.; Risher, K.; Welty, S.; Waruiru, W.; Magutshwa, S.; Motoku, J.; Kwaro, D.; Ochieng, B.; Reniers, G.; et al. Experiences and Lessons Learned from the Real-World Implementation of an HIV Recent Infection Testing Algorithm in Three Routine Service-Delivery Settings in Kenya and Zimbabwe. BMC Health Serv. Res. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Justman, J.; Reed, J.B.; Bicego, G.; Donnell, D.; Li, K.; Bock, N.; Koler, A.; Philip, N.M.; Mlambo, C.K.; Parekh, B.S.; et al. Swaziland HIV Incidence Measurement Survey (SHIMS): A Prospective National Cohort Study. Lancet HIV 2017, 4, e83–e92. [Google Scholar] [CrossRef] [Green Version]
- Nsanzimana, S.; Remera, E.; Kanters, S.; Mulindabigwi, A.; Suthar, A.B.; Uwizihiwe, J.P.; Mwumvaneza, M.; Mills, E.J.; Bucher, H.C. Household Survey of HIV Incidence in Rwanda: A National Observational Cohort Study. Lancet HIV 2017, 4, e457–e464. [Google Scholar] [CrossRef]
- Saidel, T.; Sokal, D.; Rice, J.; Buzingo, T.; Hassig, S. Validation of a Method to Estimate Age-Specific Human Immunodeficiency Virus (HIV) Incidence Rates in Developing Countries Using Population-Based Seroprevalence Data. Am. J. Epidemiol. 1996, 144, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Hallett, T.B.; Zaba, B.; Todd, J.; Lopman, B.; Mwita, W.; Biraro, S.; Gregson, S.; Boerma, J.T. Estimating Incidence from Prevalence in Generalised HIV Epidemics: Methods and Validation. PLoS Med. 2008, 5, e80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.; Gouws, E.; Wilkinson, D.; Karim, S.A. Estimating HIV Incidence Rates from Age Prevalence Data in Epidemic Situations. Stat. Med. 2001, 20, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Sakarovitch, C.; Alioum, A.; Ekouevi, D.K.; Msellati, P.; Leroy, V.; Dabis, F. Estimating Incidence of HIV Infection in Childbearing Age African Women Using Serial Prevalence Data from Antenatal Clinics. Stat. Med. 2007, 26, 320–335. [Google Scholar] [CrossRef]
- Sun, X.; Nishiura, H.; Xiao, Y. Modeling Methods for Estimating HIV Incidence: A Mathematical Review. Theor. Biol. Med. Model. 2020, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.; Bao, L.; Eaton, J.W.; Hogan, D.R.; Mahy, M.; Marsh, K.; Mathers, B.M.; Puckett, R. Improvements in Prevalence Trend Fitting and Incidence Estimation in EPP 2013. AIDS 2014, 28, S415–S425. [Google Scholar] [CrossRef] [Green Version]
- Stover, J.; Brown, T.; Puckett, R.; Peerapatanapokin, W. Updates to the Spectrum/Estimations and Projections Package Model for Estimating Trends and Current Values for Key HIV Indicators. AIDS 2017, 31, S5–S11. [Google Scholar] [CrossRef]
- Stover, J.; Glaubius, R.; Kassanjee, R.; Dugdale, C.M. Updates to the Spectrum/AIM Model for the UNAIDS 2020 HIV Estimates. J. Int. AIDS Soc. 2021, 24, e25778. [Google Scholar] [CrossRef]
- Mastro, T.D.; Kim, A.A.; Hallett, T.; Rehle, T.; Welte, A.; Laeyendecker, O.; Oluoch, T.; Garcia-Calleja, J.M. Estimating HIV Incidence in Populations Using Tests for Recent Infection: Issues, Challenges and the Way Forward. J. HIV AIDS Surveill. Epidemiol. 2010, 2, 1–14. [Google Scholar]
- Pilcher, C.D.; Fiscus, S.A.; Nguyen, T.Q.; Foust, E.; Wolf, L.; Williams, D.; Ashby, R.; O’Dowd, J.O.; McPherson, J.T.; Stalzer, B.; et al. Detection of Acute Infections during HIV Testing in North Carolina. N. Engl. J. Med. 2005, 352, 1873–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassanjee, R.; De Angelis, D.; Farah, M.; Hanson, D.; Labuschagne, J.P.L.; Laeyendecker, O.; Le Vu, S.; Tom, B.; Wang, R.; Welte, A. Cross-Sectional HIV Incidence Surveillance: A Benchmarking of Approaches for Estimating the ‘Mean Duration of Recent Infection’. Stat. Commun. Infect. Dis. 2017, 9, 20160002. [Google Scholar] [CrossRef] [PubMed]
- WHO Working Group on HIV Incidence Measurement and Data Use: Meeting Report; World Health Organization: Boston, MA, USA, 2018.
- Hargrove, J.; Eastwood, H.; Mahiane, G.; van Schalkwyk, C. How Should We Best Estimate the Mean Recency Duration for the BED Method? PLoS ONE 2012, 7, e49661. [Google Scholar] [CrossRef] [PubMed]
- Kassanjee, R.; Welte, A.; McWalter, T.A.; Keating, S.M.; Vermeulen, M.; Stramer, S.L.; Busch, M.P. Seroconverting Blood Donors as a Resource for Characterising and Optimising Recent Infection Testing Algorithms for Incidence Estimation. PLoS ONE 2011, 6, e20027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, E.H.; Brookmeyer, R. Snapshot Estimators of Recent HIV Incidence Rates. Oper. Res. 1999, 47, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Bannick, M. Statistical Considerations for Cross-sectional HIV Incidence Estimation Based on Recency Test. Stat. Med. 2022, 41, 1446–1461. [Google Scholar] [CrossRef]
- Kassanjee, R.; McWalter, T.A.; Welte, A. Short Communication: Defining Optimality of a Test for Recent Infection for HIV Incidence Surveillance. AIDS Res. Hum. Retrovir. 2014, 30, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Grant-McAuley, W.; Klock, E.; Laeyendecker, O.; Piwowar-Manning, E.; Wilson, E.; Clarke, W.; Breaud, A.; Moore, A.; Ayles, H.; Kosloff, B.; et al. Evaluation of Multi-Assay Algorithms for Identifying Individuals with Recent HIV Infection: HPTN 071 (PopART). PLoS ONE 2021, 16, e0258644. [Google Scholar] [CrossRef]
- Laeyendecker, O.; Konikoff, J.; Morrison, D.E.; Brookmeyer, R.; Wang, J.; Celum, C.; Morrison, C.S.; Abdool Karim, Q.; Pettifor, A.E.; Eshleman, S.H. Identification and Validation of a Multi-Assay Algorithm for Cross-Sectional HIV Incidence Estimation in Populations with Subtype C Infection. J. Int. AIDS Soc. 2018, 21, e25082. [Google Scholar] [CrossRef]
- Nikolopoulos, G.K.; Katsoulidou, A.; Kantzanou, M.; Rokka, C.; Tsiara, C.; Sypsa, V.; Paraskevis, D.; Psichogiou, M.; Friedman, S.; Hatzakis, A. Evaluation of the Limiting Antigen Avidity EIA (LAg) in People Who Inject Drugs in Greece. Epidemiol. Infect. 2017, 145, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Laeyendecker, O.; Brookmeyer, R.; Cousins, M.M.; Mullis, C.E.; Konikoff, J.; Donnell, D.; Celum, C.; Buchbinder, S.P.; Seage, G.R.; Kirk, G.D.; et al. HIV Incidence Determination in the United States: A Multiassay Approach. J. Infect. Dis. 2013, 207, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Voetsch, A.C.; Duong, Y.T.; Stupp, P.; Saito, S.; McCracken, S.; Dobbs, T.; Winterhalter, F.S.; Williams, D.B.; Mengistu, A.; Mugurungi, O.; et al. HIV-1 Recent Infection Testing Algorithm with Antiretroviral Drug Detection to Improve Accuracy of Incidence Estimates. J. Acquir. Immune Defic. Syndr. 2021, 87, S73–S80. [Google Scholar] [CrossRef]
- Duong, Y.T.; Dobbs, T.; Mavengere, Y.; Manjengwa, J.; Rottinghaus, E.; Saito, S.; Bock, N.; Philip, N.; Justman, J.; Bicego, G.; et al. Field Validation of Limiting-Antigen Avidity Enzyme Immunoassay to Estimate HIV-1 Incidence in Cross-Sectional Survey in Swaziland. AIDS Res. Hum. Retrovir. 2019, 35, 896–905. [Google Scholar] [CrossRef]
- Fellows, I.E.; Hladik, W.; Eaton, J.W.; Voetsch, A.C.; Parekh, B.S.; Shiraishi, R.W. Improving Biomarker Based HIV Incidence Estimation in the Treatment Era. arXiv 2022, arXiv:2204.00048. [Google Scholar]
- Kroon, E.D.M.B.; Phanuphak, N.; Shattock, A.J.; Fletcher, J.L.K.; Pinyakorn, S.; Chomchey, N.; Akapirat, S.; De Souza, M.S.; Robb, M.L.; Kim, J.H.; et al. Acute HIV Infection Detection and Immediate Treatment Estimated to Reduce Transmission by 89% among Men Who Have Sex with Men in Bangkok. J. Int. AIDS Soc. 2017, 20, 21708. [Google Scholar] [CrossRef]
- Chauhan, C.; Lakshmi, P.V.M.; Sagar, V.; Sharma, A.; Arora, S.; Kumar, R. Immunological Markers for Identifying Recent HIV Infection in North-West India. Indian J. Med. Res. 2020, 152, 227–233. [Google Scholar] [CrossRef]
- Kim, A.A.; Parekh, B.S.; Umuro, M.; Galgalo, T.; Bunnell, R.; Makokha, E.; Dobbs, T.; Murithi, P.; Muraguri, N.; De Cock, K.M.; et al. Identifying Risk Factors for Recent HIV Infection in Kenya Using a Recent Infection Testing Algorithm: Results from a Nationally Representative Population-Based Survey. PLoS ONE 2016, 11, e0155498. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.R.; Downing, M.J.; Smyrnov, P.; Nikolopoulos, G.; Schneider, J.A.; Livak, B.; Magiorkinis, G.; Slobodianyk, L.; Vasylyeva, T.I.; Paraskevis, D.; et al. Socially-Integrated Transdisciplinary HIV Prevention. AIDS Behav. 2014, 18, 1821–1834. [Google Scholar] [CrossRef]
- Smyrnov, P.; Williams, L.D.; Korobchuk, A.; Sazonova, Y.; Nikolopoulos, G.K.; Skaathun, B.; Morgan, E.; Schneider, J.; Vasylyeva, T.I.; Friedman, S.R. Risk Network Approaches to Locating Undiagnosed HIV Cases in Odessa, Ukraine. J. Int. AIDS Soc. 2018, 21, e25040. [Google Scholar] [CrossRef]
- Schueler, K.; Ferreira, M.; Nikolopoulos, G.; Skaathun, B.; Paraskevis, D.; Hatzakis, A.; Friedman, S.R.; Schneider, J.A. Pre-Exposure Prophylaxis (PrEP) Awareness and Use within High HIV Transmission Networks. AIDS Behav. 2019, 23, 1893–1903. [Google Scholar] [CrossRef]
- Morgan, E.; Skaathun, B.; Nikolopoulos, G.K.; Paraskevis, D.; Williams, L.D.; Smyrnov, P.; Friedman, S.R.; Schneider, J.A. A Network Intervention to Locate Newly HIV Infected Persons within MSM Networks in Chicago. AIDS Behav. 2019, 23, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, G.K.; Pavlitina, E.; Muth, S.Q.; Schneider, J.; Psichogiou, M.; Williams, L.D.; Paraskevis, D.; Sypsa, V.; Magiorkinis, G.; Smyrnov, P.; et al. A Network Intervention That Locates and Intervenes with Recently HIV-Infected Persons: The Transmission Reduction Intervention Project (TRIP). Sci. Rep. 2016, 6, 38100. [Google Scholar] [CrossRef] [PubMed]
- Psichogiou, M.; Giallouros, G.; Pantavou, K.; Pavlitina, E.; Papadopoulou, M.; Williams, L.D.; Hadjikou, A.; Kakalou, E.; Skoutelis, A.; Protopapas, K.; et al. Identifying, Linking, and Treating People Who Inject Drugs and Were Recently Infected with HIV in the Context of a Network-Based Intervention. AIDS Care 2019, 31, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Korobchuk, A.; Pavlitina, E.; Nikolopoulos, G.K.; Skaathun, B.; Schneider, J.; Kostaki, E.G.; Smyrnov, P.; Vasylyeva, T.I.; Psichogiou, M.; et al. Experiences of Stigma and Support Reported by Participants in a Network Intervention to Reduce HIV Transmission in Athens, Greece; Odessa, Ukraine; and Chicago, Illinois. AIDS Behav. 2019, 23, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Quinn, T.C. Estimation of Current Human Immunodeficiency Virus Incidence Rates from a Cross-Sectional Survey Using Early Diagnostic Tests. Am. J. Epidemiol. 1995, 141, 166–172. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Liu, C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV Virus. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Tian, X.; Wang, Y.; Li, L.; Yu, Y.; Zhao, S.; Zhang, J. CRISPR-Cas12a-Activated Palindrome-Catalytic Hairpin Assembly for Ultrasensitive Fluorescence Detection of HIV-1 DNA. Anal. Chim. Acta 2022, 1227, 340303. [Google Scholar] [CrossRef]
- FIND and WHO Working Group on HIV Incidence Assays: Meeting Report; World Health Organization: Geneva, Switzerland, 2017.
- Fowler, L.; Saksena, N.K. Micro-RNA: New Players in HIV-Pathogenesis, Diagnosis, Prognosis and Antiviral Therapy. AIDS Rev. 2013, 15, 3–14. [Google Scholar]
- Zhao, J.; Ao, C.; Wan, Z.; Dzakah, E.E.; Liang, Y.; Lin, H.; Wang, H.; Tang, S. A Point-of-Care Rapid HIV-1 Test Using an Isothermal Recombinase-Aided Amplification and CRISPR Cas12a-Mediated Detection. Virus Res. 2021, 303, 198505. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and Visual Detection of SARS-CoV-2 Using All-in-One Dual CRISPR-Cas12a Assay. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
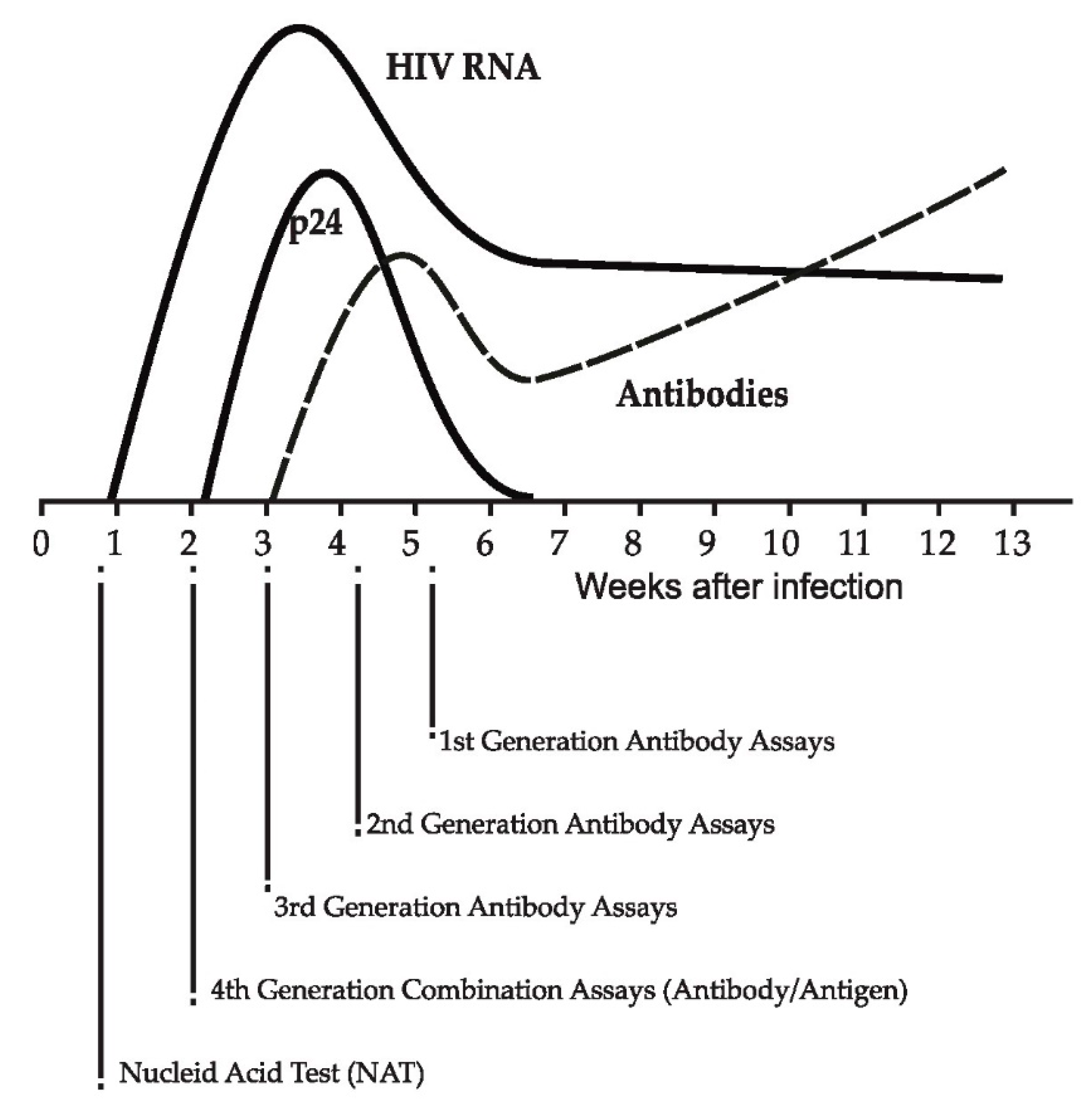
| Assay | Period | Biomarker | Example-Technique |
|---|---|---|---|
| Antigen-based test | Pre-seroconversion (0–4 weeks) | Viral protein (p24) | Fourth generation antibody/antigen combination assay [28] |
| Nucleic acid amplification test (NAT) | Pre-seroconversion (0–4 weeks) | Viral genome | HIV RNA PCR test [28] |
| Detuned: | Post-seroconversion | Host marker—rising antibody titer after seroconversion | Sensitive/less sensitive EIA (first generation EIA) [58,59,60,61,62] |
| BED-EIA: | Post-seroconversion | Host marker—Relative ratio of HIV specific IgG to total IgG | Capture enzyme immunoassay using a multi-peptide from subtypes B and D, and Circulating Recombinant Form_01 AE [62,63] |
| Avidity EIA: | Post-seroconversion | Host marker—Avidity of antibodies with antigens | Limiting Antigen Avidity assay (LAg-EIA) [64,65,66] |
| Other methods/new technologies | - Based on the genetic diversity of HIV (people with recent HIV infection probably have less genetic diversity than people with long-term HIV infection) [67,68,69] - Based on “HIV serosignature” (measuring antibody reactivity to a panel of peptides associated with recent HIV infection) [34] - Gold nanocluster immunoassay (gold nanoclusters conjugated with streptavidin are used as ultrasensitive fluorescent sensors for the detection of HIV antigens) [70] | ||
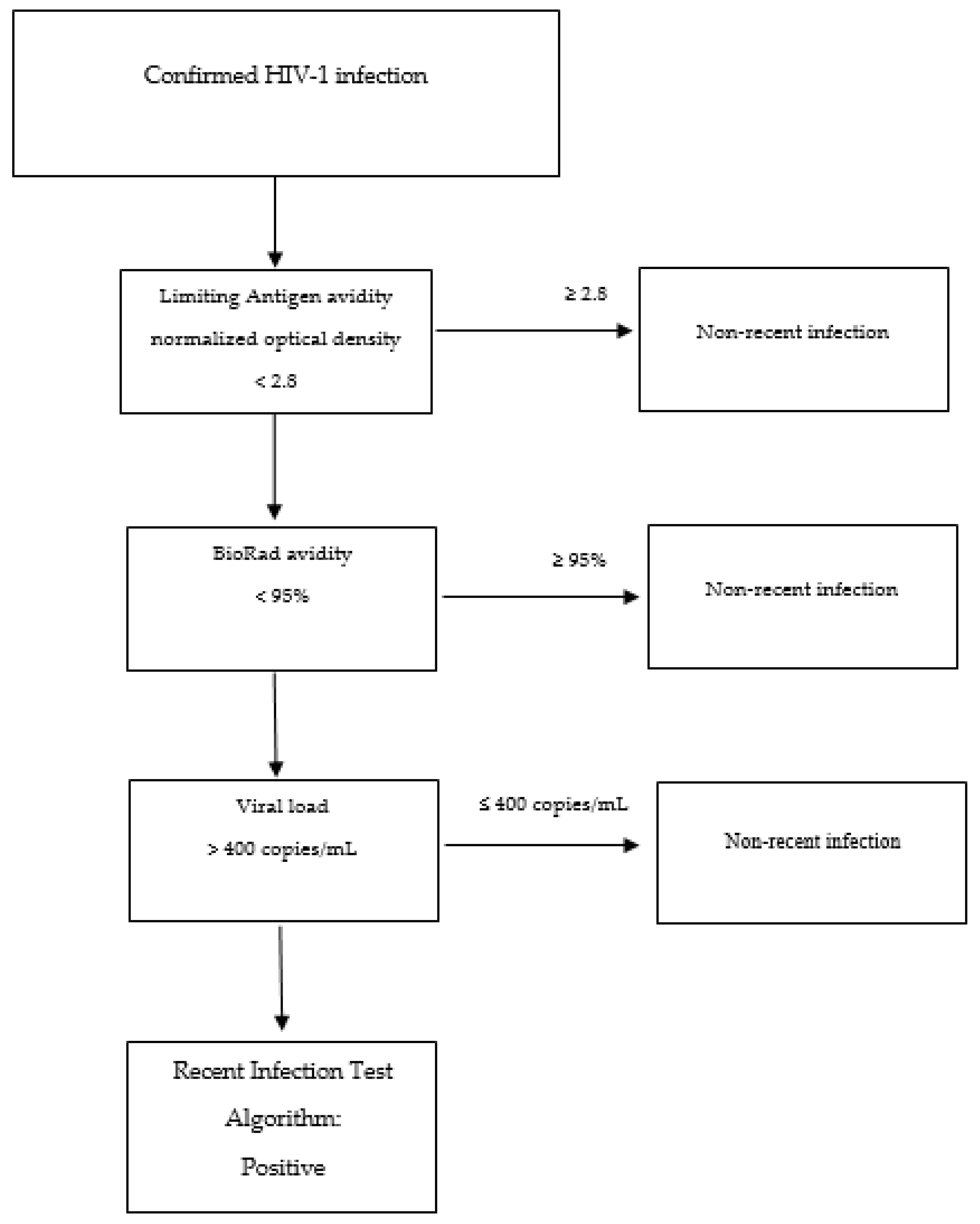
| RITA | Recency Test 1 | Recency Test 2 | Viral Load (Copies/mL) | ART | Another Marker/Condition (Cells/mm for CD4 T-Cell Count) | Reference |
|---|---|---|---|---|---|---|
| A | LAg Avidity-EIA (<1.5 ODn) | NA | >1000 | NA | NA | [56,66,95] |
| B | LAg Avidity-EIA (<1.5 ODn) | NA | >1000 | NA | NAT for HIV seronegative people | [132] |
| C | LAg Avidity-EIA (<1.5 ODn) | NA | >1000 | No ART | NA | [107,131] |
| D | LAg Avidity-EIA (<1.5 ODn) | NA | >1000 | No ART | CD4 T-cell > 200 and no AIDS | [129] |
| E | Avidity test (<80%) | BED-EIA (<1 ODn) | >400 | NA | CD4 T-cell > 200 | [130] |
| F | LAg Avidity-EIA (<2.8 ODn) | BioRad-avidity (<95%) | >400 | NA | NA | [127,128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolopoulos, G.K.; Tsantes, A.G. Recent HIV Infection: Diagnosis and Public Health Implications. Diagnostics 2022, 12, 2657. https://doi.org/10.3390/diagnostics12112657
Nikolopoulos GK, Tsantes AG. Recent HIV Infection: Diagnosis and Public Health Implications. Diagnostics. 2022; 12(11):2657. https://doi.org/10.3390/diagnostics12112657
Chicago/Turabian StyleNikolopoulos, Georgios K., and Andreas G. Tsantes. 2022. "Recent HIV Infection: Diagnosis and Public Health Implications" Diagnostics 12, no. 11: 2657. https://doi.org/10.3390/diagnostics12112657







