Challenging Diagnosis of Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery
Abstract
:1. Introduction
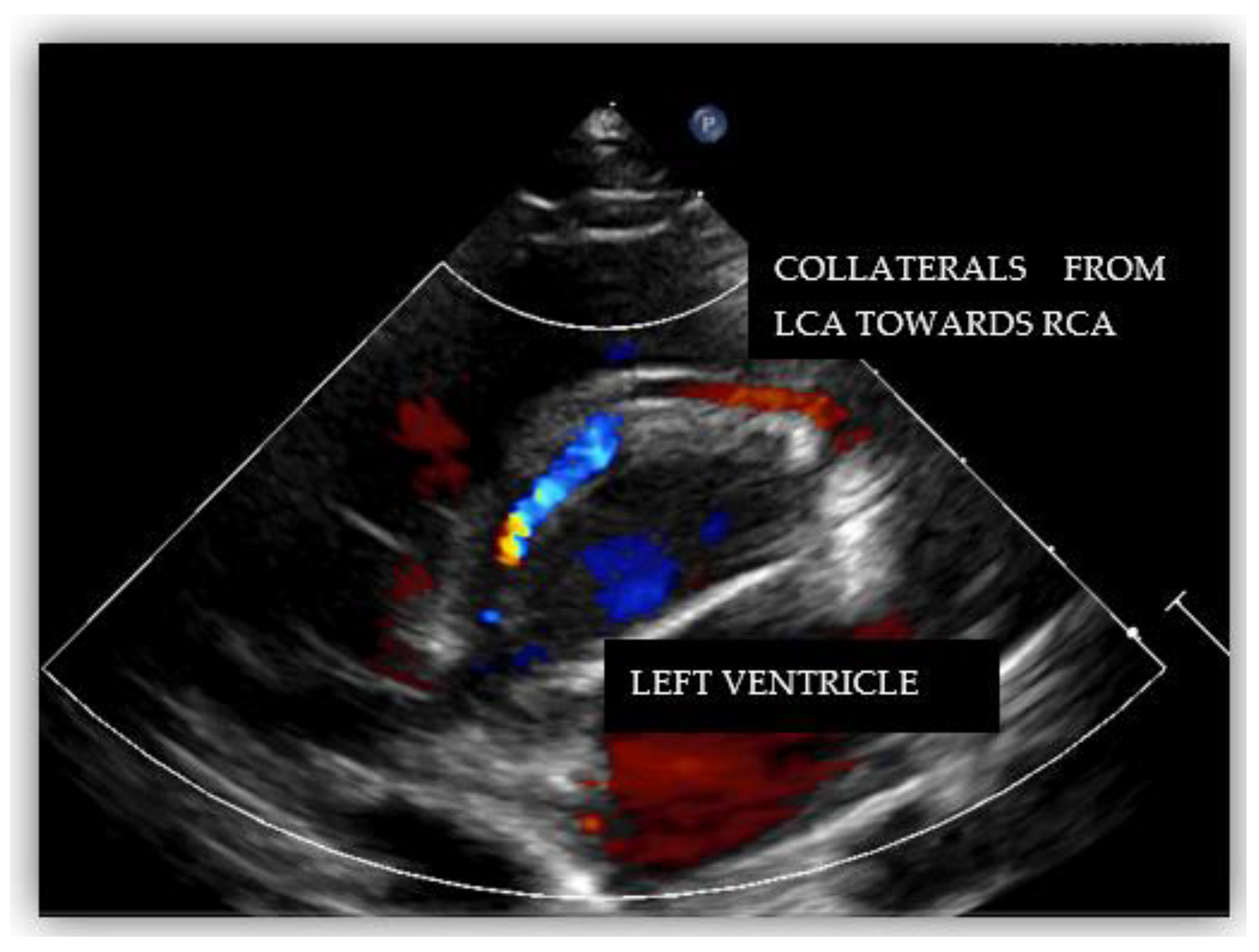
2. Case Report #1
3. Case Report #2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.D.; Sammut, E.; Nair, A.; Rajani, R.; Bonamini, R.; Chiribiri, A. Coronary artery anomalies overview: The normal and the abnormal. World J. Radiol. 2016, 8, 537–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, I.A.; Gersony, W.M.; Hellenbrand, W.E. Anomalous right coronary artery arising from the pulmonary artery: A report of 7 cases and a review of the literature. Am. Heart J. 2006, 152, 1004.e9–1004.e17. [Google Scholar] [CrossRef]
- Brooks, H.S.J. Two cases of an abnormal coronary artery of the heart, arising from the pulmonary artery, with some remarks upon the effect of this anomaly in producing cirsoid dilatation of the vessels. Trans. Acad. Med. Irel. 1885, 3, 447–449. [Google Scholar]
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet. Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. Major anomalies of coronary arterial origin seen in adulthood. Am. Heart J. 1986, 111, 941–963. [Google Scholar] [CrossRef]
- Teng, P.; Li, W.; Ni, Y. Surgical treatment for anomalous origin of the right coronary artery from the pulmonary artery: A case report with five-year follow-up. J. Cardiothorac. Surg. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Hekmat, V.; Rao, S.M.; Chhabra, M.; Chiavarelli, M.; Anderson, J.E.; Nudel, D.B. Anomalous origin of the right coronary artery from the main pulmonary artery: Diagnosis and management. Clin. Cardiol. 1998, 21, 773–776. [Google Scholar] [CrossRef]
- Heifetz, S.A.; Robinowitz, M.; Mueller, K.H.; Virmani, R. Total anomalous origin of the coronary arteries from the pulmonary artery. Pediatr. Cardiol. 1986, 7, 11–18. [Google Scholar] [CrossRef]
- Yildiz, A.; Okcun, B.; Peker, T.; Arslan, C.; Olcay, A.; Vatan, M.B. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin. Cardiol. 2010, 33, 60–64. [Google Scholar] [CrossRef]
- Kumar, P. Congenital Coronary Artery-to-Pulmonary Artery Fistula with Anomalous Origin of Right Coronary Artery from Pulmonary Artery: A Case of "Double Trouble". Radiol. Cardiothorac. Imaging 2021, 3, e210003. [Google Scholar] [CrossRef] [PubMed]
- Stefek, B.P.; Imundo, J.R.; Clark, J.B. Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery in a Neonate with Turner Syndrome and Aortic Arch Hypoplasia. Tex. Heart Inst. J. 2019, 46, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Iskandrian, A.S.; Bemis, C.E.; Mundth, E.D.; Owens, J.S. Myocardial ischemia in anomalous origin of the right coronary artery from the pulmonary trunk: Proof of a coronary steal. Am. J. Cardiol. 1983, 51, 610–612. [Google Scholar] [CrossRef]
- Guenther, T.M.; Sherazee, E.A.; Wisneski, A.D.; Gustafson, J.D.; Wozniak, C.J.; Raff, G.W. Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery: A Systematic Review. Ann. Thorac. Surg. 2020, 110, 1063–1071. [Google Scholar] [CrossRef]
- Eugster, G.S.; Oliva, P.B. Anomalous origin of the right coronary artery from the pulmonary artery. Chest 1973, 63, 294–296. [Google Scholar] [CrossRef]
- Lerberg, D.B.; Ogden, J.A.; Zuberbuhler, J.R. Anomalous origin of the right coronary artery from the pulmonary artery. Ann. Thorac. Surg. 1979, 27, 87–94. [Google Scholar] [CrossRef]
- Bregman, D.; Brennan, F.J.; Singer, A.; Vinci, J.; Parodi, E.N.; Cassarella, W.J. Anomalous origin of right coronary artery from the pulmonary artery. J. Thorac. Cardiovasc. Surg. 1976, 72, 626–630. [Google Scholar] [CrossRef]
- Goo, H.W. Anomalous Origin of the Coronary Artery from the Pulmonary Artery in Children and Adults: A Pictorial Review of Cardiac Imaging Findings. Korean J. Radiol. 2021, 22, 1441–1450. [Google Scholar] [CrossRef]
- Tsai, I.C.; Lee, T.; Chen, M.C.; Fu, Y.C.; Jan, S.L.; Wang, C.C. Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr. Radiol. 2007, 37, 818–825. [Google Scholar] [CrossRef]
- Goo, H.W.; Seo, D.M.; Yun, T.J.; Park, J.J.; Park, I.S.; Ko, J.K. Coronary artery anomalies and clinically important anatomy in patients with congenital heart disease: Multislice CT findings. Pediatr. Radiol. 2009, 39, 265–273. [Google Scholar] [CrossRef]
- Pandey, N.N.; Sinha, M.; Sharma, A.; Rajagopal, R.; Bhambri, K.; Kumar, S. Anomalies of coronary artery origin: Evaluation on multidetector CT angiography. Clin. Imaging 2019, 57, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoen, T.; Bell, A.; Hegde, S.; Hussain, T.; Beerbaum, P.; Schaeffter, T. Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology 2011, 259, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, M.T.; Robbers-Visser, D.; van der Bilt, I.; Boekholdt, S.M.; Koolbergen, D.R.; Planken, R.N.; Groenink, M. Anomalous coronary artery from the pulmonary artery diagnosed in adulthood: A case series on variations of coronary anatomy and the diagnostic value of cardiac magnetic resonance imaging. Eur. Heart J. Case Rep. 2022, 6, 1–7. [Google Scholar]
- McAlindon, E.; Johnson, T.W.; Strange, J.; Lawton, C.; Baumbach, A.; Bucciarelli-Ducci, C. Isolated anomalous right coronary artery from the pulmonary artery in adulthood: Anatomical features and ischemic burden. Circulation 2012, 125, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2022, 24, 37. [Google Scholar]
- Radke, P.W.; Messmer, B.J.; Haager, P.K.; Klues, H.G. Anomalous origin of the right coronary artery: Preoperative and postoperative hemodynamics. Ann. Thorac. Surg. 1998, 66, 1444–1449. [Google Scholar] [CrossRef]
- Kastellanos, S.; Aznaouridis, K.; Vlachopoulos, C.; Tsiamis, E.; Oikonomou, E.; Tousoulis, D. Overview of coronary artery variants, aberrations and anomalies. World J. Cardiol. 2018, 10, 127–140. [Google Scholar] [CrossRef]
- Al-Dairy, A.; Rezaei, Y.; Pouraliakbar, H.; Mahdavi, M.; Bayati, P.; Dehaki, M.-G. Surgical repair for anomalous origin of the right coronary artery from the pulmonary artery. Korean Circ. J. 2017, 47, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Guzeltas, A.; Ozturk, E.; Tanidir, I.C.; Kasar, T.; Haydin, S. Evaluation of Anomalous Coronary Arteries from the Pulmonary Artery. Braz. J. Cardiovasc. Surg. 2017, 32, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Azakie, A.; Russell, J.L.; McCrindle, B.W.; Van Arsdell, G.S.; Benson, L.N.; Coles, J.G. Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: Early survival, patterns of ventricular recovery and late outcome. Ann. Thorac. Surg. 2003, 75, 1535–1541. [Google Scholar] [CrossRef]
- Xu, J.P.; Guo, H.W.; Hu, S.S.; Sun, L.Z.; Song, Y.H.; Sun, H.S. Results of surgical correction in patients with anomalous origin of the coronary artery from the pulmonary artery. Chin. J. Surg. 2006, 44, 1525–1528. [Google Scholar] [PubMed]
- Ohashi, K.; Itagaki, R.; Mukaida, T.; Miyazaki, K.; Ohashi, K.; Kawada, M.; Abe, D. Cardiac Arrest in a 33-year-old Marathon Runner with Anomalous Right Coronary Artery Originating from the Pulmonary Artery. Intern. Med. 2022, 61, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Levine, B.D.; Washington, R.L.; Baggish, A.L.; Kovacs, R.J.; Maron, M.S. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities. J. Am. Coll. Cardiol. 2015, 66, 2356–2361. [Google Scholar] [CrossRef] [PubMed]








| Category | Characteristics |
|---|---|
| Clinical |
|
| ECG |
|
| Echocardiography |
|
| AngioCT, angioMRI |
|
| Invasive coronarography |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, H.; Cinteză, E.; Voicu, C.; Mărgărint, I.; Rotaru, I.; Aria, A.; Youssef, T.; Nicolescu, A. Challenging Diagnosis of Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery. Diagnostics 2022, 12, 2671. https://doi.org/10.3390/diagnostics12112671
Mahmoud H, Cinteză E, Voicu C, Mărgărint I, Rotaru I, Aria A, Youssef T, Nicolescu A. Challenging Diagnosis of Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery. Diagnostics. 2022; 12(11):2671. https://doi.org/10.3390/diagnostics12112671
Chicago/Turabian StyleMahmoud, Hiyam, Eliza Cinteză, Cristiana Voicu, Irina Mărgărint, Iulian Rotaru, Amelia Aria, Tammam Youssef, and Alin Nicolescu. 2022. "Challenging Diagnosis of Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery" Diagnostics 12, no. 11: 2671. https://doi.org/10.3390/diagnostics12112671
APA StyleMahmoud, H., Cinteză, E., Voicu, C., Mărgărint, I., Rotaru, I., Aria, A., Youssef, T., & Nicolescu, A. (2022). Challenging Diagnosis of Anomalous Origin of the Right Coronary Artery from the Pulmonary Artery. Diagnostics, 12(11), 2671. https://doi.org/10.3390/diagnostics12112671







