Does a Caesarean Section Scar Affect Placental Volume, Vascularity and Localization?
Abstract
1. Introduction
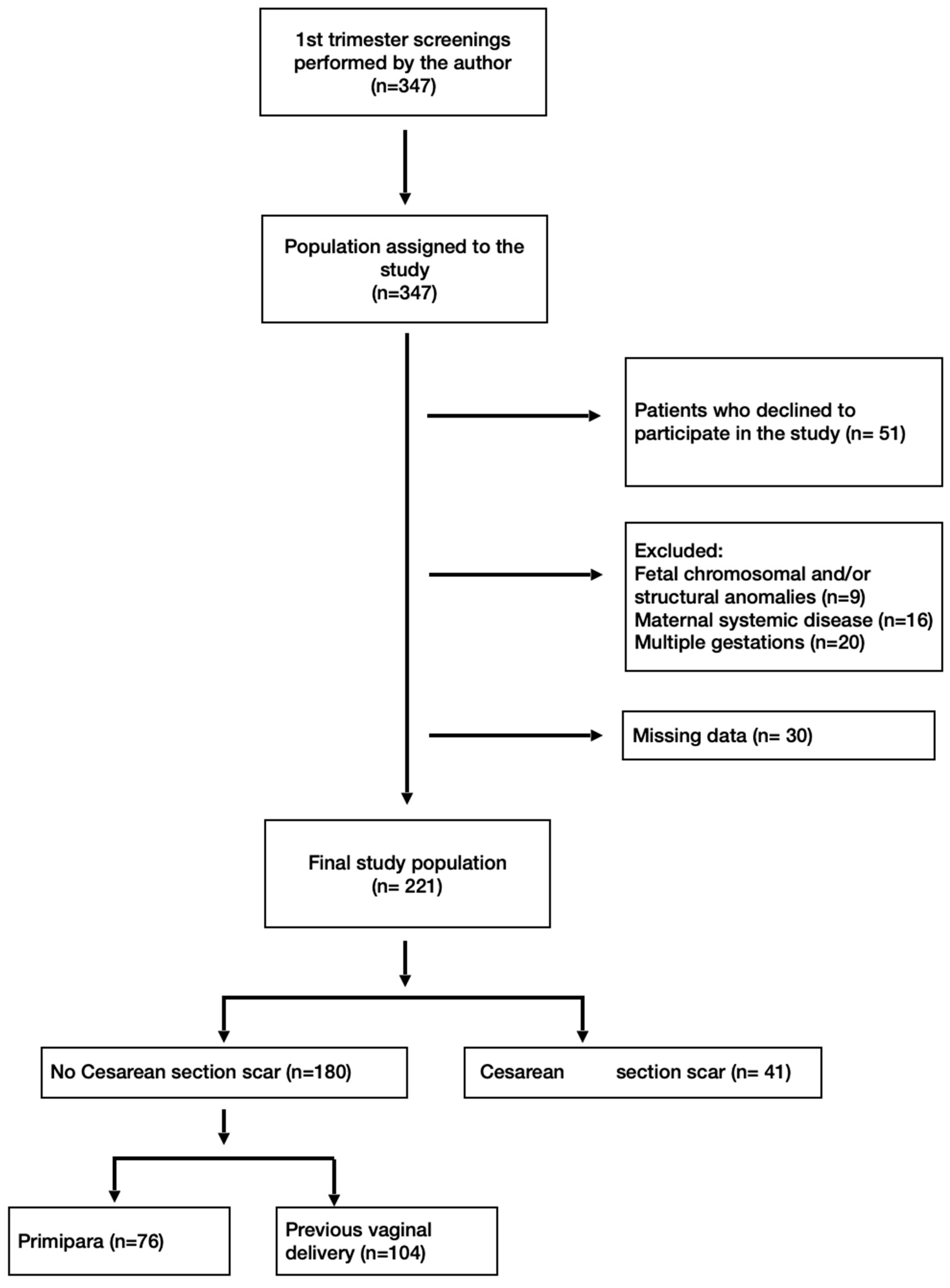
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Betran, A.P.; Ye, J.; Moller, A.B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Bhide, A.; Kennedy, A.; Woodward, P.; Hubinont, C.; Collins, S. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int. J. Gynaecol. Obstet. 2018, 140, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Pell, J.P.; Dobbie, R. Caesarean section and risk of unexplained stillbirth in subsequent pregnancy. Lancet 2003, 362, 1779–1784. [Google Scholar] [CrossRef]
- Kennare, R.; Tucker, G.; Heard, A.; Chan, A. Risks of adverse outcomes in the next birth after a first cesarean delivery. Obstet. Gynecol. 2007, 109 Pt 1, 270–276. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Bou-Antoun, S.; Lim, C.P.; Cromwell, D.A.; Mahmood, T.A.; Templeton, A.; van der Meulen, J.H. Impact of Caesarean section on subsequent fertility: A systematic review and meta-analysis. Hum. Reprod. 2013, 28, 1943–1952. [Google Scholar] [CrossRef]
- Vissers, J.; Hehenkamp, W.; Lambalk, C.B.; Huirne, J.A. Post-Caesarean section niche-related impaired fertility: Hypothetical mechanisms. Hum. Reprod. 2020, 35, 1484–1494. [Google Scholar] [CrossRef]
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel microarchitecture of human endometrial glands: Implications in endometrial regeneration and pathologies. Hum. Reprod. Update 2022, 28, 153–171. [Google Scholar] [CrossRef]
- Shepherd, A.M.; Mahdy, H. Placenta Accreta. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vissers, J.; Sluckin, T.C.; van Driel-Delprat, C.C.R.; Schats, R.; Groot, C.J.M.; Lambalk, C.B.; Twisk, J.W.R.; Huirne, J.A.F. Reduced pregnancy and live birth rates after in vitro fertilization in women with previous Caesarean section: A retrospective cohort study. Hum. Reprod. 2020, 35, 595–604. [Google Scholar] [CrossRef]
- Ben-Nagi, J.; Walker, A.; Jurkovic, D.; Yazbek, J.; Aplin, J.D. Effect of cesarean delivery on the endometrium. Int. J. Gynaecol. Obstet. 2009, 106, 30–34. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef] [PubMed]
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Visconti, F.; Quaresima, P.; Rania, E.; Palumbo, A.R.; Micieli, M.; Zullo, F.; Venturella, R.; Di Carlo, C. Difficult caesarean section: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 72–78. [Google Scholar] [CrossRef]
- Pairleitner, H.; Steiner, H.; Hasenoehrl, G.; Staudach, A. Three-dimensional power Doppler sonography: Imaging and quantifying blood flow and vascularization. Ultrasound Obstet. Gynecol. 1999, 14, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yamasato, K.; Zalud, I. Three dimensional power Doppler of the placenta and its clinical applications. J. Perinat. Med. 2017, 45, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Capponi, A.; Cavicchioni, O.; Vendola, M.; Arduini, D. First trimester uterine Doppler and three-dimensional ultrasound placental volume calculation in predicting pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 147–151. [Google Scholar] [CrossRef]
- Noguchi, J.; Hata, K.; Tanaka, H.; Hata, T. Placental vascular sonobiopsy using three-dimensional power Doppler ultrasound in normal and growth restricted fetuses. Placenta 2009, 30, 391–397. [Google Scholar] [CrossRef]
- Hafner, E.; Metzenbauer, M.; Stumpflen, I.; Waldhor, T.; Philipp, K. First trimester placental and myometrial blood perfusion measured by 3D power Doppler in normal and unfavourable outcome pregnancies. Placenta 2010, 31, 756–763. [Google Scholar] [CrossRef]
- Sagberg, K.; Eskild, A.; Sommerfelt, S.; Gjesdal, K.I.; Higgins, L.E.; Borthne, A.; Hillestad, V. Placental volume in gestational week 27 measured by three-dimensional ultrasound and magnetic resonance imaging. Acta. Obstet. Gynecol. Scand. 2021, 100, 1412–1418. [Google Scholar] [CrossRef]
- Wegrzyn, P.; Faro, C.; Falcon, O.; Peralta, C.F.; Nicolaides, K.H. Placental volume measured by three-dimensional ultrasound at 11 to 13 + 6 weeks of gestation: Relation to chromosomal defects. Ultrasound Obstet. Gynecol. 2005, 26, 28–32. [Google Scholar] [CrossRef]
- de Paula CF, R.R.; Campos, J.A.; Zugaib, M. Placental Volumes Measured by 3-Dimensional Ultrasonography in Normal Pregnancies from 12 to 40 Weeks’ Gestation. J. Ultrasound Med. 2008, 27, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, M.; Zimmer, M.; Fuchs, T.; Florjanski, J.; Pomorska, M.; Tomialowicz, M.; Milnerowicz-Nabzdyk, E. Quantitative assessment of placental vasculature and placental volume in normal pregnancies with the use of 3D Power Doppler. Adv. Med. Sci. 2014, 59, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Tanaka, H.; Noguchi, J.; Hata, K. Three-dimensional ultrasound evaluation of the placenta. Placenta 2011, 32, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Simcox, L.E.; Higgins, L.E.; Myers, J.E.; Johnstone, E.D. Intraexaminer and Interexaminer Variability in 3D Fetal Volume Measurements during the Second and Third Trimesters of Pregnancy. J. Ultrasound Med. 2017, 36, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Hafner, E.; Metzenbauer, M.; Hofinger, D.; Munkel, M.; Gassner, R.; Schuchter, K.; Dillinger-Paller, B.; Philipp, K. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta 2003, 24, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Farina, A. Systematic review on first trimester three-dimensional placental volumetry predicting small for gestational age infants. Prenat. Diagn. 2016, 36, 135–141. [Google Scholar] [CrossRef]
- Papastefanou, I.; Chrelias, C.; Siristatidis, C.; Kappou, D.; Eleftheriades, M.; Kassanos, D. Placental volume at 11 to 14 gestational weeks in pregnancies complicated with fetal growth restriction and preeclampsia. Prenat. Diagn. 2018, 38, 928–935. [Google Scholar] [CrossRef]
- Hoopmann, M.; Schermuly, S.; Abele, H.; Zubke, W.; Kagan, K.O. First trimester pregnancy volumes and subsequent small for gestational age fetuses. Arch. Gynecol. Obstet. 2014, 290, 41–46. [Google Scholar] [CrossRef]
- Schwartz, N.; Coletta, J.; Pessel, C.; Feng, R.; Timor-Tritsch, I.E.; Parry, S.; Salafia, C.N. Novel 3-dimensional placental measurements in early pregnancy as predictors of adverse pregnancy outcomes. J. Ultrasound Med. 2010, 29, 1203–1212. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, N.L.; Gonzalez Davila, E.; Padron, E.; Armas Gonzalez, M.; Plasencia, W. Value of Placental Volume and Vascular Flow Indices as Predictors of Early and Late Preeclampsia at First Trimester. Fetal Diagn. Ther. 2018, 44, 256–263. [Google Scholar] [CrossRef]
- Farina, A. Placental vascular indices (VI, FI and VFI) in intrauterine growth retardation (IUGR). A pooled analysis of the literature. Prenat. Diagn. 2015, 35, 1065–1072. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, C.P.; Sun, F.J.; Chen, C.Y. Comparison of Placental Three-Dimensional Power Doppler Vascular Indices and Placental Volume in Pregnancies with Small for Gestational Age Neonates. J. Clin. Med. 2019, 8, 1651. [Google Scholar] [CrossRef] [PubMed]
- Ballering, G.; Leijnse, J.; Eijkelkamp, N.; Peeters, L.; de Heus, R. First-trimester placental vascular development in multiparous women differs from that in nulliparous women. J. Matern. Fetal Neonatal Med. 2018, 31, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, N.L.; Gonzalez-Davila, E.; Gonzalez Marrero, L.; Padron, E.; Conde, J.R.; Plasencia, W. Value of placental volume and vascular flow indices as predictors of intrauterine growth retardation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 13–19. [Google Scholar] [CrossRef] [PubMed]


| Variable | Previous CS | p * | Previous Vaginal Birth | p ** | Nulliparas | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||||
| Age | 34.8 (4.7) | 36.0 (31.0–38.0) | 0.06 | 33.0 (5.1) | 33.0 (30.0–37.0) | <0.001 | 29.8 (5.1) | 29.0 (26.0–33.0) | 32.1 (5.4) | 32.0 (28.0–36.0) | |
| BMI | 24.5 (4.4) | 23.8 (21.8–26.7) | 0.29 | 24.0 (5.1) | 22.8 (20.3–26.7) | 0.09 | 22.6 (3.7) | 22.1 (19.9–24.3) | 23.6 (4.5) | 22.5 (20.3–25.9) | |
| Smoking status during the pregnancy, n, % | 2 | 5.7 | 0.60 | 3 | 2.9 | 0.70 | 3 | 3.9 | 8 | 3.7 | |
| Smoking status before the pregnancy, n, % | 2 | 5.7 | 0.68 | 6 | 5.8 | 0.38 | 7 | 9.2 | 15 | 7.0 | |
| Placental localisation 1st trimester | Anterior | 11 | 29.7 | 0.38 | 40 | 42.1 | 0.80 | 35 | 47.9 | 86 | 42.0 |
| Posterior | 25 | 67.6 | 51 | 53.7 | 35 | 47.9 | 111 | 54.1 | |||
| Fundus | 1 | 2.7 | 4 | 4.2 | 3 | 4.1 | 8 | 3.9 | |||
| Variable | Previous CS Median (IQR) | p * | Previous Vaginal Birth Median (IQR) | p ** | Nulliparas Median (IQR) |
|---|---|---|---|---|---|
| PV 1st trimester | 76.2 (52.8–100.8) | p = 0.53 | 78.8 (61.8–103.5) | p = 0.80 | 77.5 (54.5–102.2) |
| PV 2nd trimester | 223.0 (178.4–343.7) | p = 0.55 | 229.8 (107.3–280.9) | p = 0.92 | 223.3 (177.2–309.7) |
| PQ | 1.2 (0.7–1.8) | p = 0.99 | 1.20 (0.92–1.53) | p = 0.92 | 1.16 (0.7–1.5) |
| VI 1st trimester | 20.8 (16.5–29.3) | p = 0.16 | 26.6 (17.8–33.2) | p = 0.21 | 22.8 (15.8–32.0) |
| VI 2nd trimester | 20.0 (15.3–29.5) | p = 0.42 | 24.4 (14.5–33.3) | p = 0.14 | 14.9 (11.8–28.2) |
| FI 1st trimester | 37.1 (30.7–44.3) | p = 0.62 | 38.8 (33.3–43.5) | p = 0.01 | 35.2 (30.3–38.7) |
| FI 2nd trimester | 37.7 (32.5–42.9) | p = 0.38 | 40.3 (35.0–43.4) | p = 0.58 | 39.3 (34.3–41.3) |
| VFI 1st trimester | 8.0 (5.7–10.7) | p = 0.20 | 9.3 (6.2–13.5) | p = 0.09 | 7.6 (5.2–10.5) |
| VFI 2nd trimester | 8.2 (5.1–11.1) | p = 0.43 | 8.7 (5.8–11.6) | p = 0.12 | 5.7 (4.9–10.0) |
| Variable | Previous CS N (%) or Median (IQR) | Previous Vaginal Birth N (%) or Median (IQR) | p | |
|---|---|---|---|---|
| Placental localisation 1st trimester | Anterior Posterior Fundus | 11 (29.7) 25 (67.6) 1 (2.7) | 40 (42.1) 51 (53.7) 4 (4.2) | 0.38 |
| PV 1st trimester | 76.2 (52.8–100.8) | 78.8 (61.8–103.5) | p = 0.53 | |
| PV 2nd trimester | 223.0 (178.4–343.7) | 229.8 (107.3–280.9) | p = 0.55 | |
| PQ | 1.2 (0.7–1.8) | 1.20 (0.92–1.53) | p = 0.99 | |
| VI 1st trimester | 20.8 (16.5–29.3) | 26.6 (17.8–33.2) | p = 0.16 | |
| VI 2nd trimester | 20.0 (15.3–29.5) | 24.4 (14.5–33.3) | p = 0.42 | |
| FI 1st trimester | 37.1 (30.7–44.3) | 38.8 (33.3–43.5) | p = 0.62 | |
| FI 2nd trimester | 37.7 (32.5–42.9) | 40.3 (35.0–43.4) | p = 0.38 | |
| VFI 1st trimester | 8.0 (5.7–10.7) | 9.3 (6.2–13.5) | p = 0.20 | |
| VFI 2nd trimester | 8.2 (5.1–11.1) | 8.7 (5.8–11.6) | p = 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokučava, D.; Ķīvīte-Urtāne, A.; Domaševs, P.; Lūse, L.; Vedmedovska, N.; Donders, G.G.G. Does a Caesarean Section Scar Affect Placental Volume, Vascularity and Localization? Diagnostics 2022, 12, 2674. https://doi.org/10.3390/diagnostics12112674
Bokučava D, Ķīvīte-Urtāne A, Domaševs P, Lūse L, Vedmedovska N, Donders GGG. Does a Caesarean Section Scar Affect Placental Volume, Vascularity and Localization? Diagnostics. 2022; 12(11):2674. https://doi.org/10.3390/diagnostics12112674
Chicago/Turabian StyleBokučava, Diana, Anda Ķīvīte-Urtāne, Pavels Domaševs, Laura Lūse, Natālija Vedmedovska, and Gilbert G. G. Donders. 2022. "Does a Caesarean Section Scar Affect Placental Volume, Vascularity and Localization?" Diagnostics 12, no. 11: 2674. https://doi.org/10.3390/diagnostics12112674
APA StyleBokučava, D., Ķīvīte-Urtāne, A., Domaševs, P., Lūse, L., Vedmedovska, N., & Donders, G. G. G. (2022). Does a Caesarean Section Scar Affect Placental Volume, Vascularity and Localization? Diagnostics, 12(11), 2674. https://doi.org/10.3390/diagnostics12112674








