A Case of Early-Stage Gallbladder Cancer, Positive for ALDH1A1, Which Arose from Adenomyomatosis of the Gallbladder
Abstract
:1. Background
2. Case Presentation
- History of present illness: a 78-year-old woman was referred to our department by her family doctor for further examination of a gallbladder polyp, with gallbladder wall thickening without any symptoms;
- Medical history: she had type 2 diabetes, hypertension, and hyperuricemia as comorbidities, each of which was treated with medication;
- Social history: she had a history of smoking 10–15 cigarettes per day and drinking 60 g of alcohol per week;
- Clinical chemistry: there were no abnormal findings in her clinical chemistry, including with regard to tumor markers;
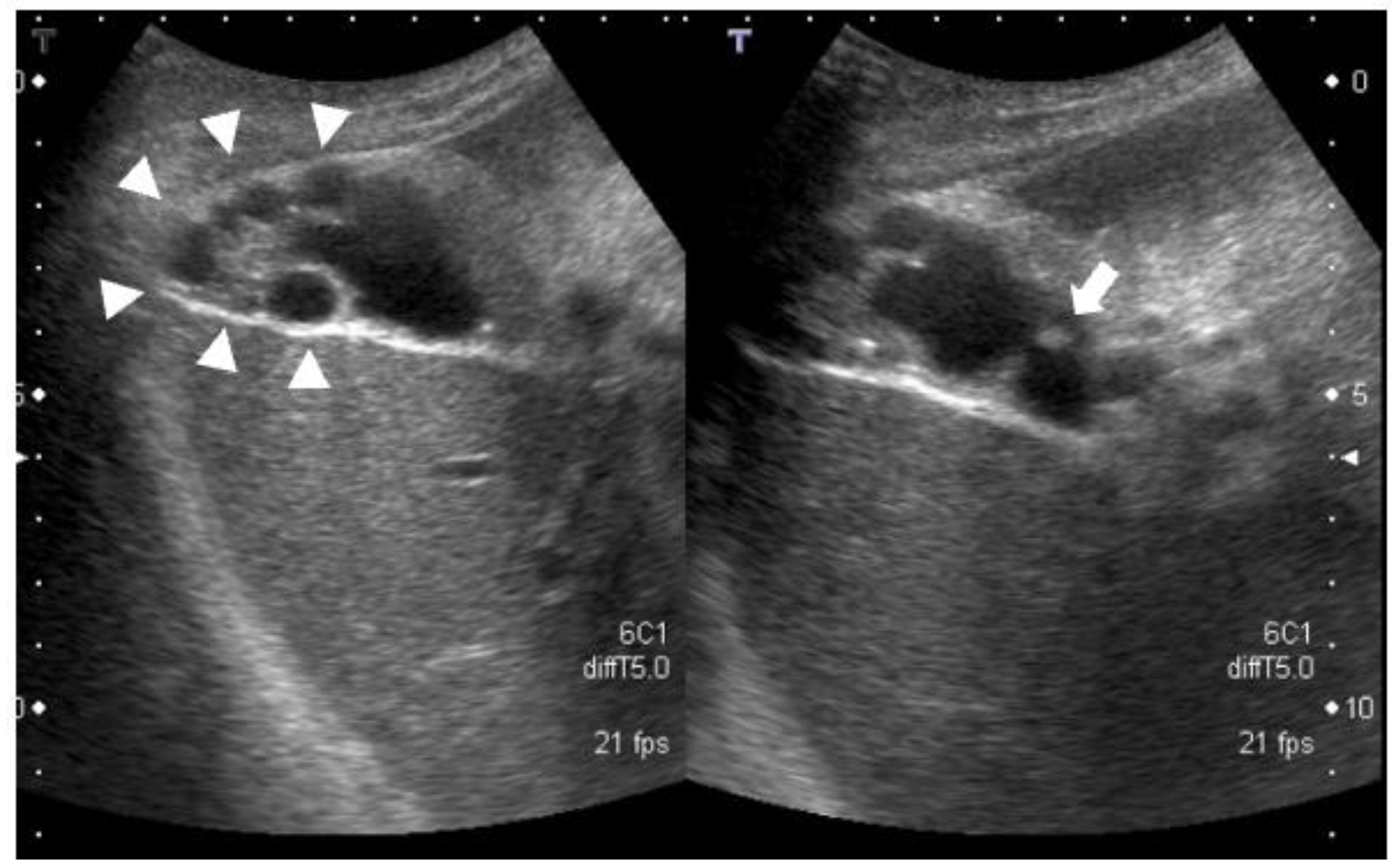
- Abdominal ultrasonography: a 7.4-mm stalked polyp on the gallbladder body and wall thickening of the fundus with aggregated multiple cystic structures was seen, suggesting RAS (Figure 1).
- Contrast-enhanced CT: there were multiple cystic structures at the fundus of the gallbladder and no hyperenhancement of the gallbladder wall or nodules (Figure 2);
- Contrast-enhanced MRI: there was a 4-mm gallbladder body polyp and no abnormal signals in the thickened wall at the fundus of the gallbladder (Figure 3);
- ○
- Based on these imaging findings, we diagnosed the patient with a gallbladder polyp and focal-type ADM;
- ○
- The patient chose surgical treatment, and laparoscopic cholecystectomy was performed. The gallbladder was easily dissected from the liver and was able to be removed laparoscopically as usual;
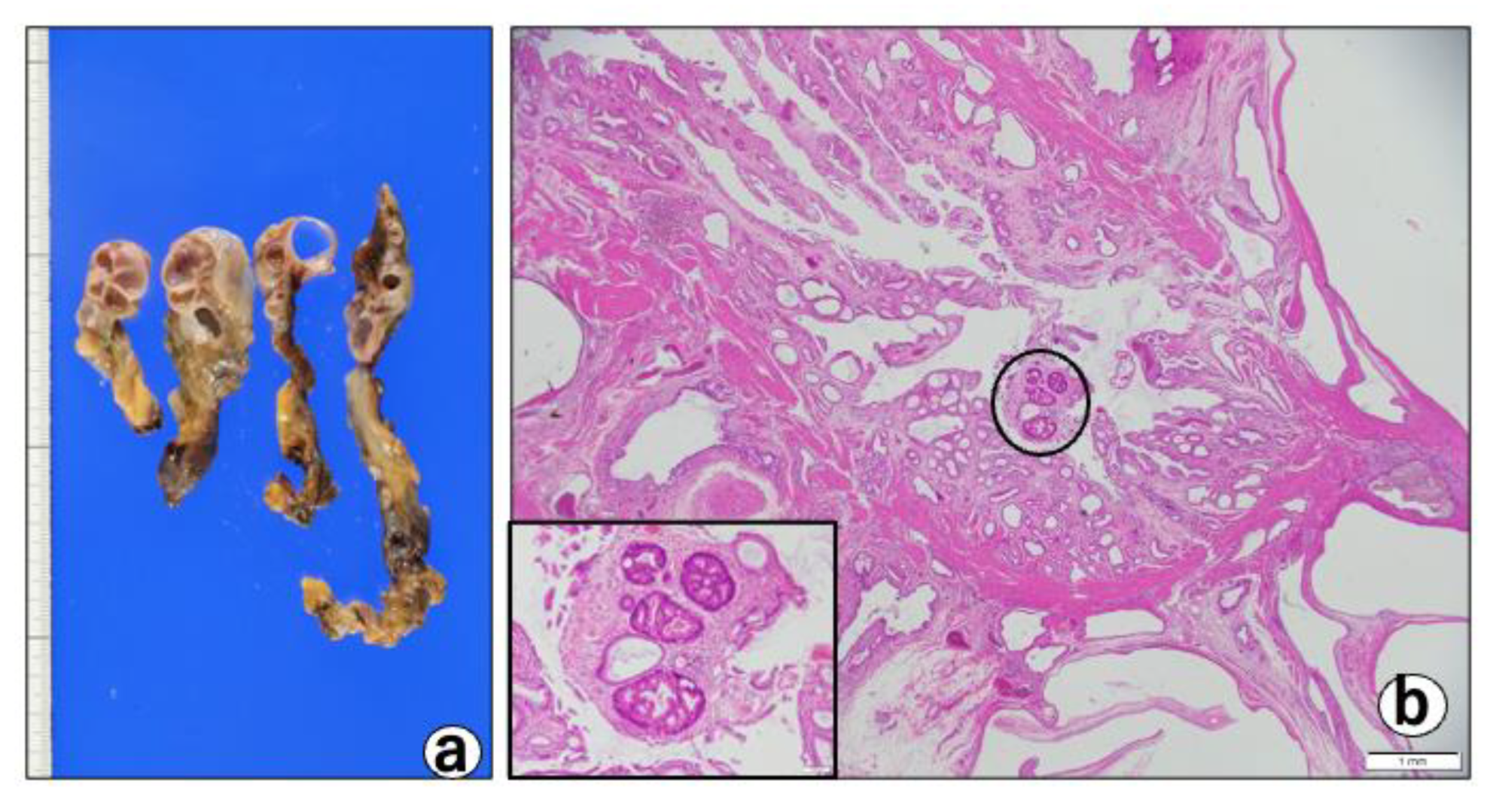
- Pathological findings: macroscopically, the gallbladder showed a thickened wall containing cystic lesions, suggesting ADM (Figure 4a).
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jutras, J.A. Hyperplastic cholecystoses; Hickey lecture, 1960. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1960, 83, 795–827. [Google Scholar] [PubMed]
- Kin, T.; Maguchi, H.; Takahashi, K.; Katanuma, A.; Osanai, M.; Yane, K.; Takaki, R.; Matsumoto, K.; Matsumori, T.; Gon, K.; et al. Clinicopathological features of gallbladder cancer with adenomyomatosis. J. Jpn. Biliary Assoc. 2014, 28, 633–640. (In Japanese) [Google Scholar]
- Basturk, O.; Aishima, S.; Esposito, I. Biliary intraepithelial neoplasia. In WHO Classification of Tumors: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 273–275. [Google Scholar]
- Fukumura, Y.; Rong, L.; Maimaitiaili, Y.; Fujisawa, T.; Isayama, H.; Nakahodo, J.; Kikuyama, M.; Yao, T. Precursor Lesions of Gallbladder Carcinoma: Disease Concept, Pathology, and Genetics. Diagnostics 2022, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Harada, K.; Sasaki, M.; Nakanuma, Y. Histological Characterization of Biliary Intraepithelial Neoplasia with respect to Pancreatic Intraepithelial Neoplasia. Int. J. Hepatol. 2014, 2014, 678260. [Google Scholar] [CrossRef] [Green Version]
- Lendvai, G.; Szekerczes, T.; Illyes, I.; Dora, R.; Kontsek, E.; Gogl, A.; Kiss, A.; Werling, K.; Kovalszky, I.; Schaff, Z.; et al. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol. Oncol. Res. 2020, 26, 3–15. [Google Scholar] [CrossRef]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharm. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chai, S.; Wang, P.; Zhang, C.; Yang, Y.; Yang, Y.; Wang, K. Aldehyde dehydrogenases and cancer stem cells. Cancer Lett. 2015, 369, 50–57. [Google Scholar] [CrossRef]
- Yue, H.; Hu, Z.; Hu, R.; Guo, Z.; Zheng, Y.; Wang, Y.; Zhou, Y. ALDH1A1 in Cancers: Bidirectional Function, Drug Resistance, and Regulatory Mechanism. Front. Oncol. 2022, 12, 918778. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016, 21, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Kida, M.; Hasegawa, R.; Matsumoto, T.; Mishima, T.; Kaneko, T.; Tokunaga, S.; Yamauchi, H.; Okuwaki, K.; Miyazawa, S.; Iwai, T.; et al. Adenomyomatosis of the gallbladder -pathogenesis, diagnosis, and management. Nihon Shokakibyo Gakkai Zasshi 2015, 112, 456–463. [Google Scholar] [CrossRef]
- Ootani, T.; Shirai, Y.; Tsukada, K.; Muto, T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer 1992, 69, 2647–2652. [Google Scholar] [CrossRef]
- Nabatame, N.; Shirai, Y.; Nishimura, A.; Yokoyama, N.; Wakai, T.; Hatakeyama, K. High risk of gallbladder carcinoma in elderly patients with segmental adenomyomatosis of the gallbladder. J. Exp. Clin. Cancer Res. 2004, 23, 593–598. [Google Scholar] [PubMed]
- Mori, K.; Kubokawa, Y.; Sai, J.; Suyama, M.; Nobukawa, B. Clinical and immunohistochemical study of adenomyomatosis of the gallbladder combined with gallbladder cancer. J. Jpn. Biliary Assoc. 2010, 24, 675–682. (In Japanese) [Google Scholar]
- Anno, K.; Jinkan, S.; Kubokawa, Y.; Kato, K.; Matsumura, Y.; Inami, K.; Chikamori, M.; Takahashi, Y.; Maruki, J.; Suyama, M. Carcinoma of segmental type adenomyomatosis, originating in the Rokitansky-Aschoff sinus―A case report. Hepato-Biliary-Pancreat. Imaging 2008, 10, 343–349. (In Japanese) [Google Scholar]
- Hattori, M.; Kunou, T.; Yokoi, S.; Saeki, S.; Okada, Y.; Shibahara, H.; Abe, T.; Satou, K.; Kawahara, T.; Hayakawa, S. A Case of Advanced Gallbladder Cancer derived from the Rokitansky-Aschoff Sinus. Jpn. J. Gastroenterol. Surg. 2006, 39, 1822–1826. (In Japanese) [Google Scholar] [CrossRef]
- Matsumoto, T.; Shimada, K. A case of gallbladder cancer arising from the Rokitansky-Aschoff sinus. Jpn. J. Clin. Oncol. 2009, 39, 776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, T.; Morita, T.; Tanaka, E.; Narasaki, H.; Kuwatani, T.; Takahashi, T. A case of gallbladder cancer derived from a Rokitansky-Aschoff sinus that was diagnosed after laparoscopic cholecystectomy. J. Jpn. Surg. Assoc. 2015, 70, 359–364. (In Japanese) [Google Scholar]
- Washida, M.; Nishihira, T.; Kaneko, T.; Ishii, T.; Iwai, A.; Ide, Y. A case of adenomyomatosis with unexpectd gallbladder cancer. J. Jpn. Surg. Assoc. 2002, 63, 2775–2779. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Yang, K.; Liang, D.; Jiang, C.; Ma, Z. Discovery and development of selective aldehyde dehydrogenase 1A1 (ALDH1A1) inhibitors. Eur. J. Med. Chem. 2021, 209, 112940. [Google Scholar] [CrossRef]
- Liu, D.C.; Yang, Z.L.; Jiang, S. Identification of musashi-1 and ALDH1 as carcinogenesis, progression, and poor-prognosis related biomarkers for gallbladder adenocarcinoma. Cancer Biomark. 2010, 8, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Gupta, A.; Rastogi, N.; Agrawal, S.; Kumar, A.; Kumar, V.; Mittal, B. Association of cancer stem cell markers genetic variants with gallbladder cancer susceptibility, prognosis, and survival. Tumour Biol. 2016, 37, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Kim, Y.J.; Park, H.S.; Jung, S.I. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J. Gastroenterol. 2020, 26, 2967–2986. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Han, J.K.; Choi, B.I.; Choi, J.Y. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. Am. J. Roentgenol. 2008, 191, 765–771. [Google Scholar] [CrossRef]
- Joo, I.; Lee, J.Y.; Kim, J.H.; Kim, S.J.; Kim, M.A.; Han, J.K.; Choi, B.I. Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound. Eur. Radiol. 2013, 23, 730–738. [Google Scholar] [CrossRef]
- Ogawa, T.; Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Koshita, S.; Kanno, Y.; Masu, K.; Sugita, R. High b-value diffusion-weighted magnetic resonance imaging for gallbladder lesions: Differentiation between benignity and malignancy. J. Gastroenterol. 2012, 47, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, A.; Piccolo, G.; Di Vita, M.; Zanghi, A.; Cardi, F.; Di Mattia, P.; Barbera, G.; Borzi, L.; Panebianco, V.; Di Carlo, I.; et al. Managing the incidentally detected gallbladder cancer: Algorithms and controversies. Int. J. Surg. 2014, 12 (Suppl. 2), S108–S119. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasa, Y.; Iwata, K.; Okuno, M.; Sugiyama, A.; Nishigaki, Y.; Ohashi, Y.; Tanaka, T.; Iwashita, T.; Shimizu, M.; Tomita, E. A Case of Early-Stage Gallbladder Cancer, Positive for ALDH1A1, Which Arose from Adenomyomatosis of the Gallbladder. Diagnostics 2022, 12, 2721. https://doi.org/10.3390/diagnostics12112721
Iwasa Y, Iwata K, Okuno M, Sugiyama A, Nishigaki Y, Ohashi Y, Tanaka T, Iwashita T, Shimizu M, Tomita E. A Case of Early-Stage Gallbladder Cancer, Positive for ALDH1A1, Which Arose from Adenomyomatosis of the Gallbladder. Diagnostics. 2022; 12(11):2721. https://doi.org/10.3390/diagnostics12112721
Chicago/Turabian StyleIwasa, Yuhei, Keisuke Iwata, Mitsuru Okuno, Akihiko Sugiyama, Yoichi Nishigaki, Yosuke Ohashi, Takuji Tanaka, Takuji Iwashita, Masahito Shimizu, and Eiichi Tomita. 2022. "A Case of Early-Stage Gallbladder Cancer, Positive for ALDH1A1, Which Arose from Adenomyomatosis of the Gallbladder" Diagnostics 12, no. 11: 2721. https://doi.org/10.3390/diagnostics12112721





