BCNet: A Deep Learning Computer-Aided Diagnosis Framework for Human Peripheral Blood Cell Identification
Abstract
:1. Introduction
- A deep learning schema of the CAD system based on the newly deep learning BCNet is proposed in order to identify multiclass blood cells rapidly and automatically.
- The multiple class identification task is conducted in terms of improving the overall classification performance.
- A comprehensive evaluation experiment is conducted to investigate the reliability and feasibility of the proposed BCNet using multiple optimizers and different state-of-the-art deep learning models such as DensNet, ResNet, Inception, and MobileNet.
2. Related Works
3. Materials and Methods
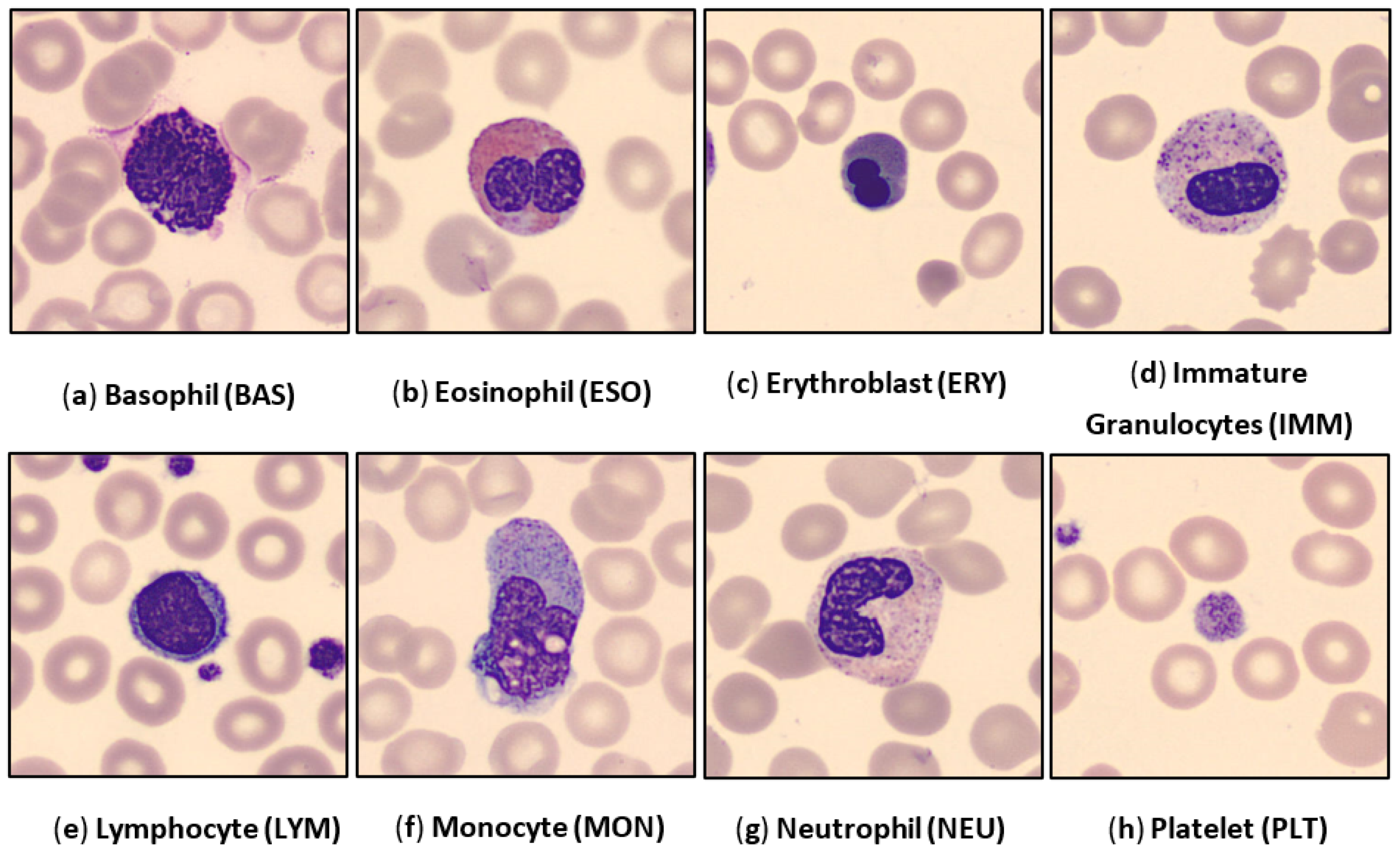
3.1. Dataset
3.2. Pre-Processing
3.3. Data Preparation: Training, Validation, and Testing
3.4. The Proposed Deep Learning Framework
3.5. BCNet Deep Learning Architecture
3.6. Performance Metrics
4. Experimental Results
5. Discussion
5.1. Comparative Results of the BCNet and Other DL Models
5.2. Comparison between Proposed BCNet and Previously Published Models
5.3. Ablation Study
5.4. Limitations and Future Work
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BAS | Basophils |
| CAD | Computer-Aided Diagnosis |
| Conv | Convolution |
| CNN | Convolution Neural Network |
| DL | Deep Learning |
| ERY | Erythroblasts |
| EOS | Eosinophils |
| FC | Fully connected |
| GAP | Global Average Pooling |
| HPBC | Human Peripheral Blood Cells |
| LRN | local response normalization |
| LYM | Lymphocytes |
| MBConv | Mobile inverted bottleneck convolution |
| ML | Machine Learning |
| NEU | Neutrophils |
| MRI | Magnetic Resonance Imaging |
| MON | Monocytes |
| PLT | Platelets |
| RBC | Red Blood Cells |
| WBC | White Blood Cells |
References
- Li, Y.; Chen, J.; Xue, P.; Tang, C.; Chang, J.; Chu, C.; Ma, K.; Li, Q.; Zheng, Y.; Qiao, Y. Computer-Aided Cervical Cancer Diagnosis Using Time-Lapsed Colposcopic Images. IEEE Trans. Med. Imaging 2020, 39, 3403–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lu, M.Y.; Wang, J.; Williamson, D.F.K.; Rodig, S.J.; Lindeman, N.I.; Mahmood, F. Pathomic Fusion: An Integrated Framework for Fusing Histopathology and Genomic Features for Cancer Diagnosis and Prognosis. IEEE Trans. Med. Imaging 2020, 41, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Raphael, R.T.; Joy, K.R. Segmentation and Classification Techniques of Leukemia Using Image Processing: An Overview. In Proceedings of the 2019 International Conference on Intelligent Sustainable Systems, Palladam, India, 21–22 February 2019; pp. 378–384. [Google Scholar] [CrossRef]
- Chin Neoh, S.; Srisukkham, W.; Zhang, L.; Todryk, S.; Greystoke, B.; Lim, C.P.; Hossain, M.A.; Aslam, N. An Intelligent Decision Support System for Leukaemia Diagnosis using Microscopic Blood Images. Sci. Rep. 2015, 5, 14938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaian, S.; Madhukar, M.; Chronopoulos, A.T. Automated Screening System for Acute Myelogenous Leukemia Detection in Blood Microscopic Images. IEEE Syst. J. 2014, 8, 995–1004. [Google Scholar] [CrossRef]
- Amin, M.M.; Kermani, S.; Talebi, A.; Oghli, M.G. Recognition of Acute Lymphoblastic Leukemia Cells in Microscopic Images Using K-Means Clustering and Support Vector Machine Classifier. J. Med. Signals Sens. 2015, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Matek, C.; Schwarz, S.; Spiekermann, K.; Marr, C. Human-level recognition of blast cells in acute myeloid leukaemia with convolutional neural networks. Nat. Mach. Intell. 2019, 1, 538–544. [Google Scholar] [CrossRef]
- Shree, K.D.; Janani, B. Classification of Leucocytes for Leukaemia Detection. Res. J. Eng. Technol. 2019, 10, 59–66. [Google Scholar] [CrossRef]
- Baig, R.; Rehman, A.; Almuhaimeed, A.; Alzahrani, A.; Rauf, H.T. Detecting Malignant Leukemia Cells Using Microscopic Blood Smear Images: A Deep Learning Approach. Appl. Sci. 2022, 12, 6317. [Google Scholar] [CrossRef]
- Bain, B.J. Diagnosis from the blood smear. N. Engl. J. Med. 2005, 353, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fey, M.F.; Buske, C. Acute myeloblastic leukaemias in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi138–vi143. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann. Intern. Med. 1985, 103, 620–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, Z.F.; Abdulla, A.A. An efficient CAD system for ALL cell identification from microscopic blood images. Multimed. Tools Appl. 2021, 80, 6355–6368. [Google Scholar] [CrossRef]
- Wang, M.; Lee, K.C.M.; Chung, B.M.F.; Bogaraju, S.V.; Ng, H.C.; Wong, J.S.J.; Shum, H.C.; Tsia, K.K.; So, H.K.H. Low-Latency In Situ Image Analytics With FPGA-Based Quantized Convolutional Neural Network. IEEE Trans. Neural Netw. Learn. Syst. 2021, 33, 2853–2866. [Google Scholar] [CrossRef]
- Rastogi, P.; Khanna, K.; Singh, V. LeuFeatx: Deep learning–based feature extractor for the diagnosis of acute leukemia from microscopic images of peripheral blood smear. Comput. Biol. Med. 2022, 142, 105236. [Google Scholar] [CrossRef]
- Atteia, G.; Alhussan, A.A.; Samee, N.A. BO-ALLCNN: Bayesian-Based Optimized CNN for Acute Lymphoblastic Leukemia Detection in Microscopic Blood Smear Images. Sensors 2022, 22, 5520. [Google Scholar] [CrossRef]
- Heyat, M.B.; Lai, D.; Khan, F.I.; Zhang, Y. Sleep Bruxism Detection Using Decision Tree Method by the Combination of C4-P4 and C4-A1 Channels of Scalp EEG. IEEE Access 2019, 7, 102542–102553. [Google Scholar] [CrossRef]
- Samee, N.A.; Atteia, G.; Meshoul, S.; Al-antari, M.A.; Kadah, Y.M. Deep Learning Cascaded Feature Selection Framework for Breast Cancer Classification: Hybrid CNN with Univariate-Based Approach. Mathematics 2022, 10, 3631. [Google Scholar] [CrossRef]
- Samee, N.A.; Alhussan, A.A.; Ghoneim, V.F.; Atteia, G.; Alkanhel, R.; Al-Antari, M.A.; Kadah, Y.M. A Hybrid Deep Transfer Learning of CNN-Based LR-PCA for Breast Lesion Diagnosis via Medical Breast Mammograms. Sensors 2022, 22, 4938. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.A.; Samee, N.M.A.; Ghoneim, V.F.; Kadah, Y.M. Evaluating Deep and Statistical Machine Learning Models in the Classification of Breast Cancer from Digital Mammograms. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 1–11. Available online: https://www.academia.edu/en/68424683/Evaluating_Deep_and_Statistical_Machine_Learning_Models_in_the_Classification_of_Breast_Cancer_from_Digital_Mammograms (accessed on 1 June 2022). [CrossRef]
- Habibzadeh Motlagh, M.; Jannesari, M.; Rezaei, Z.; Totonchi, M.; Baharvand, H. Automatic white blood cell classification using pre-trained deep learning models: ResNet and Inception. In Proceedings of the Tenth International Conference on Machine Vision (ICMV 2017), Vienna, Austria, 13–15 November 2017; p. 105. [Google Scholar] [CrossRef]
- Pan, C.; Park, D.S.; Yang, Y.; Yoo, H.M. Leukocyte image segmentation by visual attention and extreme learning machine. Neural Comput. Appl. 2012, 21, 1217–1227. [Google Scholar] [CrossRef]
- Mohapatra, S.; Patra, D.; Satpathi, S. Image analysis of blood microscopic images for acute leukemia detection. In Proceedings of the 2010 International Conference on Industrial Electronics, Control and Robotics, Rourkela, India, 27–29 December 2010; pp. 215–219. [Google Scholar] [CrossRef]
- Hegde, R.B.; Prasad, K.; Hebbar, H.; Singh, B.M.K.; Sandhya, I. Automated Decision Support System for Detection of Leukemia from Peripheral Blood Smear Images. J. Digit. Imaging 2020, 33, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Goutam, D.; Sailaja, S. Classification of acute myelogenous leukemia in blood microscopic images using supervised classifier. In Proceedings of the 2015 IEEE International Conference on Engineering and Technology (ICETECH), Coimbatore, India, 20 March 2015. [Google Scholar] [CrossRef]
- Qin, F.; Gao, N.; Peng, Y.; Wu, Z.; Shen, S.; Grudtsin, A. Fine-grained leukocyte classification with deep residual learning for microscopic images. Comput. Methods Programs Biomed. 2018, 162, 243–252. [Google Scholar] [CrossRef]
- Mundhra, D.; Cheluvaraju, B.; Rampure, J.; Rai Dastidar, T. Analyzing microscopic images of peripheral blood smear using deep learning. In Deep Learning in Medical Image Analysis and Multimodal Learning for Clinical Decision Support; Springer: Cham, Switzerland, 2017; pp. 178–185. [Google Scholar] [CrossRef]
- Çınar, A.; Arslan, S. And neutrophils on white blood cells using hybrid Alexnet—GoogleNet—SVM. SN Appl. Sci. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Acevedo, A.; Alférez, S.; Merino, A.; Puigví, L.; Rodellar, J. Recognition of peripheral blood cell images using convolutional neural networks. Comput. Methods Programs Biomed. 2019, 180, 105020. [Google Scholar] [CrossRef]
- Elhassan, T.A.M.; Rahim, M.S.M.; Swee, T.T.; Hashim, S.Z.M.; Aljurf, M. Feature Extraction of White Blood Cells Using CMYK-Moment Localization and Deep Learning in Acute Myeloid Leukemia Blood Smear Microscopic Images. IEEE Access 2022, 10, 16577–16591. [Google Scholar] [CrossRef]
- Jung, C.; Abuhamad, M.; Mohaisen, D.; Han, K.; Nyang, D.H. WBC image classification and generative models based on convolutional neural network. BMC Med. Imaging 2022, 22, 94. [Google Scholar] [CrossRef]
- Cheuque, C.; Querales, M.; León, R.; Salas, R.; Torres, R. An Efficient Multi-Level Convolutional Neural Network Approach for White Blood Cells Classification. Diagnostics 2022, 12, 248. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, S.; Wang, S.H.; Górriz, J.M.; Zhang, Y.D. BCNet: A Novel Network for Blood Cell Classification. Front. Cell Dev. Biol. 2022, 9, 813996. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, W.; Wu, T.; Wang, M.; Wan, S.; Liu, J. AIMIC: Deep Learning for Microscopic Image Classification. Comput. Methods Programs Biomed. 2022, 226, 107162. [Google Scholar] [CrossRef]
- Liang, G.; Hong, H.; Xie, W.; Zheng, L. Combining Convolutional Neural Network With Recursive Neural Network for Blood Cell Image Classification. IEEE Access 2018, 6, 36188–36197. [Google Scholar] [CrossRef]
- Almezhghwi, K.; Serte, S. Improved Classification of White Blood Cells with the Generative Adversarial Network and Deep Convolutional Neural Network. Comput. Intell. Neurosci. 2020, 2020, 6490479. [Google Scholar] [CrossRef]
- Ma, L.; Shuai, R.; Ran, X.; Liu, W.; Ye, C. Combining DC-GAN with ResNet for blood cell image classification. Med. Biol. Eng. Comput. 2020, 58, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Abbasi, R.; Bin Heyat, M.B.; Akhtar, F.; Abdelgeliel, A.S.; Albogami, S.; Fayad, E.; Iqbal, M.A. Recognition of mRNA N4 Acetylcytidine (ac4C) by Using Non-Deep vs. Deep Learning. Appl. Sci. 2022, 12, 1344. [Google Scholar] [CrossRef]
- An Effective and Lightweight Deep Electrocardiography Arrhythmia Recognition Model Using Novel Special and Native Structural Regularization Techniques on Cardiac Signal. Available online: https://www.hindawi.com/journals/jhe/2022/3408501/ (accessed on 11 October 2022).
- Wijesinghe, C.B.; Wickramarachchi, D.N.; Kalupahana, I.N.; De Seram, L.R.; Silva, I.D.; Nanayakkara, N.D. Fully Automated Detection and Classification of White Blood Cells. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 1816–1819. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Zhou, Z.; Chu, J.; Cao, F. Automatic detection and classification of leukocytes using convolutional neural networks. Med. Biol. Eng. Comput. 2017, 55, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Wang, G.; Liu, J. Fast and robust segmentation of white blood cell images by self-supervised learning. Micron 2018, 107, 55–71. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Jan, Z.; Muhammad, K.; Moon, H.; Kwak, J.T.; Rho, S.; Baik, S.W.; Mehmood, I. Leukocytes Classification and Segmentation in Microscopic Blood Smear: A Resource-Aware Healthcare Service in Smart Cities. IEEE Access 2017, 5, 3475–3489. [Google Scholar] [CrossRef]
- Chola, C.; Benifa, J.V.B.; Guru, D.S.; Muaad, A.Y.; Hanumanthappa, J.; Al-antari, M.A.; Alsalman, H.; Gumaei, A.H. Gender Identification and Classification of Drosophila melanogaster Flies Using Machine Learning Techniques. Comput. Math. Methods Med. 2022, 2022, 4593330. [Google Scholar] [CrossRef]
- Al-masni, M.A.; Al-antari, M.A.; Choi, M.T.; Han, S.M.; Kim, T.S. Skin lesion segmentation in dermoscopy images via deep full resolution convolutional networks. Comput. Methods Programs Biomed. 2018, 162, 221–231. [Google Scholar] [CrossRef] [PubMed]
- He, K. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Zagoruyko, S.; Komodakis, N. Wide Residual Networks. arXiv 2016, arXiv:1605.07146. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking model scaling for convolutional neural networks. In Proceedings of the 36th International Conference on Machine Learning ICML, Long Beach, CA, USA, 9–15 June 2019; pp. 10691–10700. [Google Scholar]
- Lin, M.; Chen, Q.; Yan, S. Network in network. In Proceedings of the 2nd International Conference on Learning Representations, ICLR 2014, Banff, AB, Canada, 14–16 April 2014; pp. 1–10. [Google Scholar]
- Al-masni, M.A.; Al-antari, M.A.; Park, J.M.; Gi, G.; Kim, T.Y.; Rivera, P.; Valarezo, E.; Choi, M.T.; Han, S.M.; Kim, T.S. Simultaneous detection and classification of breast masses in digital mammograms via a deep learning YOLO-based CAD system. Comput. Methods Programs Biomed. 2018, 157, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Al-antari, M.A.; Al-masni, M.A.; Choi, M.T.; Han, S.M.; Kim, T.S. A fully integrated computer-aided diagnosis system for digital X-ray mammograms via deep learning detection, segmentation, and classification. Int. J. Med. Inform. 2018, 117, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Chola, C.; Mallikarjuna, P.; Muaad, A.Y.; Bibal Benifa, J.V.; Hanumanthappa, J.; Al-antari, M.A. A Hybrid Deep Learning Approach for COVID-19 Diagnosis via CT and X-ray Medical Images. Comput. Sci. Math. Forum 2021, 2, 13. [Google Scholar]
- Al-antari, M.A.; Hua, C.H.; Bang, J.; Lee, S. Fast deep learning computer-aided diagnosis of COVID-19 based on digital chest x-ray images. Appl. Intell. 2021, 51, 2890–2907. [Google Scholar] [CrossRef]
- Ukwuoma, C.C.; Qin, Z.; Belal Bin Heyat, M.; Akhtar, F.; Bamisile, O.; Muad, A.Y.; Addo, D.; Al-antari, M.A. A Hybrid Explainable Ensemble Transformer Encoder for Pneumonia Identification from Chest X-ray Images. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Bin Heyat, M.B.; Akhtar, F.; Abbas, S.J.; Al-Sarem, M.; Alqarafi, A.; Stalin, A.; Abbasi, R.; Muaad, A.Y.; Lai, D.; Wu, K. Wearable Flexible Electronics Based Cardiac Electrode for Researcher Mental Stress Detection System Using Machine Learning Models on Single Lead Electrocardiogram Signal. Biosensors 2022, 12, 427. [Google Scholar] [CrossRef]
- Heyat, M.B.; Akhtar, F.; Khan, A.; Noor, A.; Benjdira, B.; Qamar, Y.; Abbas, S.J.; Lai, D. A novel hybrid machine learning classification for the detection of bruxism patients using physiological signals. Appl. Sci. 2020, 10, 7410. [Google Scholar] [CrossRef]
- Sultana, A.; Rahman, K.; Heyat, M.B.; Sumbul; Akhtar, F.; Muaad, A.Y. Role of Inflammation, Oxidative Stress, and Mitochondrial Changes in Premenstrual Psychosomatic Behavioral Symptoms with Anti-Inflammatory, Antioxidant Herbs, and Nutritional Supplements. Oxid. Med. Cell. Longev. 2022, 2022, 3599246. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Begum, W.; Saeedi, R.; Rahman, K.; Bin Heyat, M.B.; Akhtar, F.; Son, N.T.; Ullah, H. Experimental and Computational Approaches for the Classification and Correlation of Temperament (Mizaj) and Uterine Dystemperament (Su’-I-Mizaj Al-Rahim) in Abnormal Vaginal Discharge (Sayalan Al-Rahim) Based on Clinical Analysis Using Support Vector Mach. Complexity 2022, 2022, 5718501. [Google Scholar] [CrossRef]
- Tripathi, P.; Ansari, M.A.; Gandhi, T.K.; Heyat MB, B.; Akhtar, F.; Ukwuoma, C.C.; Muaad, A.Y.; Kadah, Y.M.; Al-Antari, M.A.; Li, J.P.; et al. Ensemble Computational Intelligent for Insomnia Sleep Stage Detection via the Sleep ECG Signal. IEEE Access 2022, 10, 108710–108721. [Google Scholar] [CrossRef]
- Mestetskiy, L.M.; Guru, D.S.; Benifa, J.V.B.; Nagendraswamy, H.S.; Chola, C. Gender identification of Drosophila melanogaster based on morphological analysis of microscopic images. Vis. Comput. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Journal, A.I.; Yao, X.; Sun, K.; Bu, X.; Zhao, C.; Jin, Y. Classification of white blood cells using weighted optimized deformable convolutional neural networks convolutional neural networks. Artif. Cells Nanomed. Biotechnol. 2021, 49, 147–155. [Google Scholar] [CrossRef]
- Baydilli, Y.Y.; Atila, Ü. Classification of white blood cells using capsule networks. Comput. Med. Imaging Graph. 2020, 80, 101699. [Google Scholar] [CrossRef]







| Stage | Operatory | Spatial Resolution Hi × Wi | Channel, Ci | Layer, Li |
|---|---|---|---|---|
| 1 | Conv., k3 × 3 | 224 × 224 | 32 | 1 |
| 2 | MBConv1, k3 × 3 | 112 × 112 | 16 | 1 |
| 3 | MBConv6, k3 × 3 | 112 × 112 | 24 | 2 |
| 4 | MBConv6, k3 × 3 | 56 × 56 | 40 | 2 |
| 5 | MBConv6, k3 × 3 | 28 × 28 | 80 | 3 |
| 6 | MBConv6, k3 × 3 | 14 × 14 | 112 | 3 |
| 7 | MBConv6, k3 × 3 | 14 × 14 | 192 | 4 |
| 8 | MBConv6, k3 × 3 | 7 × 7 | 320 | 1 |
| 9 | Conv1 × 1, | 7 × 7 | 1280 | 1 |
| 10 | GAP | |||
| 11 | 2 Dense Layers | 8 nodes | ||
| 12 | SoftMax | 8 nodes: Number of blood cell classes. | ||
| No. of Fold | Optimizer | SE | SP | Az. | MCC | F1-Score | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Fold 1 | ADAM | 93.89 | 98.14 | 97.53 | 90.93 | 91.66 | 90.44 | 98.55 |
| RMSP | 95.53 | 98.9 | 98.47 | 95.11 | 95.61 | 95.8 | 98.84 | |
| SGD | 93.12 | 98.53 | 97.83 | 92.55 | 93.36 | 93.89 | 98.47 | |
| Fold 2 | ADAM | 97.91 | 99.26 | 99.04 | 97.74 | 97.74 | 98.02 | 99.12 |
| RMSP | 98.3 | 99.26 | 99.13 | 98.15 | 98.3 | 98.33 | 99.22 | |
| SGD | 93.12 | 98.53 | 97.83 | 92.55 | 93.36 | 93.89 | 98.47 | |
| Fold 3 | ADAM | 96.8 | 98.88 | 98.53 | 96.28 | 96.79 | 96.82 | 98.87 |
| RMSP | 95.09 | 98.64 | 98.13 | 94.37 | 95.1 | 95.21 | 98.63 | |
| SGD | 96.6 | 98.85 | 98.49 | 96.05 | 96.59 | 96.61 | 98.84 | |
| Fold 4 | ADAM | 97.16 | 98.99 | 98.7 | 96.73 | 97.15 | 97.15 | 98.86 |
| RMSP | 97.1 | 98.96 | 98.67 | 96.66 | 97.09 | 97.12 | 98.96 | |
| SGD | 96.63 | 98.93 | 98.57 | 96.13 | 96.62 | 96.63 | 98.88 | |
| Fold 5 | ADAM | 96.8 | 98.88 | 98.53 | 96.28 | 96.79 | 96.82 | 98.87 |
| RMSP | 95.09 | 98.64 | 98.13 | 94.37 | 95.1 | 95.21 | 98.63 | |
| SGD | 96.6 | 98.85 | 98.49 | 96.05 | 96.59 | 96.61 | 98.84 | |
| Avg. (%) | ADAM | 96.51 | 98.83 | 98.47 | 95.59 | 96.03 | 95.85 | 98.85 |
| RMSP | 96.22 | 98.88 | 98.51 | 95.73 | 96.24 | 96.33 | 98.86 | |
| SGD | 95.21 | 98.74 | 98.24 | 94.67 | 95.30 | 95.53 | 98.70 |
| AI Model | Number of Trainable Parameters (million) | Training Time Per Epoch (sec.) | Testing Time/Image (msec.) |
|---|---|---|---|
| DenseNet 201 | 18.10 | 286 | 18.13 |
| ResNet 50 | 23.55 | 148 | 11.41 |
| Inception V3 | 21.78 | 128 | 9.18 |
| MobileNet V2 | 2.23 | 123 | 7.36 |
| The proposed BCNet | 4.017 | 122 | 7.15 |
| Reference | Data | Methods | Az. (%) |
|---|---|---|---|
| Zhao et al., 2017 [44] | Cell vision, ALL-IDB, Jiashan | CNN, SVM, and random forest | 92.80 |
| Journal et al., 2021 [66] | Collected, BCCD data set | Two DCNN | 95.17 (Precession) |
| Acevedo et al., 2019 [32] | Private | CNN + Transfer learning | 96 |
| Qin et al., 2018 [29] | Private | CNN | 76.84 |
| Ma et al., 2020 [40] | BCCD | DCGAN + Transfer learning | 91.7 |
| Baydilli and Atila 2020, [67] | LISC | Capsule network | 96.86 |
| Rui Liu et al., 2022 [37] | HPBC | Transfer Learning | 96.83 |
| The proposed BCNet | HPBC images | BCNet | 98.51 |
| AI Models | SE | SP | Az. | MCC | F1-Score | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Baseline Model | ADAM | 95.8 | 97.88 | 96.53 | 93.38 | 94.89 | 94.82 | 96.88 |
| RMSP | 94.09 | 95.66 | 95.18 | 94.37 | 94.11 | 94.21 | 95.63 | |
| SGD | 93.50 | 96.65 | 96.59 | 95.05 | 94.55 | 95.61 | 97.84 | |
| The Proposed BCNet | ADAM | 96.51 | 98.83 | 98.47 | 95.59 | 96.03 | 95.85 | 98.85 |
| RMSP | 96.22 | 98.88 | 98.51 | 95.73 | 96.24 | 96.33 | 98.86 | |
| SGD | 95.21 | 98.74 | 98.24 | 94.67 | 95.30 | 95.53 | 98.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chola, C.; Muaad, A.Y.; Bin Heyat, M.B.; Benifa, J.V.B.; Naji, W.R.; Hemachandran, K.; Mahmoud, N.F.; Samee, N.A.; Al-Antari, M.A.; Kadah, Y.M.; et al. BCNet: A Deep Learning Computer-Aided Diagnosis Framework for Human Peripheral Blood Cell Identification. Diagnostics 2022, 12, 2815. https://doi.org/10.3390/diagnostics12112815
Chola C, Muaad AY, Bin Heyat MB, Benifa JVB, Naji WR, Hemachandran K, Mahmoud NF, Samee NA, Al-Antari MA, Kadah YM, et al. BCNet: A Deep Learning Computer-Aided Diagnosis Framework for Human Peripheral Blood Cell Identification. Diagnostics. 2022; 12(11):2815. https://doi.org/10.3390/diagnostics12112815
Chicago/Turabian StyleChola, Channabasava, Abdullah Y. Muaad, Md Belal Bin Heyat, J. V. Bibal Benifa, Wadeea R. Naji, K. Hemachandran, Noha F. Mahmoud, Nagwan Abdel Samee, Mugahed A. Al-Antari, Yasser M. Kadah, and et al. 2022. "BCNet: A Deep Learning Computer-Aided Diagnosis Framework for Human Peripheral Blood Cell Identification" Diagnostics 12, no. 11: 2815. https://doi.org/10.3390/diagnostics12112815








