The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussions
5. Limitations of the Study
6. Conclusions and Future Work
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining vitamin D insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
- Thomas, M.K.; Lloyd-Jones, D.M.; Thadhani, R.I.; Shaw, A.C.; Deraska, D.J.; Kitch, B.T.; Vamvakas, E.C.; Dick, I.M.; Prince, R.L.; Finkelstein, J.S. Hypovitaminosis D in medical inpatients. N. Engl. J. Med. 1998, 338, 777–783. [Google Scholar] [CrossRef]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3215–3224. [Google Scholar] [CrossRef]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016. Endocr. Pract. 2016, 22 (Suppl. S4), 1–42. [Google Scholar] [CrossRef]
- Heaney, R.P. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1706S–1709S. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Schott, A.M.; Garnero, P.; Hans, D.; Delmas, P.D.; Meunier, P.J. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J. Clin. Endocrinol. Metab. 1996, 81, 1129–1133. [Google Scholar] [PubMed]
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.M.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Intern. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Greene-Finestone, L.S.; Berger, C.; de Groh, M.; Hanley, D.A.; Hidiroglou, N.; Sarafin, K.; Poliquin, S.; Krieger, J.; Richards, J.B.; Goltzman, D.; et al. 25-Hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. Osteoporos Int. 2011, 22, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Shah, S.; Chiang, C.; Sikaris, K.; Lu, Z.; Bui, M.; Zebaze, R.; Seeman, E. Serum 25-Hydroxyvitamin D Insufficiency in Search of a Bone Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2321–2328. [Google Scholar] [CrossRef]
- Plotnikoff, G.A.; Quigley, J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin. Proc. 2003, 78, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, P.; Ghetu, M.V.; Langan, R. Recognition and Management of Vitamin D Deficiency. Am. Fam. Phys. 2009, 80, 841–846. [Google Scholar]
- Arvold, D.S.; Odean, M.J.; Dornfeld, M.P.; Regal, R.R.; Arvold, J.G.; Karwoski, G.C.; Mast, D.J.; Sanford, P.B.; Sjoberg, R.J. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: A randomized controlled trial. Endocr. Pract. 2009, 15, 203–212. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, Y.; Ryu, S.; Cho, I.Y.; Kwon, M.J.; Wild, S.H.; Byrne, C.D. Serum 25-hydroxy vitamin D and the risk of low muscle mass in young and middle-aged Korean adults. Eur. J. Endocrinol. 2022, 186, 477–487. [Google Scholar] [CrossRef]
- Choi, R.; Cho, S.E.; Lee, S.G.; Lee, E.H. Recent Information on Vitamin D Deficiency in an Adult Korean Population Visiting Local Clinics and Hospitals. Nutrients 2022, 14, 1978. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Porojnicu, A.C.; Dahlback, A.; Setlow, R.B. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc. Natl. Acad. Sci. USA 2008, 105, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Pfeiffer, C.M.; Lacher, D.A.; Schleicher, R.L.; Picciano, M.F.; Yetley, E.A. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am. J. Clin. Nutr. 2008, 88, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, L.Y.; Ide, L.; Wortsman, J.; MacLaughlin, J.A.; Holick, M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J. Clin. Endocrinol. Metab. 1987, 64, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Clemens, T.L.; Adams, J.S.; Henderson, S.L.; Holick, M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1982, 1, 74–76. [Google Scholar] [CrossRef]
- Hintzpeter, B.; Scheidt-Nave, C.; Muller, M.J.; Schenk, L.; Mensink, G.B. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J. Nutr. 2008, 138, 1482–1490. [Google Scholar] [CrossRef]
- Heaney, R.P.; Dowell, M.S.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar] [CrossRef]
- Nesby-O’Dell, S.; Scanlon, K.S.; Cogswell, M.E.; Gillespie, C.; Hollis, B.W.; Looker, A.C.; Allen, C.; Doughertly, C.; Gunter, E.W.; Bowman, B.A. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 2002, 76, 187–192. [Google Scholar] [CrossRef]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Saliba, W.; Barnett, O.; Rennert, H.S.; Lavi, I.; Rennert, G. The relationship between serum 25(OH)D and parathyroid hormone levels. Am. J. Med. 2011, 124, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Carter, G.D.; Mishra, G.D.; O’Shea, D.; Jones, J.; Prentice, A. In a population study, can parathyroid hormone aid the definition of adequate vitamin D status? A study of people aged 65 years and over from the British National Diet and Nutrition Survey. Osteoporos. Int. 2003, 14, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef] [PubMed]
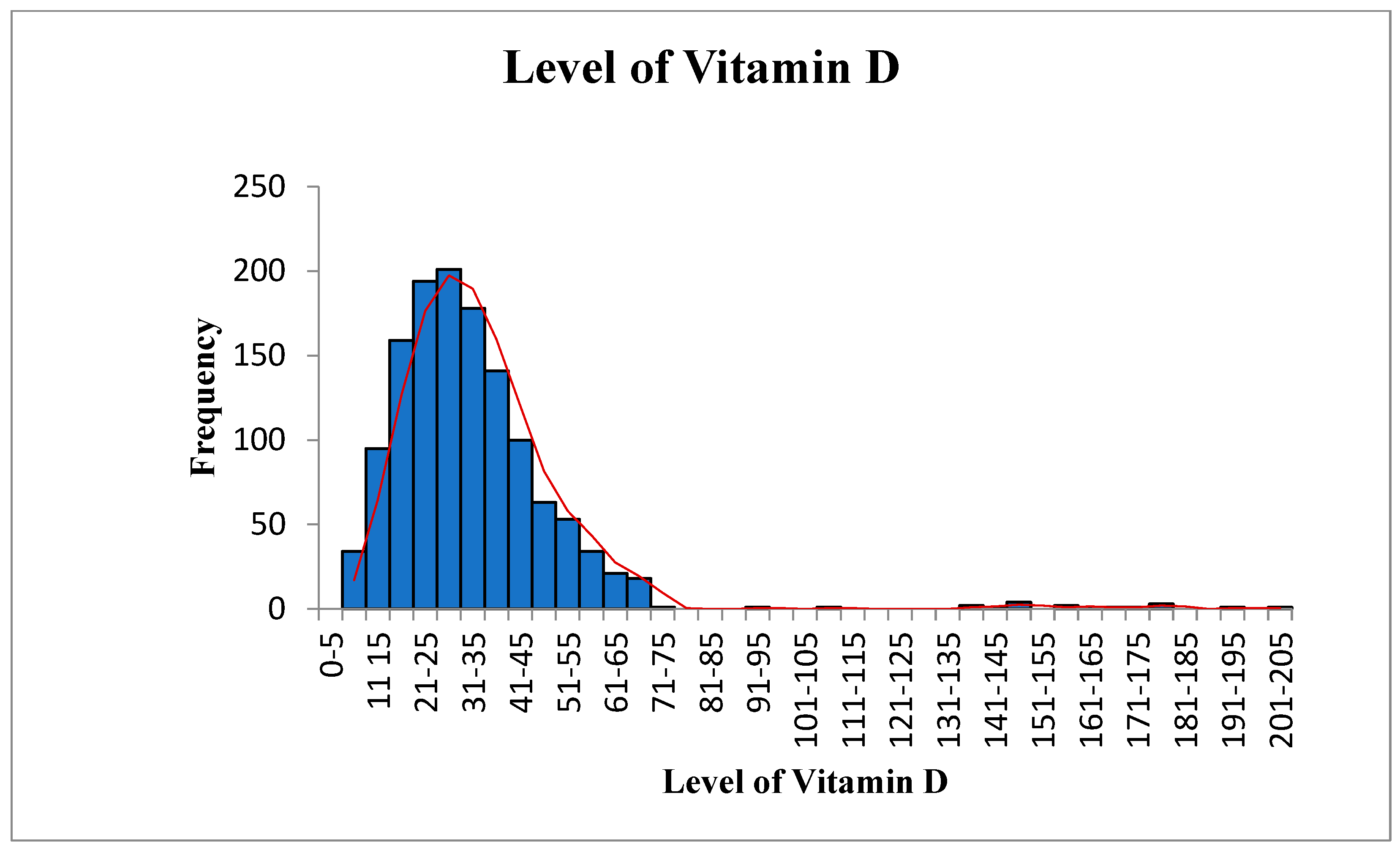
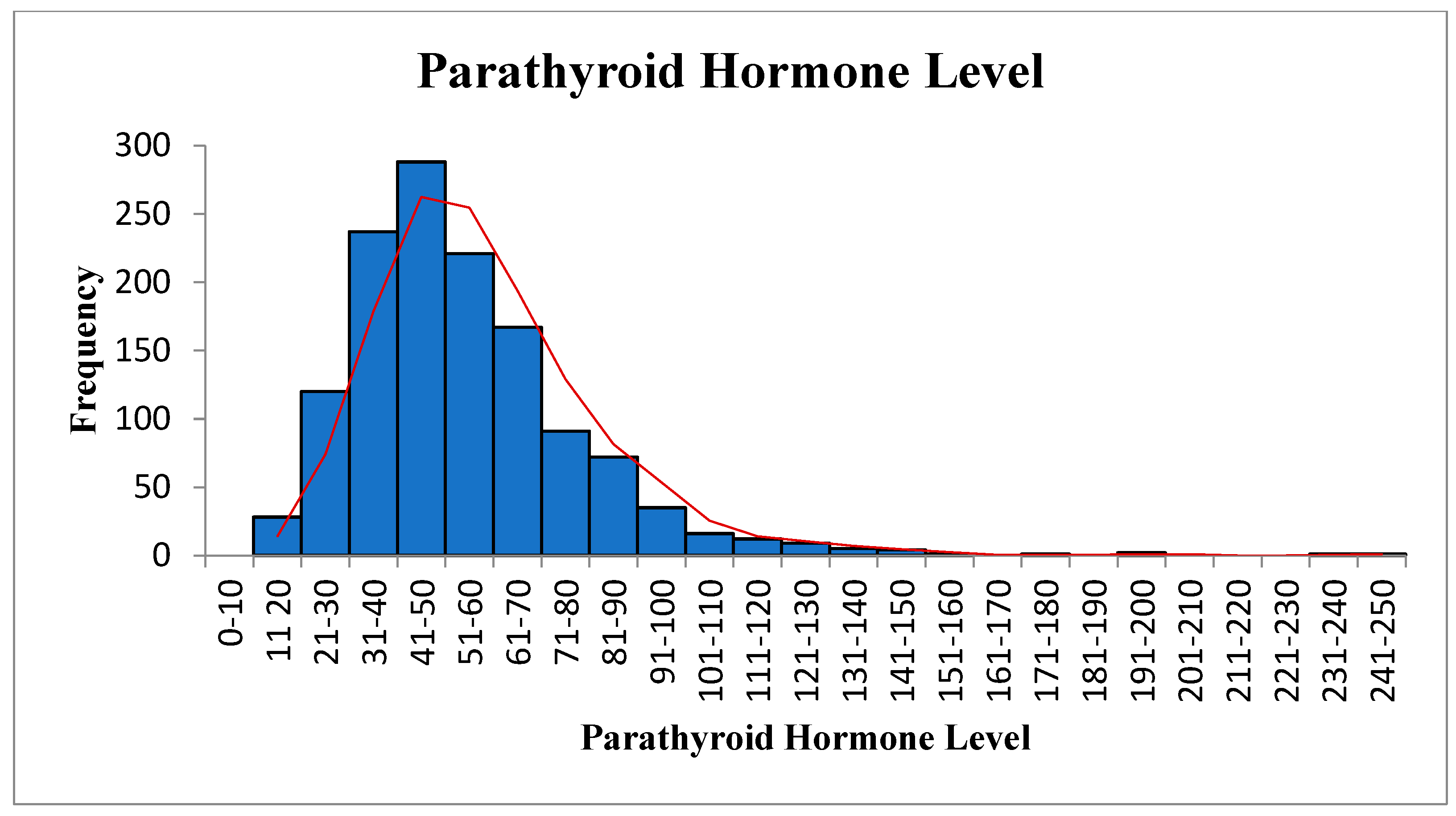


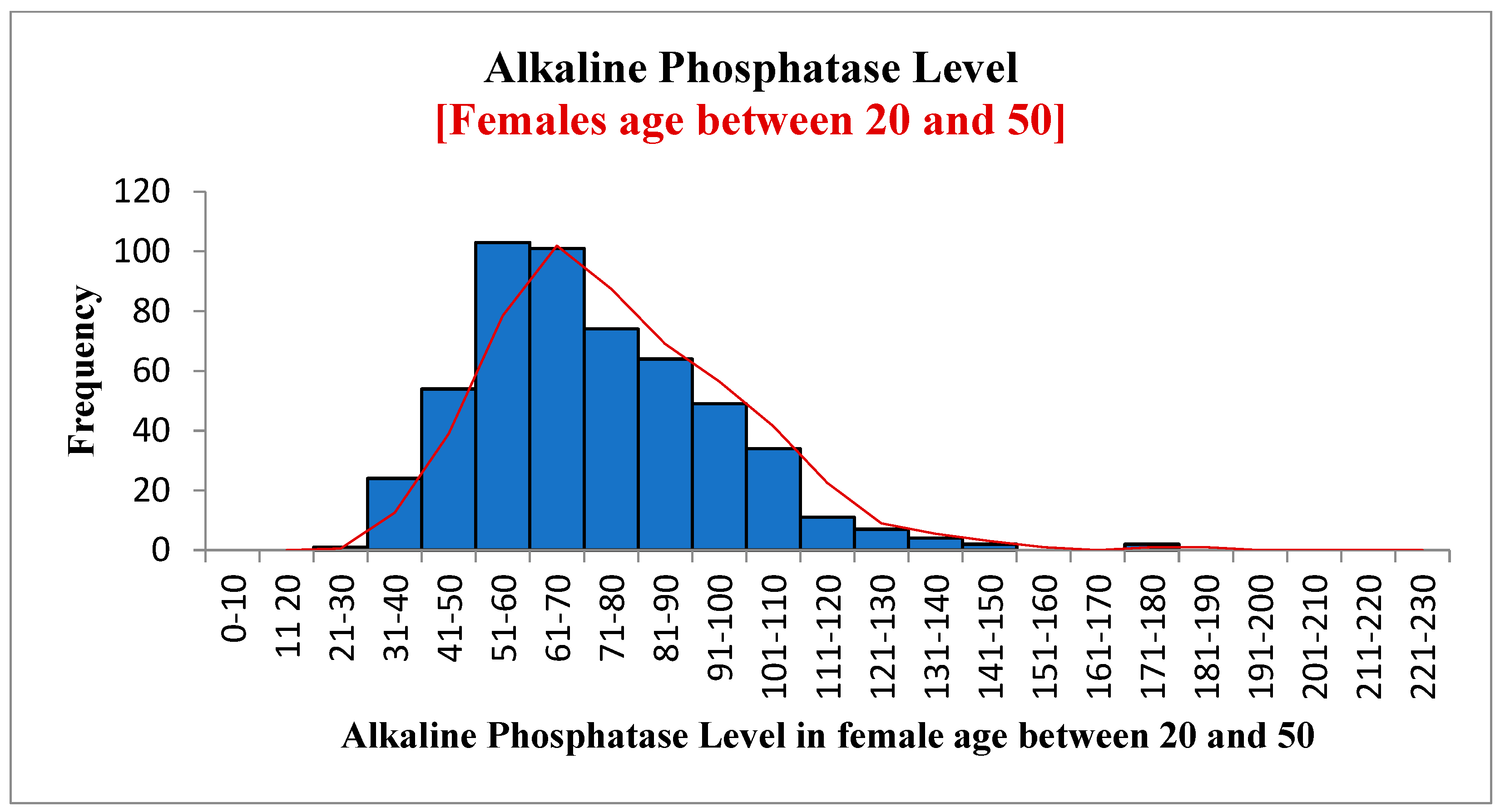
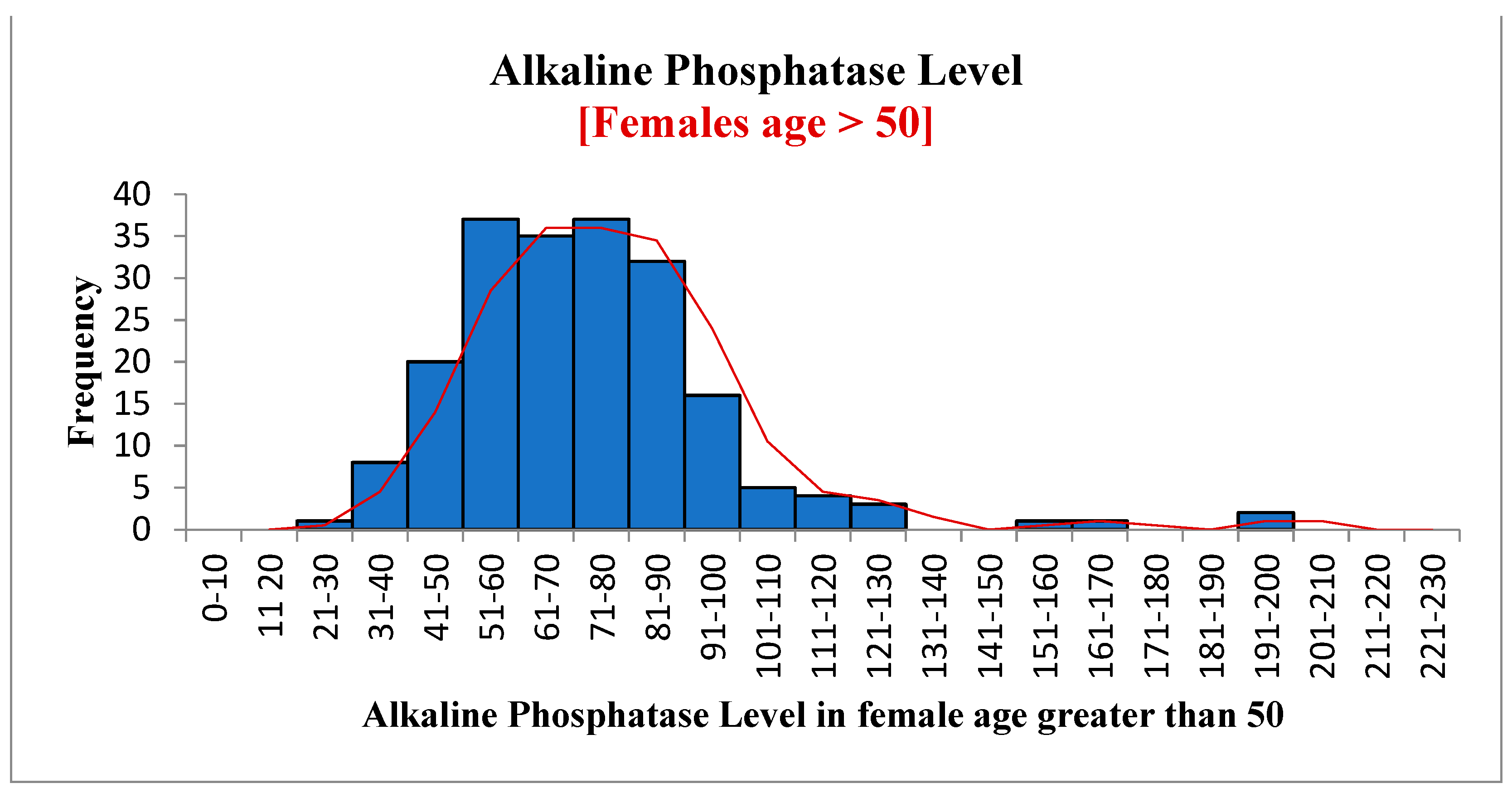

| Variables | Statistic | Std. Error | ||
|---|---|---|---|---|
| 25OHD (ng/mL) | Mean | 32.52 | 0.56 | |
| 95% Confidence Interval for Mean | Lower Bound | 31.43 | ||
| Upper Bound | 33.61 | |||
| 5% Trimmed Mean | 30.88 | |||
| Median | 29.30 | |||
| Variance | 405.75 | |||
| Std. Deviation | 20.14 | |||
| Range | 197 | |||
| Interquartile Range | 17.77 | |||
| PTH (pg/mL) | Mean | 53.84 | 0.67 | |
| 95% Confidence Interval for Mean | Lower Bound | 52.53 | ||
| Upper Bound | 55.15 | |||
| 5% Trimmed Mean | 52.54 | |||
| Median | 49.20 | |||
| Variance | 582.83 | |||
| Std. Deviation | 24.13 | |||
| Range | 238 | |||
| Interquartile Range | 17.77 | |||
| ALK (U/L) | Mean | 73.14 | 0.67 | |
| 95% Confidence Interval for Mean | Lower Bound | 71.83 | ||
| Upper Bound | 74.45 | |||
| 5% Trimmed Mean | 71.79 | |||
| Median | 69.0 | |||
| Variance | 586.83 | |||
| Std. Deviation | 24.22 | |||
| Range | 221 | |||
| Interquartile Range | 30.65 | |||
| Classification | Levels | Frequency (%) |
|---|---|---|
| 25OHD (ng/mL) | ||
| Severe deficiency | <10 | 34 (3) |
| Moderate deficiency | 10–<15 | 95 (7) |
| Mild deficiency | 15–<20 | 159 (12) |
| Insufficient | 20–<30 | 395 (30) |
| Normal | 31–100 | 610 (47) |
| Increased | >101 | 18 (1) |
| PTH (pg/mL) | ||
| Deficient | <15 | 8 (1) |
| Normal | 15–65 | 976 (74) |
| Increased | >65 | 327 (25) |
| ALP (IU/L) | ||
| Low | <35 | 9 (1) |
| Normal | 35–104 | 1188 (91) |
| Increased | >104 | 114 (9) |
| 25OHD (ng/mL) | PTH (pg/mL) | p Value | |||
| <15 pg/mL (Deficient) # Of patients (%) | 15–65 pg/mL (Normal) # Of patients (%) | >65 pg/mL (Increased) # Of patients (%) | Total (%) | ||
| Severe deficiency (<10) | 0 | 12 (35) | 22 (65) | 34 (100) | <0.001 |
| Moderate deficiency (10–<15) | 1 (1) | 39 (41) | 55 (58) | 95 (100) | |
| Mild deficiency (15–<20) | 0 | 111 (70) | 48 (30) | 159 (100) | |
| Insufficient (20–<30) | 3 (0.7) | 291 (73.6) | 101 (25.5) | 395 (100) | |
| Normal (30–100) | 4 (1) | 506 (83) | 100 (16) | 610 (100) | |
| Increased (>100) | 0 | 17 (94) | 1 (6) | 18 (100) | |
| 25OHD (ng/mL) | ALP (IU/L) | p Value | |||
| <35 IU/L (Low) # Of patients (%) | 35–104 IU/L (Normal) # Of patients (%) | >104 IU/L (Increased) # Of patients (%) | Total (%) | ||
| Severe deficiency (<10) | 0 | 27 (79) | 7 (21) | 34 (100) | <0.001 |
| Moderate deficiency (10–<15) | 0 | 80 (84) | 15 (16) | 95 (100) | |
| Mild deficiency (15–<20) | 0 | 139 (87) | 20 (13) | 159 (100) | |
| Insufficient (20–<30) | 0 | 362 (92) | 33 (8) | 395 (100) | |
| Normal (30–100) | 8 (1) | 563(93) | 39 (6) | 610 (100) | |
| Increased (>100) | 1 (6) | 17 (94) | 0 | 18 (100) | |
| Vitamin D (ng/mL) | PTH (Mean ± SD) | ALP (Mean ± SD) |
|---|---|---|
| Severe deficiency (<10) | 85.56 ± 38.80 | 82.61 ± 30.12 |
| Moderate deficiency (10–<15) | 70.24 ± 29.90 | 81.67 ± 28.06 |
| Mild deficiency (15–< 20) | 59.09 ± 28.24 | 78.54 ± 24.36 |
| Insufficient (20–<30) | 53.80 ± 21.20 | 75.47 ± 24.26 |
| Normal (30–100) | 48.63 ± 19.63 | 68.89 ± 22.15 |
| Increased (>100) | 38.49 ± 20.34 | 55.52 ± 20.15 |
| Total | 53.84 ± 24.14 | 73.14 ± 24.22 |
| Variables | Level of Vitamin D (ng/mL) | Level of PTH (pg/mL) | Level of ALK (IU/L) | |
|---|---|---|---|---|
| Level of vitamin D (ng/mL) | Correlation Coefficient (Rs) | 1.000 | −0.24 * | −0.18 * |
| Sig. (2-tailed) | ---- | <0.001 | <0.001 | |
| Level of PTH (pg/mL) | Correlation Coefficient (Rs) | −0.24 ** | 1.000 | 0.11 ** |
| Sig. (2-tailed) | <0.001 | . | <0.001 | |
| Level of ALP (IU/L) | Correlation Coefficient (Rs) | −0.18 ** | 0.11 ** | 1.000 |
| Sig. (2-tailed) | <0.001 | <0.001 | ---- | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajab, H.A. The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase. Diagnostics 2022, 12, 2828. https://doi.org/10.3390/diagnostics12112828
Rajab HA. The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase. Diagnostics. 2022; 12(11):2828. https://doi.org/10.3390/diagnostics12112828
Chicago/Turabian StyleRajab, Hussein Abdullah. 2022. "The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase" Diagnostics 12, no. 11: 2828. https://doi.org/10.3390/diagnostics12112828
APA StyleRajab, H. A. (2022). The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase. Diagnostics, 12(11), 2828. https://doi.org/10.3390/diagnostics12112828






