Deep Learning-Based Glaucoma Screening Using Regional RNFL Thickness in Fundus Photography
Abstract
1. Introduction
2. Materials and Methods
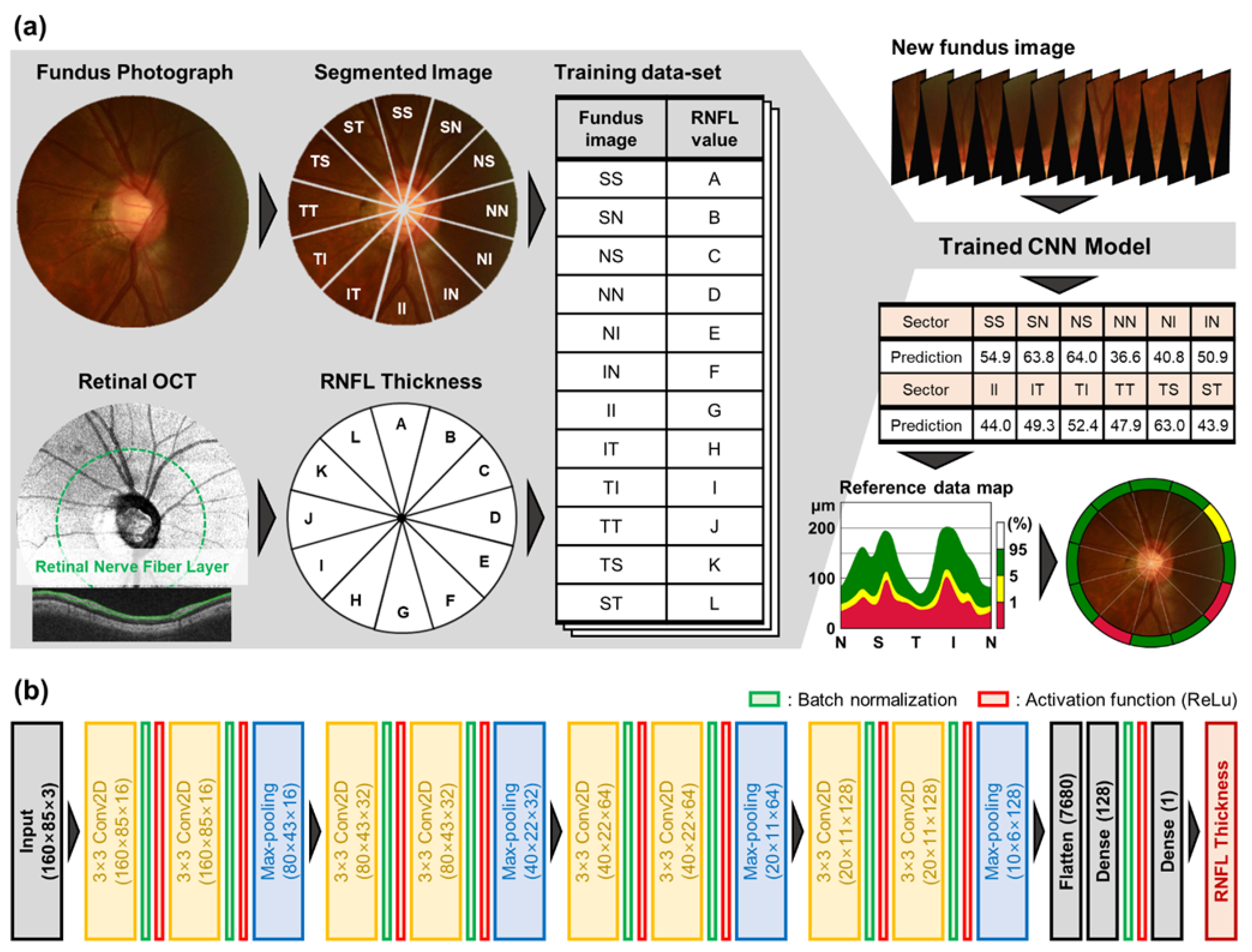
2.1. Data Preparation
2.2. CNN Architecture for RNFL Prediction and Training
2.3. Estimation and Categorization of RNFL Thickness for Glaucoma Screening
3. Results
3.1. Model Evaluation and Regional RNFL Thinning Level
3.2. Regional RNFL Thinning and Glaucoma Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Greco, A.; Rizzo, M.I.; De Virgilio, A.; Gallo, A.; Fusconi, M.; de Vincentiis, M. Emerging Concepts in Glaucoma and Review of the Literature. Am. J. Med. 2016, 129, 1000.e1007–1000.e1013. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Haleem, M.S.; Han, L.; van Hemert, J.; Li, B. Automatic extraction of retinal features from colour retinal images for glaucoma diagnosis: A review. Comput. Med. Imaging Graph. 2013, 37, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.S.; Fudemberg, S.J.; Lee, D. Evolution of optic nerve photography for glaucoma screening: A review. Clin. Exp. Ophthalmol. 2018, 46, 169–176. [Google Scholar] [CrossRef]
- Bock, R.; Meier, J.; Nyúl, L.G.; Hornegger, J.; Michelson, G. Glaucoma risk index:Automated glaucoma detection from color fundus images. Med. Image Anal. 2010, 14, 471–481. [Google Scholar] [CrossRef]
- Badalà, F.; Nouri-Mahdavi, K.; Raoof, D.A.; Leeprechanon, N.; Law, S.K.; Caprioli, J. Optic Disk and Nerve Fiber Layer Imaging to Detect Glaucoma. Am. J. Ophthalmol. 2007, 144, 724–732. [Google Scholar] [CrossRef]
- Niemeijer, M.; Ginneken, B.v.; Cree, M.J.; Mizutani, A.; Quellec, G.; Sanchez, C.I.; Zhang, B.; Hornero, R.; Lamard, M.; Muramatsu, C.; et al. Retinopathy Online Challenge: Automatic Detection of Microaneurysms in Digital Color Fundus Photographs. IEEE Trans. Med. Imaging 2010, 29, 185–195. [Google Scholar] [CrossRef]
- Jin, K.; Lu, H.; Su, Z.; Cheng, C.; Ye, J.; Qian, D. Telemedicine screening of retinal diseases with a handheld portable non-mydriatic fundus camera. BMC Ophthalmol. 2017, 17, 89. [Google Scholar] [CrossRef]
- Upadhyaya, S.; Agarwal, A.; Rengaraj, V.; Srinivasan, K.; Newman Casey, P.A.; Schehlein, E. Validation of a portable, non-mydriatic fundus camera compared to gold standard dilated fundus examination using slit lamp biomicroscopy for assessing the optic disc for glaucoma. Eye 2021, 36, 441–447. [Google Scholar] [CrossRef]
- Reis, A.S.C.; O’Leary, N.; Yang, H.; Sharpe, G.P.; Nicolela, M.T.; Burgoyne, C.F.; Chauhan, B.C. Influence of Clinically Invisible, but Optical Coherence Tomography Detected, Optic Disc Margin Anatomy on Neuroretinal Rim Evaluation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1852–1860. [Google Scholar] [CrossRef]
- Adhi, M.; Duker, J.S. Optical coherence tomography--current and future applications. Curr. Opin. Ophthalmol. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Thomas, D.; Duguid, G. Optical coherence tomography—A review of the principles and contemporary uses in retinal investigation. Eye 2004, 18, 561–570. [Google Scholar] [CrossRef]
- Wu, H.; De Boer, J.F.; Chen, T.C. Diagnostic capability of spectral-domain optical coherence tomography for glaucoma. Am. J. Ophthalmol. 2012, 153, 815–826.e812. [Google Scholar] [CrossRef]
- Povazay, B.; Hofer, B.; Hermann, B.M.; Unterhuber, A.; Morgan, J.E.; Glittenberg, C.; Binder, S.; Drexler, W. Minimum distance mapping using three-dimensional optical coherence tomography for glaucoma diagnosis. J. Biomed. Opt. 2007, 12, 041204. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98, ii15–ii19. [Google Scholar] [CrossRef]
- Chen, T.C. Spectral domain optical coherence tomography in glaucoma: Qualitative and quantitative analysis of the optic nerve head and retinal nerve fiber layer (an AOS thesis). Trans. Am. Ophthalmol. Soc. 2009, 107, 254–281. [Google Scholar]
- Moreno, P.A.M.; Konno, B.; Lima, V.C.; Castro, D.P.E.; Castro, L.C.; Leite, M.T.; Pacheco, M.A.M.M.; Lee, J.M.; Prata, T.S. Spectral-domain optical coherence tomography for early glaucoma assessment: Analysis of macular ganglion cell complex versus peripapillary retinal nerve fiber layer. Can. J. Ophthalmol. 2011, 46, 543–547. [Google Scholar] [CrossRef]
- Sakata, K.; Sakata, L.M.; Sakata, V.M.; Santini, C.; Hopker, L.M.; Bernardes, R.; Yabumoto, C.; Moreira, A.T. Prevalence of glaucoma in a South brazilian population: Projeto Glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4974–4979. [Google Scholar] [CrossRef]
- Takahashi, H.; Tampo, H.; Arai, Y.; Inoue, Y.; Kawashima, H. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS ONE 2017, 12, e0179790. [Google Scholar] [CrossRef]
- Sahlsten, J.; Jaskari, J.; Kivinen, J.; Turunen, L.; Jaanio, E.; Hietala, K.; Kaski, K. Deep learning fundus image analysis for diabetic retinopathy and macular edema grading. Sci. Rep. 2019, 9, 10750. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Pagidimarri, V.; Barreto, R.; Kadam, A.; Kasivajjala, V.; Aswath, A. Development of a deep learning algorithm for automatic diagnosis of diabetic retinopathy. In MEDINFO 2017: Precision Healthcare through Informatics; IOS Press: Amsterdam, The Netherlands, 2017; pp. 559–563. [Google Scholar]
- Li, Z.; He, Y.; Keel, S.; Meng, W.; Chang, R.T.; He, M. Efficacy of a deep learning system for detecting glaucomatous optic neuropathy based on color fundus photographs. Ophthalmology 2018, 125, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Singh, A.; Leopold, H.A.; Gulati, T.; Lakshminarayanan, V. Ophthalmic diagnosis using deep learning with fundus images–a critical review. Artif. Intell. Med. 2020, 102, 101758. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.; Hoseini, P.; Walker, E.; Proudfoot, J.A.; Bowd, C.; Fazio, M.A.; Girkin, C.A.; De Moraes, C.G.; Liebmann, J.M.; Weinreb, R.N. A deep learning approach to improve retinal structural predictions and aid glaucoma neuroprotective clinical trial design. Ophthalmol. Glaucoma 2022. [Google Scholar] [CrossRef]
- Ali, R.; Hardie, R.C.; Narayanan, B.N.; Kebede, T.M. IMNets: Deep Learning Using an Incremental Modular Network Synthesis Approach for Medical Imaging Applications. Appl. Sci. 2022, 12, 5500. [Google Scholar] [CrossRef]
- Jammal, A.A.; Thompson, A.C.; Mariottoni, E.B.; Berchuck, S.I.; Urata, C.N.; Estrela, T.; Wakil, S.M.; Costa, V.P.; Medeiros, F.A. Human Versus Machine: Comparing a Deep Learning Algorithm to Human Gradings for Detecting Glaucoma on Fundus Photographs. Am. J. Ophthalmol. 2020, 211, 123–131. [Google Scholar] [CrossRef]
- Jampel, H.D.; Friedman, D.; Quigley, H.; Vitale, S.; Miller, R.; Knezevich, F.; Ding, Y. Agreement among glaucoma specialists in assessing progressive disc changes from photographs in open-angle glaucoma patients. Am. J. Ophthalmol. 2009, 147, 39–44.e31. [Google Scholar] [CrossRef]
- Varma, R.; Steinmann, W.C.; Scott, I.U. Expert agreement in evaluating the optic disc for glaucoma. Ophthalmology 1992, 99, 215–221. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Katz, J.; Quigley, H.A.; Miller, N.R.; Sommer, A. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology 1988, 95, 350–356. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Jammal, A.A.; Thompson, A.C. From machine to machine: An OCT-trained deep learning algorithm for objective quantification of glaucomatous damage in fundus photographs. Ophthalmology 2019, 126, 513–521. [Google Scholar] [CrossRef]
- Thompson, A.C.; Jammal, A.A.; Medeiros, F.A. A deep learning algorithm to quantify neuroretinal rim loss from optic disc photographs. Am. J. Ophthalmol. 2019, 201, 9–18. [Google Scholar] [CrossRef]
- Zhao, J.; Solano, M.M.; Oldenburg, C.E.; Liu, T.; Wang, Y.; Wang, N.; Lin, S.C. Prevalence of normal-tension glaucoma in the Chinese population: A systematic review and meta-analysis. Am. J. Ophthalmol. 2019, 199, 101–110. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.-W.; Park, K.H.; Kim, J.M. Risk factors for primary open-angle glaucoma in South Korea: The Namil study. Jpn. J. Ophthalmol. 2012, 56, 324–329. [Google Scholar] [CrossRef]
- Shields, M.B. Normal-tension glaucoma: Is it different from primary open-angle glaucoma? Curr. Opin. Ophthalmol. 2008, 19, 85–88. [Google Scholar] [CrossRef]
- Woo, S.; Park, K.H.; Kim, D. Comparison of localised nerve fibre layer defects in normal tension glaucoma and primary open angle glaucoma. Br. J. Ophthalmol. 2003, 87, 695–698. [Google Scholar] [CrossRef]
- Thonginnetra, O.; Greenstein, V.C.; Chu, D.; Liebmann, J.M.; Ritch, R.; Hood, D.C. Normal versus high tension glaucoma: A comparison of functional and structural defects. J. Glaucoma 2010, 19, 151. [Google Scholar] [CrossRef]
- Suh, M.; Kim, D.; Kim, Y.; Kim, T.; Park, K. Patterns of progression of localized retinal nerve fibre layer defect on red-free fundus photographs in normal-tension glaucoma. Eye 2010, 24, 857–863. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y. Convolutional networks for images, speech, and time series. Handb. Brain Theory Neural Netw. 1995, 3361, 1995. [Google Scholar]
- Ioffe, S.; Szegedy, C. Batch normalization: Accelerating deep network training by reducing internal covariate shift. In Proceedings of International Conference on Machine Learning, Virtual Event, 18–24 July 2021; pp. 448–456. [Google Scholar]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. In Proceedings of ICML, Baltimore, MD, USA, 17–23 July 2022. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Bendschneider, D.; Tornow, R.P.; Horn, F.K.; Laemmer, R.; Roessler, C.W.; Juenemann, A.G.; Kruse, F.E.; Mardin, C.Y. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. J. Glaucoma 2010, 19, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chaglasian, M.; Fingeret, M.; Davey, P.G.; Huang, W.-C.; Leung, D.; Ng, E.; Reisman, C.A. The development of a reference database with the Topcon 3D OCT-1 Maestro. Clin. Ophthalmol. (Auckl. NZ) 2018, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Sung, K.R.; Song, M.K. Ganglion cell-inner plexiform layer and retinal nerve fiber layer changes in glaucoma suspects enable prediction of glaucoma development. Am. J. Ophthalmol. 2020, 210, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, Y.; Chung, J.K.; Lee, K.B. Glaucomatous progression in the retinal nerve fibre and retinal ganglion cell-inner plexiform layers determined using optical coherence tomography-guided progression analysis. Clin. Exp. Optom. 2018, 101, 666–673. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, Y.K.; Park, K.H.; Jeoung, J.W. Trend-based analysis of ganglion cell–inner plexiform layer thickness changes on optical coherence tomography in glaucoma progression. Ophthalmology 2017, 124, 1383–1391. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Jammal, A.A.; Mariottoni, E.B. Detection of progressive glaucomatous optic nerve damage on fundus photographs with deep learning. Ophthalmology 2021, 128, 383–392. [Google Scholar] [CrossRef]
- Phene, S.; Dunn, R.C.; Hammel, N.; Liu, Y.; Krause, J.; Kitade, N.; Schaekermann, M.; Sayres, R.; Wu, D.J.; Bora, A. Deep learning and glaucoma specialists: The relative importance of optic disc features to predict glaucoma referral in fundus photographs. Ophthalmology 2019, 126, 1627–1639. [Google Scholar] [CrossRef]
- Prince, J.; Thompson, A.; Mwanza, J.-C.; Tolleson-Rinehart, S.; Budenz, D.L. Glaucoma Screening Using an iPad-Based Visual Field Test in a West African Population. Ophthalmol. Glaucoma 2022, 5, 275–283. [Google Scholar] [CrossRef]







| Collected Dataset | |
|---|---|
| Number of patients | 303 |
| Number of eye examinations | 557 |
| Number of Fundus-OCT paired data Number of segmented Fundus and OCT value pairs | 940 11,280 |
| Patients’ age (years) | 54.7 ± 13.9 |
| Gender (% of female) | 48.3 |
| Intraocular pressure (mmHg) | 13.87 ± 3.01 |
| Mean deviation (dB) | −4.69 ± 5.37 |
| Visual field index (%) | 88.39 ± 15.63 |
| Global RNFL (µm) | 84.31 ± 16.21 |
| Model | Predict | MAE | R-Squared | Pearson’s Correlation |
|---|---|---|---|---|
| Previous study [33] | Global RNFL | 7.39 m | 0.693 | 0.832 |
| Proposed CNN | Global RNFL | 9.38m | 0.502 | 0.710 |
| Proposed CNN | Regional RNFL | 16.22m | 0.578 | 0.760 |
| Region | Acronym | OCT Measurement | CNN Prediction |
|---|---|---|---|
| Global | G | 83.9 ± 16.1 | 85.0 ± 13.3 |
| Superior temporal | ST | 106.0 ± 36.3 | 105.3 ± 24.7 |
| Superior | SS | 107.0 ± 31.3 | 105.0 ± 26.9 |
| Superior nasal | SN | 101.0 ± 25.7 | 87.8 ± 20.8 |
| Nasal superior | NS | 78.7 ± 21.1 | 76.9 ± 16.9 |
| Nasal | NN | 57.6 ± 12.7 | 61.2 ± 11.0 |
| Nasal inferior | NI | 65.4 ± 16.7 | 71.3 ± 13.1 |
| Inferior nasal | IN | 93.9 ± 24.4 | 91.7 ± 18.4 |
| Inferior | II | 104.1 ± 38.2 | 101.4 ± 26.2 |
| Inferior temporal | IT | 87.2 ± 41.2 | 88.3 ± 22.5 |
| Temporal inferior | TI | 64.2 ± 16.8 | 71.8 ± 11.6 |
| Temporal | TT | 61.6 ± 13.4 | 70.2 ± 11.4 |
| Temporal superior | TS | 79.9 ± 22.2 | 79.7 ± 15.4 |
| G | ST | SS | SN | NS | NN | NI | IN | II | IT | TI | TT | TS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glaucoma | 85.0 | 105.2 | 104.9 | 87.7 | 76.9 | 61.2 | 71.2 | 91.7 | 101.3 | 88.2 | 71.8 | 70.1 | 79.7 |
| 13.3 | 24.8 | 27.0 | 20.8 | 16.9 | 10.9 | 13.2 | 18.4 | 26.2 | 22.5 | 11.6 | 11.4 | 15.4 | |
| Suspicious | 86.5 | 100.8 | 108.7 | 94.8 | 83.9 | 70.6 | 80.7 | 94.8 | 107.7 | 90.4 | 72.2 | 71.5 | 78.5 |
| 10.2 | 20.3 | 17.1 | 15.5 | 13.5 | 10.6 | 12.7 | 15.6 | 19.9 | 18.1 | 8.4 | 8.2 | 9.9 | |
| Normal | 104.1 | 133.2 | 128.2 | 114.8 | 99.4 | 78.7 | 90.1 | 115.5 | 133.6 | 124.9 | 81.8 | 80.2 | 91.5 |
| 7.5 | 17.8 | 16.8 | 15.9 | 15.4 | 12.4 | 14.5 | 14.8 | 17.2 | 16.8 | 8.7 | 8.3 | 11.2 |
| Section | Area Under Curve | Sensitivity for Specificity at 95% | Sensitivity for Specificity at 80% | Sensitivity for Specificity at 86.9% | Threshold RNFL for Specificity at 95% | Threshold RNFL for Specificity at 80% | Threshold RNFL for Specificity at 86.9% |
|---|---|---|---|---|---|---|---|
| Global | 0.861 | 34.5% | 71.3 | 60.7 | 105.57 | 96.73 | 99.41 |
| ST | 0.779 | 17.6% | 53.7% | 41.2% | 144.11 | 123.65 | 130.56 |
| SS | 0.765 | 8.6% | 56.2% | 37.1% | 149.43 | 121.19 | 131.83 |
| SN ** | 0.835 | 24.0% | 70.6% | 50.5% | 125.85 | 105.22 | 112.96 |
| NS | 0.816 | 24.6% | 62.9% | 48.6% | 108.95 | 92.12 | 97.58 |
| NN | 0.807 | 18.2% | 63.3% | 43.1% | 87.23 | 72.65 | 77.27 |
| NI | 0.815 | 23.3% | 66.1% | 50.5% | 100.66 | 84.20 | 89.56 |
| IN | 0.826 | 30.4% | 70.0% | 59.4% | 122.32 | 106.19 | 110.97 |
| II | 0.830 | 19.8% | 61.0% | 40.3% | 144.03 | 125.10 | 133.11 |
| IT * | 0.848 | 21.1% | 88.2% | 60.4% | 129.52 | 101.24 | 112.08 |
| TI | 0.725 | 16.3% | 49.2% | 35.5% | 89.20 | 79.97 | 83.57 |
| TT | 0.713 | 16.3% | 43.8% | 34.2% | 86.08 | 79.60 | 81.77 |
| TS | 0.731 | 10.9% | 49.2% | 31.0% | 103.68 | 88.41 | 93.26 |
| S | 0.862 | 29.7% | 77.0% | 60.7% | 128.93 | 113.14 | 118.34 |
| N | 0.854 | 33.6% | 73.5% | 56.6% | 92.94 | 81.79 | 86.37 |
| I | 0.899 | 36.4% | 85.6% | 71.9% | 124.22 | 108.37 | 113.79 |
| T | 0.765 | 18.9% | 54.3% | 43.8% | 89.59 | 81.23 | 83.60 |
| S + I † | 0.913 | 47.6% | 90.7% | 80.5% | 122.07 | 108.73 | 113.02 |
| N + T | 0.868 | 32.6% | 76.7% | 60.7% | 88.86 | 80.00 | 83.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ahn, Y.; Askaruly, S.; You, J.S.; Kim, S.W.; Jung, W. Deep Learning-Based Glaucoma Screening Using Regional RNFL Thickness in Fundus Photography. Diagnostics 2022, 12, 2894. https://doi.org/10.3390/diagnostics12112894
Yang H, Ahn Y, Askaruly S, You JS, Kim SW, Jung W. Deep Learning-Based Glaucoma Screening Using Regional RNFL Thickness in Fundus Photography. Diagnostics. 2022; 12(11):2894. https://doi.org/10.3390/diagnostics12112894
Chicago/Turabian StyleYang, Hyunmo, Yujin Ahn, Sanzhar Askaruly, Joon S. You, Sang Woo Kim, and Woonggyu Jung. 2022. "Deep Learning-Based Glaucoma Screening Using Regional RNFL Thickness in Fundus Photography" Diagnostics 12, no. 11: 2894. https://doi.org/10.3390/diagnostics12112894
APA StyleYang, H., Ahn, Y., Askaruly, S., You, J. S., Kim, S. W., & Jung, W. (2022). Deep Learning-Based Glaucoma Screening Using Regional RNFL Thickness in Fundus Photography. Diagnostics, 12(11), 2894. https://doi.org/10.3390/diagnostics12112894









