Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Inclusion of Patients
2.2. Laboratory Analysis of Circulating Clusterin in Patients’ Plasma
2.3. Statistical Analysis
3. Results
3.1. Clusterin Concentrations Are Increased in Critically Ill Patients but Significantly Lower in ICU Patients with Sepsis
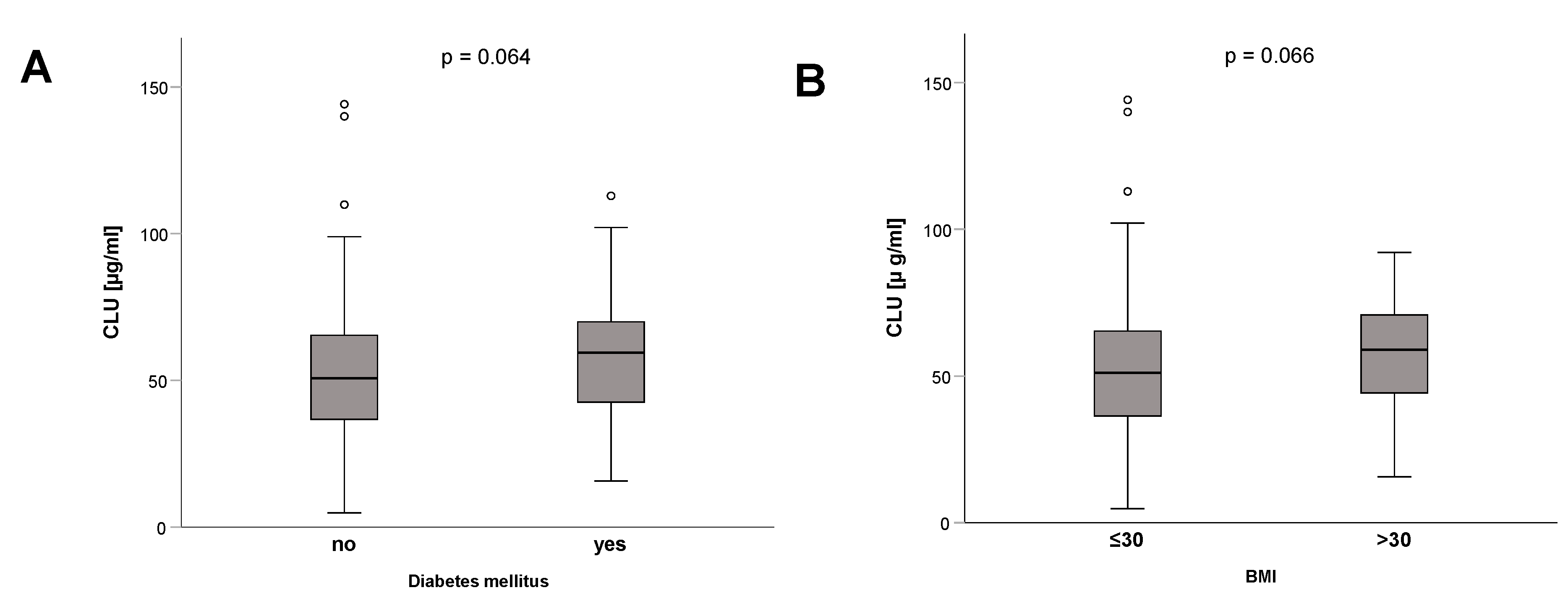
3.2. Clusterin Is Not Associated with Age, Sex, Disease Etiology or Severity, Vasopressor Therapy, or Mechanical Ventilation
3.3. Association of Clusterin Plasma Concentrations at ICU Admission with Metabolic Alterations and Inflammatory Response
3.4. Clusterin Plasma Concentrations at ICU Admission Have No Association with Short-Term or Long-Term Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzi, F.; Coletta, M.; Bettuzzi, S. Chapter 2: Clusterin (CLU): From one gene and two transcripts to many proteins. Adv. Cancer Res. 2009, 104, 9–23. [Google Scholar] [CrossRef]
- Boggs, L.N.; Fuson, K.S.; Baez, M.; Churgay, L.; McClure, D.; Becker, G.; May, P.C. Clusterin (Apo J) protects against in vitro amyloid-beta (1–40) neurotoxicity. J. Neurochem. 1996, 67, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons from Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, A.L.; Emami Khoonsari, P.; Sjödin, M.; Katila, L.; Wetterhall, M.; Gordh, T.; Kultima, K. Spinal Cord Stimulation Alters Protein Levels in the Cerebrospinal Fluid of Neuropathic Pain Patients: A Proteomic Mass Spectrometric Analysis. Neuromodulation 2016, 19, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, A.N.; Wu, M.; Gosmanova, A.K.; Becker, J.O.; Wijsman, E.M.; Brunzell, J.D.; Kahn, S.E.; Knopp, R.H.; Lyons, T.J.; Heinecke, J.W. Low clusterin levels in high-density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2528–2534. [Google Scholar] [CrossRef] [Green Version]
- Jenne, D.E.; Tschopp, J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: Identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc. Natl. Acad. Sci. USA 1989, 86, 7123–7127. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, D.T.; Carver, J.A.; Easterbrook-Smith, S.B.; Wilson, M.R. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J. Biol. Chem. 1999, 274, 6875–6881. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Eckel, R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014, 25, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Yoo, J.C.; Han, J.Y.; Hwang, E.M.; Kim, Y.S.; Jeong, E.Y.; Sun, C.H.; Yi, G.S.; Roh, G.S.; Kim, H.J.; et al. Human nuclear clusterin mediates apoptosis by interacting with Bcl-XL through C-terminal coiled coil domain. J. Cell. Physiol. 2012, 227, 1157–1167. [Google Scholar] [CrossRef]
- Murphy, B.F.; Saunders, J.R.; O’Bryan, M.K.; Kirszbaum, L.; Walker, I.D.; d’Apice, A.J. SP-40,40 is an inhibitor of C5b-6-initiated haemolysis. Int. Immunol. 1989, 1, 551–554. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Hao, F.; Hao, J.; Pan, L.; Zhao, Q.; Wo, J. Clusterin Reduces Cold Ischemia-Reperfusion Injury in Heart Transplantation Through Regulation of NF-kB Signaling and Bax/Bcl-xL Expression. Cell. Physiol. Biochem. 2018, 45, 1003–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santilli, G.; Aronow, B.J.; Sala, A. Essential requirement of apolipoprotein J (clusterin) signaling for IkappaB expression and regulation of NF-kappaB activity. J. Biol. Chem. 2003, 278, 38214–38219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, T.E.; Laping, N.J.; Rozovsky, I.; Oda, T.; Hogan, T.H.; Finch, C.E.; Pasinetti, G.M. Clusterin expression by astrocytes is influenced by transforming growth factor beta 1 and heterotypic cell interactions. J. Neuroimmunol. 1995, 58, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.J.; Kang, B.H.; Choi, B.K.; Park, I.S.; Min, B.H. Clusterin induces the secretion of TNF-α and the chemotactic migration of macrophages. Biochem. Biophys. Res. Commun. 2012, 422, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sonn, C.H.; Yu, Y.B.; Hong, Y.J.; Shim, Y.J.; Bluestone, J.A.; Min, B.H.; Lee, K.M. Clusterin synergizes with IL-2 for the expansion and IFN-γ production of natural killer cells. J. Leukoc. Biol. 2010, 88, 955–963. [Google Scholar] [CrossRef]
- Kirszbaum, L.; Bozas, S.E.; Walker, I.D. SP-40,40, a protein involved in the control of the complement pathway, possesses a unique array of disulphide bridges. FEBS Lett. 1992, 297, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.R.; Leskov, K.; Hosley-Eberlein, K.; Criswell, T.; Pink, J.J.; Kinsella, T.J.; Boothman, D.A. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 5907–5912. [Google Scholar] [CrossRef] [Green Version]
- Rohne, P.; Prochnow, H.; Koch-Brandt, C. The CLU-files: Disentanglement of a mystery. Biomol. Concepts 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Rodríguez-Rivera, C.; Garcia, M.M.; Molina-Álvarez, M.; González-Martín, C.; Goicoechea, C. Clusterin: Always protecting. Synthesis, function and potential issues. Biomed. Pharmacother. 2021, 134, 111174. [Google Scholar] [CrossRef]
- Choi-Miura, N.H.; Oda, T. Relationship between multifunctional protein “clusterin” and Alzheimer disease. Neurobiol. Aging 1996, 17, 717–722. [Google Scholar]
- Kang, S.W.; Shin, Y.J.; Shim, Y.J.; Jeong, S.Y.; Park, I.S.; Min, B.H. Clusterin interacts with SCLIP (SCG10-like protein) and promotes neurite outgrowth of PC12 cells. Exp. Cell Res. 2005, 309, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Akasaka, Y.; Ishii, T.; Komiyama, K.; Masuda, S.; Asuwa, N.; Choi-Miura, N.H.; Tomita, M. Distribution and synthesis of apolipoprotein J in the atherosclerotic aorta. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Gelissen, I.C.; Hochgrebe, T.; Wilson, M.R.; Easterbrook-Smith, S.B.; Jessup, W.; Dean, R.T.; Brown, A.J. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: A potential anti-atherogenic function? Biochem. J. 1998, 331 Pt 1, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, D.; Blaszczak, A.; Yin, Z.; Liu, J.; Joseph, J.J.; Wright, V.; Anandani, K.; Needleman, B.; Noria, S.; Renton, D.; et al. Clusterin Impairs Hepatic Insulin Sensitivity and Adipocyte Clusterin Associates with Cardiometabolic Risk. Diabetes Care 2019, 42, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, J.; Moon, M.K.; Kim, H.; Park, M.; Cho, S.Y.; Lee, J.; Lee, J.Y.; Kim, E. Plasma Clusterin as a Potential Link Between Diabetes and Alzheimer Disease. J. Clin. Endocrinol. Metab. 2020, 105, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Monte, S.V.; Sia, C.L.; Abuaysheh, S.; Green, K.; Caruana, J.A.; Dandona, P. Reduction in inflammation and the expression of amyloid precursor protein and other proteins related to Alzheimer’s disease following gastric bypass surgery. J. Clin. Endocrinol. Metab. 2012, 97, E1197–E1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, D. Clusterin as a Potential Biomarker of Obesity-Related Alzheimer’s Disease Risk. Biomark. Insights 2020, 15, 1177271920964108. [Google Scholar] [CrossRef]
- Miwa, Y.; Takiuchi, S.; Kamide, K.; Yoshii, M.; Horio, T.; Tanaka, C.; Banno, M.; Miyata, T.; Sasaguri, T.; Kawano, Y. Insertion/deletion polymorphism in clusterin gene influences serum lipid levels and carotid intima-media thickness in hypertensive Japanese females. Biochem. Biophys. Res. Commun. 2005, 331, 1587–1593. [Google Scholar] [CrossRef]
- Gil, S.Y.; Youn, B.S.; Byun, K.; Huang, H.; Namkoong, C.; Jang, P.G.; Lee, J.Y.; Jo, Y.H.; Kang, G.M.; Kim, H.K.; et al. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat. Commun. 2013, 4, 1862. [Google Scholar] [CrossRef] [Green Version]
- Kujiraoka, T.; Hattori, H.; Miwa, Y.; Ishihara, M.; Ueno, T.; Ishii, J.; Tsuji, M.; Iwasaki, T.; Sasaguri, Y.; Fujioka, T.; et al. Serum apolipoprotein j in health, coronary heart disease and type 2 diabetes mellitus. J. Atheroscler. Thromb. 2006, 13, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Thambisetty, M.; Simmons, A.; Velayudhan, L.; Hye, A.; Campbell, J.; Zhang, Y.; Wahlund, L.O.; Westman, E.; Kinsey, A.; Güntert, A.; et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch. Gen. Psychiatry 2010, 67, 739–748. [Google Scholar] [CrossRef]
- Zhang, Q.; Yue, Y.; Zheng, R. Clusterin as a serum biomarker candidate contributes to the lung fibroblasts activation in chronic obstructive pulmonary disease. Chin. Med. J. 2022, 135, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Habiel, D.M.; Camelo, A.; Espindola, M.; Burwell, T.; Hanna, R.; Miranda, E.; Carruthers, A.; Bell, M.; Coelho, A.L.; Liu, H.; et al. Divergent roles for Clusterin in Lung Injury and Repair. Sci. Rep. 2017, 7, 15444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel, Z.; Khoury, N.; Betley, M.J.; Lehallier, B.; Willoughby, D.; Olsson, N.; Yang, A.C.; Hahn, O.; Lu, N.; Vest, R.T.; et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 2021, 600, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Buendgens, L.; Yagmur, E.; Bruensing, J.; Herbers, U.; Baeck, C.; Trautwein, C.; Koch, A.; Tacke, F. Growth Differentiation Factor-15 Is a Predictor of Mortality in Critically Ill Patients with Sepsis. Dis. Markers 2017, 2017, 5271203. [Google Scholar] [CrossRef] [Green Version]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Koch, A.; Weiskirchen, R.; Kunze, J.; Dückers, H.; Bruensing, J.; Buendgens, L.; Matthes, M.; Luedde, T.; Trautwein, C.; Tacke, F. Elevated asymmetric dimethylarginine levels predict short- and long-term mortality risk in critically ill patients. J. Crit. Care 2013, 28, 947–953. [Google Scholar] [CrossRef]
- Koch, A.; Voigt, S.; Kruschinski, C.; Sanson, E.; Dückers, H.; Horn, A.; Yagmur, E.; Zimmermann, H.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care 2011, 15, R63. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Obregon, S.; Azkargorta, M.; Seijas, I.; Pilar-Orive, J.; Borrego, F.; Elortza, F.; Boyano, M.D.; Astigarraga, I. Identification of a panel of serum protein markers in early stage of sepsis and its validation in a cohort of patients. J. Microbiol. Immunol. Infect. 2018, 51, 465–472. [Google Scholar] [CrossRef]
- DeCoux, A.; Tian, Y.; DeLeon-Pennell, K.Y.; Nguyen, N.T.; de Castro Brás, L.E.; Flynn, E.R.; Cannon, P.L.; Griswold, M.E.; Jin, Y.F.; Puskarich, M.A.; et al. Plasma Glycoproteomics Reveals Sepsis Outcomes Linked to Distinct Proteins in Common Pathways. Crit. Care Med. 2015, 43, 2049–2058. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.A.; Kang, M.C.; Ciaraldi, T.P.; Kim, S.S.; Park, K.S.; Choe, C.; Hwang, W.M.; Lim, D.M.; Farr, O.; Mantzoros, C.; et al. Circulating ApoJ is closely associated with insulin resistance in human subjects. Metabolism 2018, 78, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.A.; Kang, M.C.; Yang, W.M.; Hwang, W.M.; Kim, S.S.; Hong, S.H.; Heo, J.I.; Vijyakumar, A.; Pereira de Moura, L.; Uner, A.; et al. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat. Commun. 2020, 11, 2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Häring, H.U. The role of hepatokines in metabolism. Nat. Rev. Endocrinol. 2013, 9, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, J.K.; Coppari, R.; Balthasar, N.; Ichinose, M.; Lowell, B.B. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 2005, 493, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Coppari, R.; Ichinose, M.; Lee, C.E.; Pullen, A.E.; Kenny, C.D.; McGovern, R.A.; Tang, V.; Liu, S.M.; Ludwig, T.; Chua, S.C., Jr.; et al. The hypothalamic arcuate nucleus: A key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005, 1, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Won, J.C.; Park, C.Y.; Oh, S.W.; Lee, E.S.; Youn, B.S.; Kim, M.S. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS ONE 2014, 9, e103351. [Google Scholar] [CrossRef]




| Parameter | All Patients | Non-Sepsis | Sepsis | p-Value * |
|---|---|---|---|---|
| Number | 200 | 67 | 133 | |
| Sex (male/female) | 122/78 | 44/23 | 78/55 | 0.360 |
| Age median (range) [years] | 64 (18–90) | 62 (18–85) | 65 (20–90) | 0.612 |
| APACHE-II score median (range) | 18 (2–43) | 14 (2–33) | 19 (4–43) | 0.002 |
| SOFA score median (range) | 9 (0–17) | 8 (0–17) | 9 (2–17) | 0.055 |
| SAPS-2 score median (range) | 41 (0–73) | 41 (13–72) | 41.5 (0–73) | 0.280 |
| ICU days median (range) | 7 (1–137) | 6 (1–45) | 9 (1–137) | 0.013 |
| Death during ICU n(%) | 43 (21.5%) | 9 (13.4%) | 34 (25.6%) | 0.067 |
| Death during follow up (total) n(%) | 78 (41.3%) | 21 (33.3%) | 57 (45.2%) | 0.158 |
| Mechanical ventilation n(%) | 134 (67.7%) | 46 (68.7%) | 91 (44.8%) | 0.915 |
| Ventilation time median (range) [h] | 116 (0–3628) | 66 (0–3628) | 123.5 (0–2966) | 0.350 |
| Vasopressor therapy n(%) | 123 (61.5%) | 33 (16.3%) | 92 (45.3%) | 0.011 |
| Pre-existing diabetes n(%) | 62 (31.6%) | 22 (33.8%) | 43 (32.1%) | 0.061 |
| BMI median (range) [m²/kg] | 25.8 (15.3–86.5) | 25.7 (15.9–40.5) | 25.8 (15.3–86.5) | 0.621 |
| WBC median (range) [×10³/µL] | 12.7 (0–208) | 12.1 (1.8–29.6) | 13.6 (0–208) | 0.041 |
| CRP median (range) [mg/dL] | 96 (0–230) | 17 (5–230) | 154 (0–230) | 0.001 |
| Procalcitonin median (range) [µg/L] | 0.6 (0–207.5) | 0.2 (0.03–17.4) | 1.8 (0–207.5) | 0.001 |
| Cystatin C median (range) [mg/dL] | 1.6 (0–7.3) | 1.17 (0.41–7.3) | 2.06 (0–6.33) | 0.001 |
| GFR cystatin C median (range) [mL/min] | 35 (0–379) | 63 (5–379) | 21.5 (0–218) | 0.001 |
| INR median (range) | 1.16 (0–6.73) | 1.16 (0.95–6.73) | 1.16 (0–3.67) | 0.975 |
| PTT median (range) [s] | 31 (0–150) | 27.5 (20–150) | 33 (0–150) | 0.001 |
| Clusterin day 1 median (range) [µg/mL] | 52.9 (4.8–144.1) | 60.0 (17.7–112.9) | 48.9 (4.8–144.1) | 0.026 |
| ALT day 1 median (range) [U/L] | 29.8 (0–6550) | 36.5 (7–6550) | 24.5 (0–5890) | 0.034 |
| AST day 1 median (range) [U/L] | 41 (0–20,332) | 49 (11–20,332) | 39 (0–7832) | 0.081 |
| NT-pro-BNP day 1 median (range) [ng/L] | 2353 (0–39,442) | 476 (18–14,690) | 3274 (0–39,442) | 0.001 |
| Sepsis | Non-Sepsis | |
|---|---|---|
| n = 133 | n = 67 | |
| Etiology of sepsis critical illness Site of infection n (%) | ||
| pulmonary | 68 (51.1%) | |
| abdominal | 26 (19.1%) | |
| urogenital | 8 (5.9%) | |
| other | 31 (22.8%) | |
| Etiology of non-sepsis critical illness n (%) | ||
| cardio-pulmonary disorder | 28 (41.8%) | |
| acute pancreatitis | 9 (13.4%) | |
| acute liver failure | 3 (4.5%) | |
| decompensated liver cirrhosis | 8 (11.9%) | |
| severe gastrointestinal hemorrhage | 4 (6.0%) | |
| non-sepsis other | 15 (22.4%) |
| ICU Patients | ||
|---|---|---|
| Parameters | r | p |
| Markers of inflammatory response | ||
| IL-6 | −0.268 | 0.001 |
| PCT | −0.243 | 0.004 |
| Markers of coagulation | ||
| Prothrombin time | 0.183 | 0.011 |
| INR | −0.171 | 0.017 |
| PTT | −0.243 | 0.001 |
| Fibrinogen | −0.241 | 0.007 |
| AT III | 0.242 | 0.007 |
| Markers of organ function | ||
| Bilirubin conjugated | −0.315 | 0.001 |
| PCHE | 0.19 | 0.011 |
| Albumin | 0.296 | 0.002 |
| Protein | 0.218 | 0.005 |
| Lipase | −0.203 | 0.011 |
| Marker of diabetes | ||
| Glucose | 0.201 | 0.004 |
| Markers of lipid metabolism | ||
| Triglycerides | 0.237 | 0.003 |
| Total cholesterol | 0.288 | <0.001 |
| LDL cholesterol | 0.384 | 0.002 |
| HDL cholesterol | 0.292 | 0.02 |
| Adipocytokines/metabolic markers | ||
| RBP4 | 0.336 | 0.007 |
| Myostatin | 0.261 | 0.037 |
| Sclerostin | 0.287 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagmur, E.; Abu Jhaisha, S.; Buendgens, L.; Sapundzhieva, N.; Brozat, J.F.; Hohlstein, P.; Pollmanns, M.R.; Koek, G.H.; Weiskirchen, R.; Trautwein, C.; et al. Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation. Diagnostics 2022, 12, 3010. https://doi.org/10.3390/diagnostics12123010
Yagmur E, Abu Jhaisha S, Buendgens L, Sapundzhieva N, Brozat JF, Hohlstein P, Pollmanns MR, Koek GH, Weiskirchen R, Trautwein C, et al. Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation. Diagnostics. 2022; 12(12):3010. https://doi.org/10.3390/diagnostics12123010
Chicago/Turabian StyleYagmur, Eray, Samira Abu Jhaisha, Lukas Buendgens, Nadezhda Sapundzhieva, Jonathan F. Brozat, Philipp Hohlstein, Maike R. Pollmanns, Ger H. Koek, Ralf Weiskirchen, Christian Trautwein, and et al. 2022. "Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation" Diagnostics 12, no. 12: 3010. https://doi.org/10.3390/diagnostics12123010
APA StyleYagmur, E., Abu Jhaisha, S., Buendgens, L., Sapundzhieva, N., Brozat, J. F., Hohlstein, P., Pollmanns, M. R., Koek, G. H., Weiskirchen, R., Trautwein, C., Tacke, F., Wirtz, T. H., & Koch, A. (2022). Clusterin Plasma Concentrations Are Decreased in Sepsis and Inversely Correlated with Established Markers of Inflammation. Diagnostics, 12(12), 3010. https://doi.org/10.3390/diagnostics12123010








