Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Preanalytical Sample Processing
2.3. BD Onclarity™ HPV Assay
2.4. Statistical Analysis
3. Results
3.1. Study Population
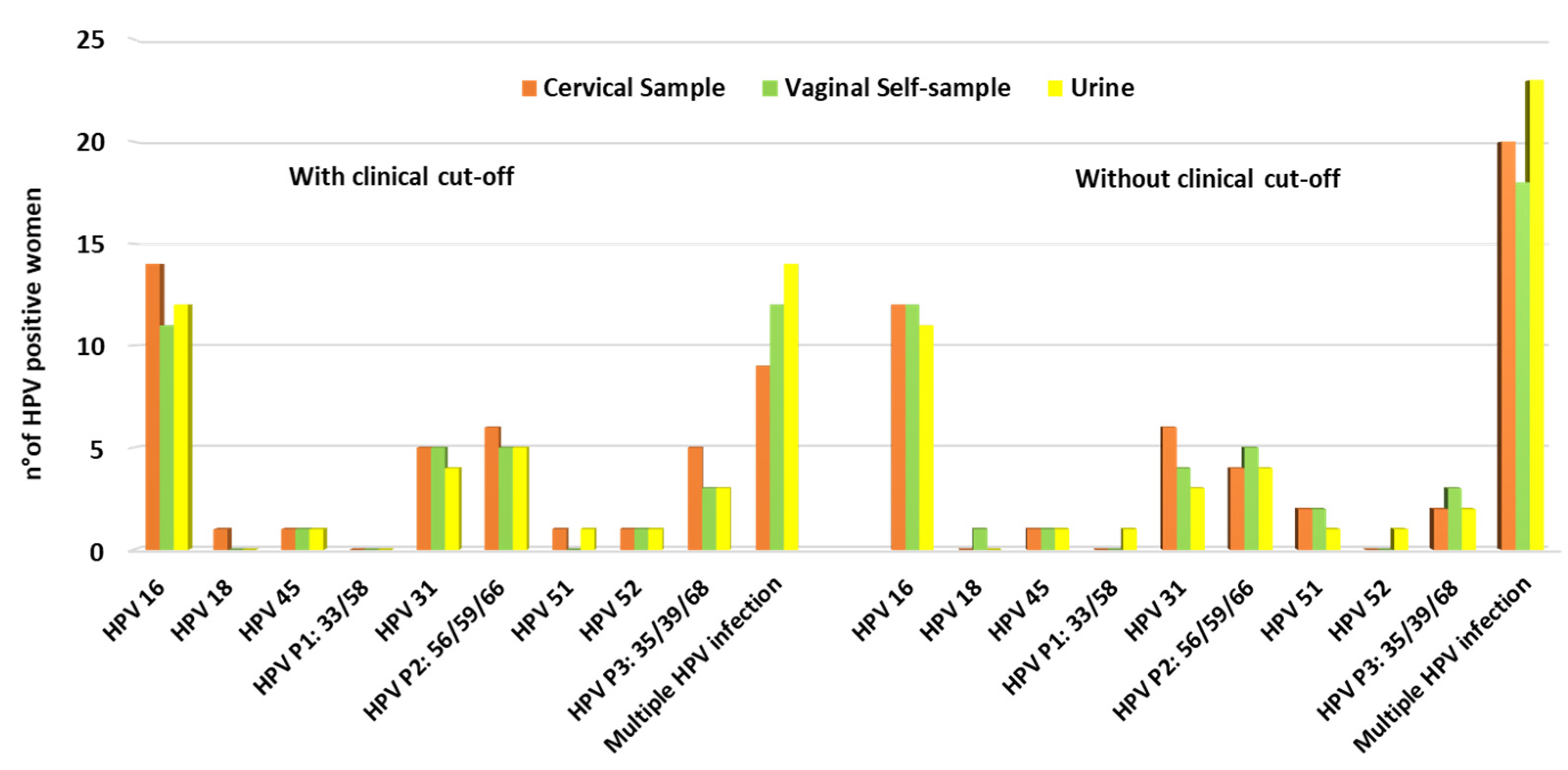
3.2. HPV Positivity and Genotype Distribution among Samples Collected
3.3. HPV Overall Positive Agreement between Cervical and Vaginal Self-Samples
3.4. HPV Overall Positive Agreement between Cervical and First-Void Urine Samples
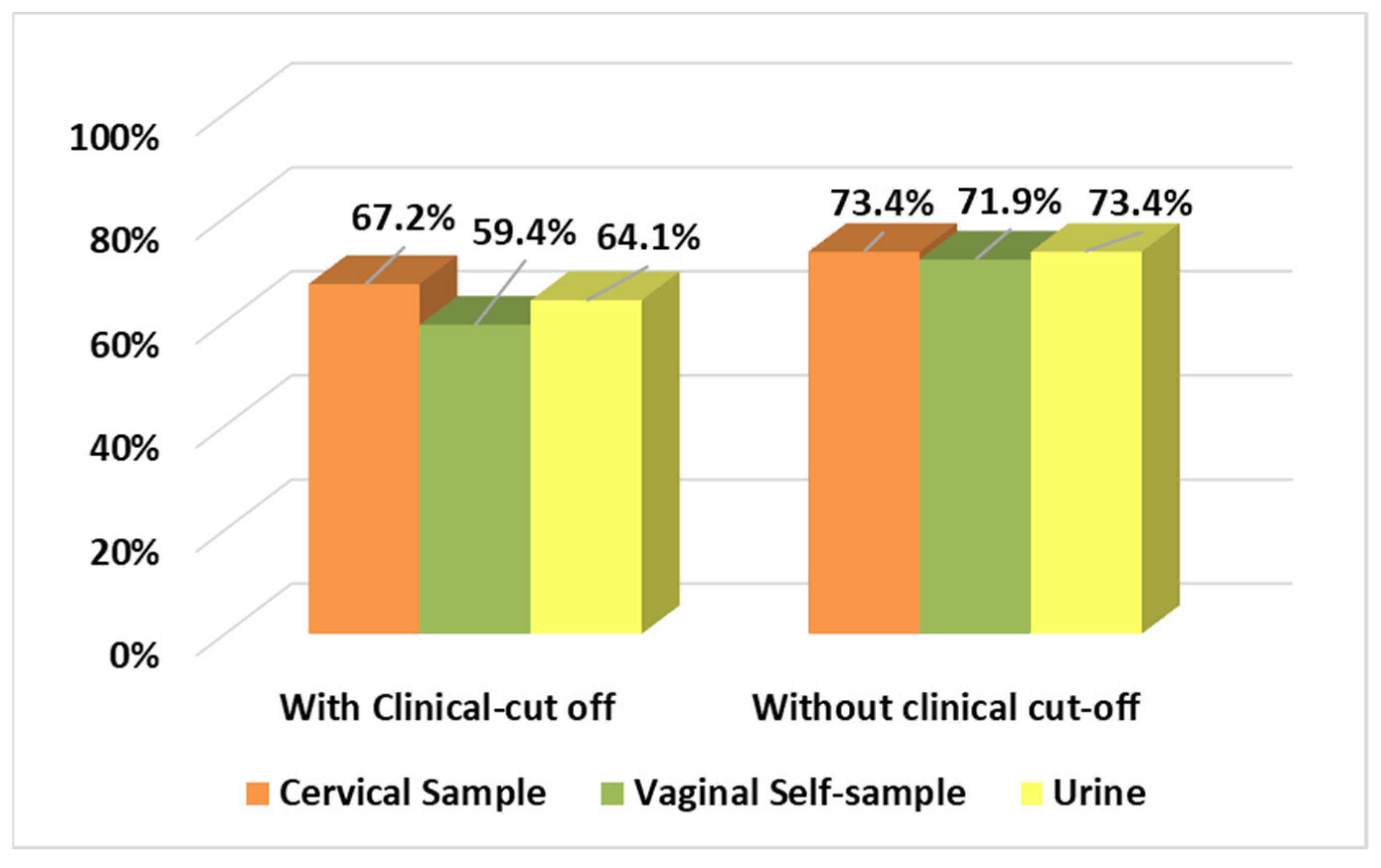
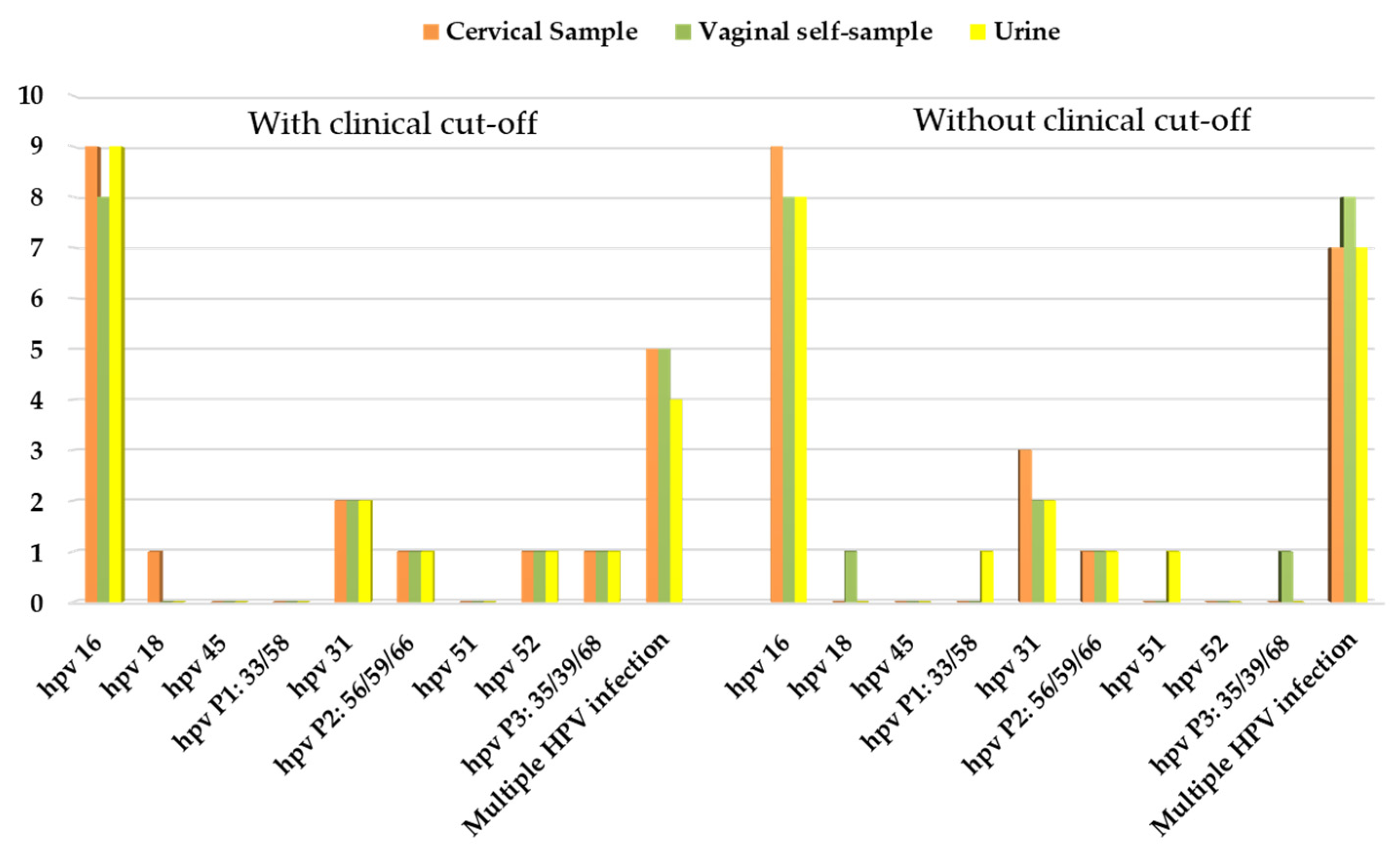
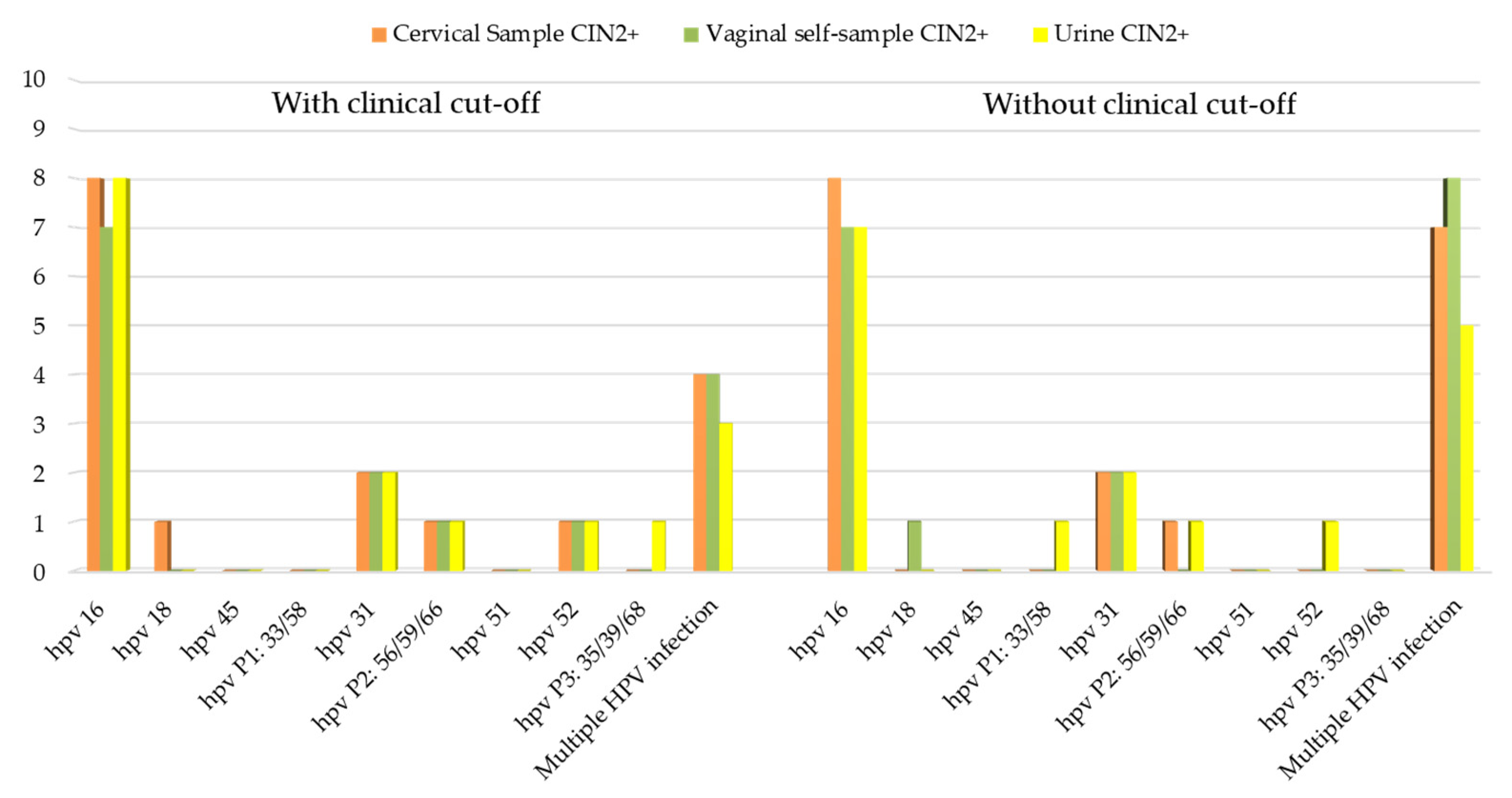
3.5. Correlation between HPV Positivity and Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 28 September 2021).
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide Use of HPV Self-Sampling for Cervical Cancer Screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef]
- Lam, J.U.H.; Elfström, K.M.; Ejegod, D.M.; Pedersen, H.; Rygaard, C.; Rebolj, M.; Lynge, E.; Juul, K.E.; Kjær, S.K.; Dillner, J.; et al. High-Grade Cervical Intraepithelial Neoplasia in Human Papillomavirus Self-Sampling of Screening Non-Attenders. Br. J. Cancer 2018, 118, 138–144. [Google Scholar] [CrossRef]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.S.; Snijders, P.J.F.; Arbyn, M. Reaching Women Who Do Not Participate in the Regular Cervical Cancer Screening Programme by Offering Self-Sampling Kits: A Systematic Review and Meta-Analysis of Randomised Trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef]
- Chorley, A.J.; Marlow, L.A.V.; Forster, A.S.; Haddrell, J.B.; Waller, J. Experiences of Cervical Screening and Barriers to Participation in the Context of an Organised Programme: A Systematic Review and Thematic Synthesis: Experiences of Cervical Screening and Barriers to Participation. Psycho-Oncology 2017, 26, 161–172. [Google Scholar] [CrossRef]
- Arbyn, M.; Verdoodt, F.; Snijders, P.J.F.; Verhoef, V.M.J.; Suonio, E.; Dillner, L.; Minozzi, S.; Bellisario, C.; Banzi, R.; Zhao, F.-H.; et al. Accuracy of Human Papillomavirus Testing on Self-Collected versus Clinician-Collected Samples: A Meta-Analysis. Lancet Oncol. 2014, 15, 172–183. [Google Scholar] [CrossRef]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting Cervical Precancer and Reaching Underscreened Women by Using HPV Testing on Self Samples: Updated Meta-Analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef]
- Snijders, P.J.F.; Verhoef, V.M.J.; Arbyn, M.; Ogilvie, G.; Minozzi, S.; Banzi, R.; van Kemenade, F.J.; Heideman, D.A.M.; Meijer, C.J.L.M. High-Risk HPV Testing on Self-Sampled versus Clinician-Collected Specimens: A Review on the Clinical Accuracy and Impact on Population Attendance in Cervical Cancer Screening. Int. J. Cancer 2013, 132, 2223–2236. [Google Scholar] [CrossRef]
- Hawkes, D.; Keung, M.H.T.; Huang, Y.; McDermott, T.L.; Romano, J.; Saville, M.; Brotherton, J.M.L. Self-Collection for Cervical Screening Programs: From Research to Reality. Cancers 2020, 12, 1053. [Google Scholar] [CrossRef]
- Frati, E.; Fasoli, E.; Martinelli, M.; Colzani, D.; Bianchi, S.; Carnelli, L.; Amendola, A.; Olivani, P.; Tanzi, E. Sexually Transmitted Infections: A Novel Screening Strategy for Improving Women’s Health in Vulnerable Populations. Int. J. Mol. Sci. 2017, 18, 1311. [Google Scholar] [CrossRef]
- De Baetselier, I.; Smet, H.; Abdellati, S.; De Deken, B.; Cuylaerts, V.; Reyniers, T.; Vuylsteke, B.; Crucitti, T. Evaluation of the “Colli-Pee”, a First-Void Urine Collection Device for Self-Sampling at Home for the Detection of Sexually Transmitted Infections, versus a Routine Clinic-Based Urine Collection in a One-to-One Comparison Study Design: Efficacy and Acceptability among MSM in Belgium. BMJ Open 2019, 9, e028145. [Google Scholar] [CrossRef]
- Gaydos, C.A.; Quinn, T.C. Urine Nucleic Acid Amplification Tests for the Diagnosis of Sexually Transmitted Infections in Clinical Practice. Curr. Opin. Infect. Dis. 2005, 18, 55–66. [Google Scholar] [CrossRef]
- Vorsters, A.; Van Damme, P.; Clifford, G. Urine Testing for HPV: Rationale for Using First Void. BMJ 2014, 349, g6252. [Google Scholar] [CrossRef]
- Rohner, E.; Rahangdale, L.; Sanusi, B.; Knittel, A.K.; Vaughan, L.; Chesko, K.; Faherty, B.; Tulenko, S.E.; Schmitt, J.W.; Romocki, L.S.; et al. Test Accuracy of Human Papillomavirus in Urine for Detection of Cervical Intraepithelial Neoplasia. J. Clin. Microbiol. 2020, 58, e01443-19. [Google Scholar] [CrossRef]
- Pattyn, J.; Van Keer, S.; Biesmans, S.; Ieven, M.; Vanderborght, C.; Beyers, K.; Vankerckhoven, V.; Bruyndonckx, R.; Van Damme, P.; Vorsters, A. Human Papillomavirus Detection in Urine: Effect of a First-Void Urine Collection Device and Timing of Collection. J. Virol. Methods 2019, 264, 23–30. [Google Scholar] [CrossRef]
- Van Keer, S.; Tjalma, W.A.A.; Pattyn, J.; Biesmans, S.; Pieters, Z.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. Human Papillomavirus Genotype and Viral Load Agreement between Paired First-Void Urine and Clinician-Collected Cervical Samples. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 859–869. [Google Scholar] [CrossRef]
- Van Keer, S.; Peeters, E.; Vanden Broeck, D.; De Sutter, P.; Donders, G.; Doyen, J.; Tjalma, W.A.A.; Weyers, S.; Vorsters, A.; Arbyn, M. Clinical and Analytical Evaluation of the RealTime High Risk HPV Assay in Colli-Pee Collected First-Void Urine Using the VALHUDES Protocol. Gynecol. Oncol. 2021, 162, 575–583. [Google Scholar] [CrossRef]
- Leeman, A.; del Pino, M.; Molijn, A.; Rodriguez, A.; Torné, A.; de Koning, M.; Ordi, J.; van Kemenade, F.; Jenkins, D.; Quint, W. HPV Testing in First-Void Urine Provides Sensitivity for CIN2+ Detection Comparable with a Smear Taken by a Clinician or a Brush-Based Self-Sample: Cross-Sectional Data from a Triage Population. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1356–1363. [Google Scholar] [CrossRef]
- Cuschieri, K.; Geraets, D.T.; Moore, C.; Quint, W.; Duvall, E.; Arbyn, M. Clinical and Analytical Performance of the Onclarity HPV Assay Using the VALGENT Framework. J. Clin. Microbiol. 2015, 53, 3272–3279. [Google Scholar] [CrossRef]
- Ejegod, D.M.; Junge, J.; Franzmann, M.; Kirschner, B.; Bottari, F.; Sideri, M.; Sandri, M.-T.; Bonde, J. Clinical and Analytical Performance of the BD OnclarityTM HPV Assay for Detection of CIN2+ Lesions on SurePath Samples. Papillomavirus Res. 2016, 2, 31–37. [Google Scholar] [CrossRef]
- Bottari, F.; Iacobone, A.D. Profile of the BD HPV OnclarityTM Assay. Expert Rev. Mol. Diagn. 2019, 19, 565–570. [Google Scholar] [CrossRef]
- Arbyn, M.; Snijders, P.J.F.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Kocjan, B.J.; Poljak, M. Which High-Risk HPV Assays Fulfil Criteria for Use in Primary Cervical Cancer Screening? Clin. Microbiol. Infect. 2015, 21, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Racey, C.S.; Withrow, D.R.; Gesink, D. Self-Collected HPV Testing Improves Participation in Cervical Cancer Screening: A Systematic Review and Meta-Analysis. Can. J. Public Health 2013, 104, e159–e166. [Google Scholar] [CrossRef] [PubMed]
- Sechi, I.; Cocuzza, C.; Martinelli, M.; Muresu, N.; Castriciano, S.; Sotgiu, G.; Piana, A. Comparison of Different Self-Sampling Devices for Molecular Detection of Human Papillomavirus (HPV) and Other Sexually Transmitted Infections (STIs): A Pilot Study. Healthcare 2022, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Ertik, F.C.; Kampers, J.; Hülse, F.; Stolte, C.; Böhmer, G.; Hillemanns, P.; Jentschke, M. CoCoss-Trial: Concurrent Comparison of Self-Sampling Devices for HPV-Detection. Int. J. Environ. Res. Public Health 2021, 18, 10388. [Google Scholar] [CrossRef] [PubMed]
- Bokan, T.; Ivanus, U.; Jerman, T.; Takac, I.; Arko, D. Long Term Results of Follow-up after HPV Self-Sampling with Devices Qvintip and HerSwab in Women Non-Attending Cervical Screening Programme. Radiol. Oncol. 2021, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Simon, M.; Peeters, E.; Xu, L.; Meijer, C.J.L.M.; Berkhof, J.; Cuschieri, K.; Bonde, J.; Ostrbenk Vanlencak, A.; Zhao, F.-H.; et al. 2020 List of Human Papillomavirus Assays Suitable for Primary Cervical Cancer Screening. Clin. Microbiol. Infect. 2021, 27, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Peeters, E.; Benoy, I.; Vanden Broeck, D.; Bogers, J.; De Sutter, P.; Donders, G.; Tjalma, W.; Weyers, S.; Cuschieri, K.; et al. VALHUDES: A Protocol for Validation of Human Papillomavirus Assays and Collection Devices for HPV Testing on Self-Samples and Urine Samples. J. Clin. Virol. 2018, 107, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Castle, P.E.; Schiffman, M.; Wentzensen, N.; Heckman-Stoddard, B.; Sahasrabuddhe, V.V. Meta-analysis of Agreement/Concordance Statistics in Studies Comparing Self- vs Clinician-collected Samples for HPV Testing in Cervical Cancer Screening. Int. J. Cancer 2022, 151, 308–312. [Google Scholar] [CrossRef]
- Bianchi, S.; Frati, E.R.; Panatto, D.; Martinelli, M.; Amicizia, D.; Zotti, C.M.; Martinese, M.; Bonanni, P.; Boccalini, S.; Coppola, R.C.; et al. Detection and Genotyping of Human Papillomavirus in Urine Samples from Unvaccinated Male and Female Adolescents in Italy. PLoS ONE 2013, 8, e79719. [Google Scholar] [CrossRef]
- Daponte, A.; Michail, G.; Daponte, A.-I.; Daponte, N.; Valasoulis, G. Urine HPV in the Context of Genital and Cervical Cancer Screening—An Update of Current Literature. Cancers 2021, 13, 1640. [Google Scholar] [CrossRef]
- Cadman, L.; Reuter, C.; Jitlal, M.; Kleeman, M.; Austin, J.; Hollingworth, T.; Parberry, A.L.; Ashdown-Barr, L.; Patel, D.; Nedjai, B.; et al. A Randomized Comparison of Different Vaginal Self-Sampling Devices and Urine for Human Papillomavirus Testing—Predictors 5.1. Cancer Epidemiol. Biomark. Prev. 2021, 30, 661–668. [Google Scholar] [CrossRef]
- Cho, H.-W.; Hong, J.H.; Min, K.J.; Ouh, Y.-T.; Seong, S.J.; Moon, J.H.; Cho, S.H.; Lee, J.K. Performance and Diagnostic Accuracy of Human Papillomavirus Testing on Self-Collected Urine and Vaginal Samples in a Referral Population. Cancer Res. Treat. 2021, 53, 829–836. [Google Scholar] [CrossRef]
- Ørnskov, D.; Jochumsen, K.; Steiner, P.H.; Grunnet, I.M.; Lykkebo, A.W.; Waldstrøm, M. Clinical Performance and Acceptability of Self-Collected Vaginal and Urine Samples Compared with Clinician-Taken Cervical Samples for HPV Testing among Women Referred for Colposcopy. A Cross-Sectional Study. BMJ Open 2021, 11, e041512. [Google Scholar] [CrossRef]
- Lozar, T.; Nagvekar, R.; Rohrer, C.; Dube Mandishora, R.S.; Ivanus, U.; Fitzpatrick, M.B. Cervical Cancer Screening Postpandemic: Self-Sampling Opportunities to Accelerate the Elimination of Cervical Cancer. Int. J. Women Health 2021, 13, 841–859. [Google Scholar] [CrossRef]
| Pap Test Result | n (64) | % |
| ASCUS | 15 | 23.4% |
| LSIL | 24 | 37.5% |
| AGCUS | 5 | 7.8% |
| ASCH | 6 | 9.4% |
| HSIL | 14 | 21.9% |
| Colposcopy Result | n (64) | % |
| ABNORMAL | 23 | 35.9% |
| NORMAL | 41 | 64.1% |
| Biopsy Result | n (23) | % |
| NEG | 2 | 8.7% |
| CIN 1 | 3 | 13.0% |
| CIN 2 | 3 | 13.0% |
| CIN 3 | 14 | 60.9% |
| Cervical Cancer | 1 | 4.3% |
| Vaginal Self-Sample | Urine | |||||||
|---|---|---|---|---|---|---|---|---|
| Ct Cut-Off Value | PPA% (n) | PNA% (n) | OPA% (n) | κ | PPA% (n) | PNA% (n) | OPA% (n) | κ |
| <38.3 Ct for HPV 16 (for all sample types) | 83.7% (36/43) | 90.5% (19/21) | 85.9% (55/64) | 0.699 | 90.7% (39/43) | 90.5% (19/21) | 90.6% (58/64) | 0.792 |
| <34.2 Ct for the other HPVs (for all sample types) | ||||||||
| <40 Ct for HPV 16 and other HPVs (for all sample types) | 95.7% (45/47) | 94.1% (16/17) | 95.3% (61/64) | 0.882 | 91.5% (43/47) | 76.5% (13/17) | 87.5% (56/64) | 0.680 |
| <38.3 Ct for HPV 16 (for cervical samples) | 100% (43/43) | 85.7% (18/21) | 95.3% (61/64) | 0.890 | 97.7% (42/43) | 76.2% (16/21) | 90.6 (58/64) | 0.776 |
| <34.2 Ct for the other HPVs (for cervical samples) | ||||||||
| <40 Ct for HPV 16 and other HPVs (for self-samples) | ||||||||
| With Clinical Cut-Off | Without Clinical Cut-Off | ||||
|---|---|---|---|---|---|
| Colposcopy Results (Tot. 64) | Total HPV-Positive Women (n) | Total HPV-Positive Women (%) | Total HPV-Positive Women (n) | Total HPV-Positive Women (%) | |
| Cervical Sample | ABNORMAL | 20 | 31.3% | 20 | 31.3% |
| NORMAL | 23 | 35.9% | 25 | 39.1% | |
| Vaginal self-sample | ABNORMAL | 18 | 28.1% | 21 | 32.8% |
| NORMAL | 20 | 31.3% | 25 | 39.1% | |
| Urine | ABNORMAL | 18 | 28.1% | 20 | 31.3% |
| NORMAL | 23 | 35.9% | 27 | 42.2% | |
| With Clinical Cut-Off | Without Clinical Cut-Off | ||||
|---|---|---|---|---|---|
| Biopsy Results (Tot. 23) | Total HPV-Positive Women (n) | Total HPV-Positive Women (%) | Total HPV-Positive Women (n) | Total HPV-Positive Women (%) | |
| Cervical Sample | CIN2+ | 17 | 73.9% | 18 | 78.3% |
| CIN2− | 3 | 13.0% | 4 | 17.4% | |
| Vaginal self-sample | CIN2+ | 15 | 65.2% | 18 | 78.3% |
| CIN2− | 3 | 13.0% | 3 | 13.0% | |
| Urine | CIN2+ | 16 | 69.6% | 17 | 73.9% |
| CIN2− | 2 | 8.7% | 3 | 13.0% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, M.; Giubbi, C.; Sechi, I.; Bottari, F.; Iacobone, A.D.; Musumeci, R.; Perdoni, F.; Muresu, N.; Piana, A.; Fruscio, R.; et al. Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study. Diagnostics 2022, 12, 3075. https://doi.org/10.3390/diagnostics12123075
Martinelli M, Giubbi C, Sechi I, Bottari F, Iacobone AD, Musumeci R, Perdoni F, Muresu N, Piana A, Fruscio R, et al. Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study. Diagnostics. 2022; 12(12):3075. https://doi.org/10.3390/diagnostics12123075
Chicago/Turabian StyleMartinelli, Marianna, Chiara Giubbi, Illari Sechi, Fabio Bottari, Anna Daniela Iacobone, Rosario Musumeci, Federica Perdoni, Narcisa Muresu, Andrea Piana, Robert Fruscio, and et al. 2022. "Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study" Diagnostics 12, no. 12: 3075. https://doi.org/10.3390/diagnostics12123075
APA StyleMartinelli, M., Giubbi, C., Sechi, I., Bottari, F., Iacobone, A. D., Musumeci, R., Perdoni, F., Muresu, N., Piana, A., Fruscio, R., Landoni, F., & Cocuzza, C. E. (2022). Evaluation of BD Onclarity™ HPV Assay on Self-Collected Vaginal and First-Void Urine Samples as Compared to Clinician-Collected Cervical Samples: A Pilot Study. Diagnostics, 12(12), 3075. https://doi.org/10.3390/diagnostics12123075










