Advances in Bone Joint Imaging-Metal Artifact Reduction
Abstract
:1. Introduction
2. Materials and Methods
3. Metal Artifact Reduction Strategies
3.1. Digital Tomosynthesis
3.2. Computed Tomography
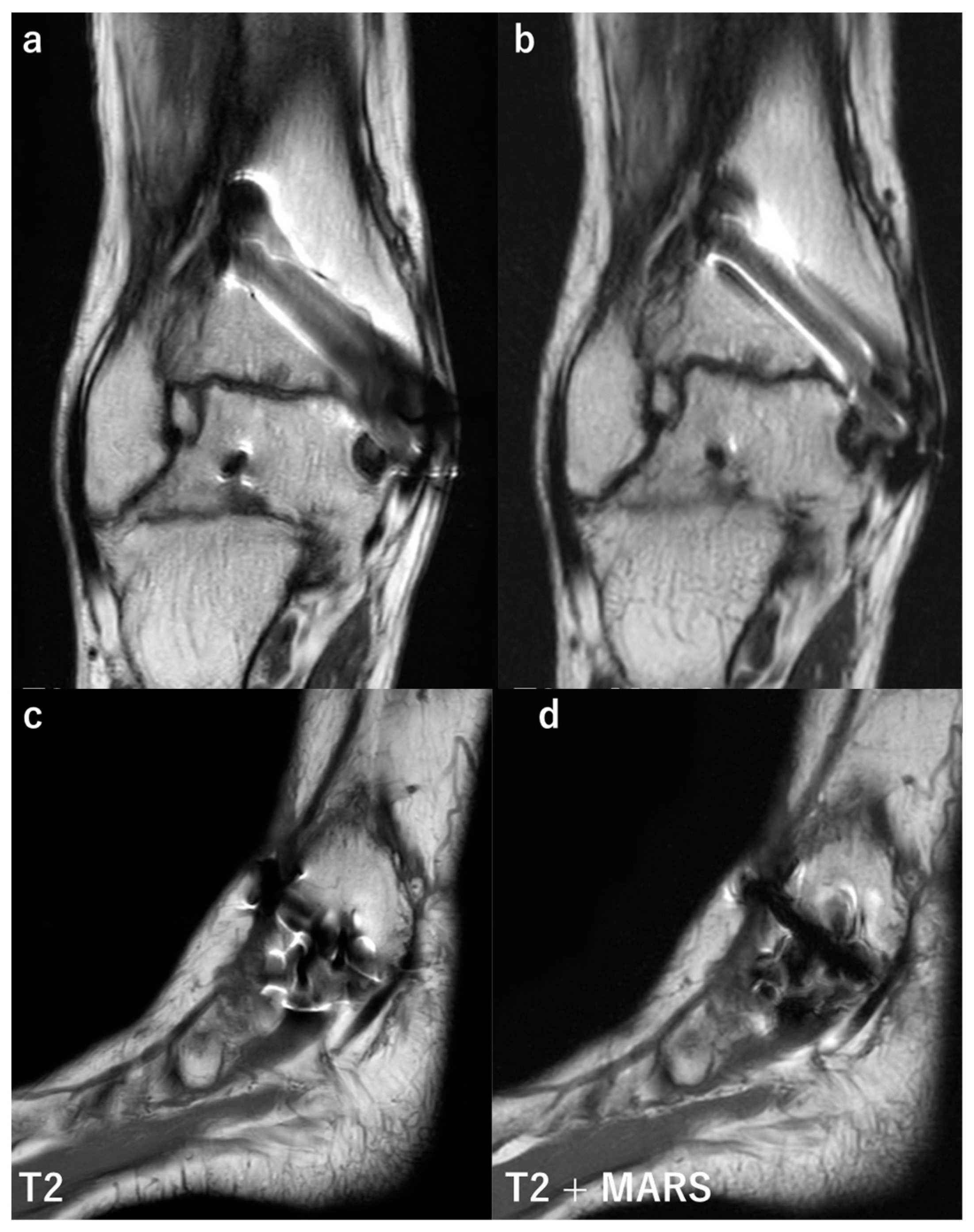
3.3. Magnetic Resonance Imaging
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tang, H.; Yang, D.; Guo, S.; Tang, J.; Liu, J.; Wang, D.; Zhou, Y. Digital tomosynthesis with metal artifact reduction for assessing cementless hip arthroplasty: A diagnostic cohort study of 48 patients. Skelet. Radiol. 2016, 45, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Aoyama, T.; Oda, N.; Yamauchi-Kawaura, C. Radiation dose evaluation in tomosynthesis and C-arm cone-beam CT examinations with an anthropomorphic phantom. Med. Phys. 2010, 37, 4298–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Yin, X.-R.; Wu, J.-T.; Wu, H.-T. Comparative study of DTS and CT in the skeletal trauma imaging diagnosis evaluation and radiation dose. Eur. J. Radiol. 2013, 82, e76–e80. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; E Bible, J.; Bohan, M.; Simpson, A.K.; Whang, P.G.; Grauer, J.N. Radiation Exposure from Musculoskeletal Computerized Tomographic Scans. J. Bone Jt. Surg. 2009, 91, 1882–1889. [Google Scholar] [CrossRef]
- Minoda, Y.; Yoshida, T.; Sugimoto, K.; Baba, S.; Ikebuchi, M.; Nakamura, H. Detection of Small Periprosthetic Bone Defects after Total Knee Arthroplasty. J. Arthroplast. 2014, 29, 2280–2284. [Google Scholar] [CrossRef]
- Solomon, L.B.; Stamenkov, R.B.; MacDonald, A.J.; Yaikwavong, N.; Neale, S.D.; Moss, M.J.; Howie, D.W. Imaging Periprosthetic Osteolysis around Total Knee Arthroplasties Using a Human Cadaver Model. J. Arthroplast. 2012, 27, 1069–1074. [Google Scholar] [CrossRef]
- Blum, A.; Noël, A.; Regent, D.; Villani, N.; Gillet, R.; Teixeira, P.G. Tomosynthesis in musculoskeletal pathology. Diagn. Interv. Imaging 2018, 99, 423–441. [Google Scholar] [CrossRef]
- Nelson, G.; Wu, M.; Hinkel, C.; Krishna, G.; Funk, T.; Rosenberg, J.; Fahrig, R. Improved targeting accuracy of lung tumor biopsies with scanning-beam digital X-ray tomosynthesis image guidance. Med. Phys. 2016, 43, 6282–6290. [Google Scholar] [CrossRef] [Green Version]
- Tucker, L.; Gilbert, F.J.; Astley, S.M.; Dibden, A.; Seth, A.; Morel, J.; Bundred, S.; Litherland, J.; Klassen, H.; Lip, G.; et al. Does Reader Performance with Digital Breast Tomosynthesis Vary according to Experience with Two-dimensional Mammography? Radiology 2017, 283, 371–380. [Google Scholar] [CrossRef] [Green Version]
- McAdams, H.P.; Samei, E.; Dobbins, J., III; Tourassi, G.; Ravin, C.E. Recent Advances in Chest Radiography. Radiology 2006, 241, 663–683. [Google Scholar] [CrossRef]
- Dobbins, J.T.; Godfrey, D.J. Digital X-ray tomosynthesis: Current state of the art and clinical potential. Phys. Med. Biol. 2003, 48, R65–R106. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, T.; Nishino, K. Metal artifact reduction in tomosynthesis by metal extraction and ordered subset-expectation maximization (OS-EM) reconstruction. In Medical Imaging 2013: Physics of Medical Imaging; SPIE: Bellingham, WA, USA, 2013; Volume 8668. [Google Scholar] [CrossRef]
- Machida, H.; Yuhara, T.; Mori, T.; Ueno, E.; Moribe, Y.; Sabol, J.M. Optimizing Parameters for Flat-Panel Detector Digital Tomosynthesis. RadioGraphics 2010, 30, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.G.; Campbell, D.G.; Woodman, R. Detection of Periprosthetic Osteolysis around Total Knee Arthroplasties: An in vitro study. J. Arthroplast. 2012, 27, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Yuhara, T.; Tamura, M.; Ishikawa, T.; Tate, E.; Ueno, E.; Nye, K.; Sabol, J.M. Whole-Body Clinical Applications of Digital Tomosynthesis. RadioGraphics 2016, 36, 735–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobres, J.; Chahine, N.; Reimer, B. Effects of ambient illumination, contrast polarity, and letter size on text legibility under glance-like reading. Appl. Ergon. 2017, 60, 68–73. [Google Scholar] [CrossRef]
- Ottenin, M.-A.; Jacquot, A.; Grospretre, O.; Noël, A.; Lecocq, S.; Louis, M.; Blum, A. Evaluation of the Diagnostic Performance of Tomosynthesis in Fractures of the Wrist. Am. J. Roentgenol. 2012, 198, 180–186. [Google Scholar] [CrossRef]
- Petraszko, A.; Siegal, D.; Flynn, M.; Rao, S.D.; Peterson, E.; van Holsbeeck, M. The advantages of tomosynthesis for evaluating bisphosphonate-related atypical femur fractures compared to radiography. Skelet. Radiol. 2016, 45, 615–623. [Google Scholar] [CrossRef]
- Geijer, M.; Gunnlaugsson, E.; Gotestrand, S.; Weber, L.; Geijer, H. Tomosynthesis of the thoracic spine: Added value in diagnosing vertebral fractures in the elderly. Eur. Radiol. 2017, 27, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Huang, X.; Cheng, X.; Yang, D.; Huang, Y.; Zhou, Y. Evaluation of peri-prosthetic radiolucent lines surrounding the cementless femoral stem using digital tomosynthesis with metal artifact reduction: A cadaveric study in comparison with radiography and computed tomography. Quant. Imaging Med. Surg. 2020, 10, 1786–1800. [Google Scholar] [CrossRef]
- Guo, S.; Tang, H.; Zhou, Y.; Huang, Y.; Shao, H.; Yang, D. Accuracy of Digital Tomosynthesis with Metal Artifact Reduction for Detecting Osteointegration in Cementless Hip Arthroplasty. J. Arthroplast. 2018, 33, 1579–1587. [Google Scholar] [CrossRef]
- Gillet, R.; Teixeira, P.; Bonarelli, C.; Coudane, H.; Sirveaux, F.; Louis, M.; Blum, A. Comparison of radiographs, tomosynthesis and CT with metal artifact reduction for the detection of hip prosthetic loosening. Eur. Radiol. 2019, 29, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, S.; Masuda, H.; Nishihara, N.; Shimazaki, N.; Ando, S.; Kawano, H.; Nakagawa, T. Tomosynthesis Is Equivalent to Computed Tomography for Evaluating Osseous Integration after Anterior Cruciate Ligament Reconstruction. Arthrosc. Sports Med. Rehabil. 2020, 2, e105–e112. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Sasaki, E.; Wijaya, E.; Yamauchi, S.; Sasaki, S.; Kimura, Y.; Yamamoto, Y.; Shimbo, T.; Tamai, K.; Ishibashi, Y. A Novel Quantitative Evaluation of Bone Formation after Opening Wedge High Tibial Osteotomy Using Tomosynthesis. J. Digit. Imaging 2022, 35, 1373–1381. [Google Scholar] [CrossRef]
- Okano, E.; Hara, Y.; Ito, A.; Mataki, K.; Totoki, Y.; Noguchi, H.; Nagashima, K.; Matsumoto, Y.; Yanagisawa, Y.; Mutsuzaki, H.; et al. Novel method for selecting slices of the same cross-sectional view from digital tomosynthesis for monitoring posterior spinal instrumentation. J. Clin. Neurosci. 2021, 92, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mataki, K.; Hara, Y.; Okano, E.; Nagashima, K.; Noguchi, H.; Shibao, Y.; Miura, K.; Takahashi, H.; Funayama, T.; Koda, M.; et al. Development of a quantitative method to evaluate pedicle screw loosening after spinal instrumentation using digital tomosynthesis. BMC Musculoskelet. Disord. 2022, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Gomi, T.; Sakai, R.; Hara, H.; Watanabe, Y.; Mizukami, S. Usefulness of a Metal Artifact Reduction Algorithm in Digital Tomosynthesis Using a Combination of Hybrid Generative Adversarial Networks. Diagnostics 2021, 11, 1629. [Google Scholar] [CrossRef] [PubMed]
- Gazaille, R.E.; Flynn, M.J.; Page, W.; Finley, S.; Van Holsbeeck, M. Technical innovation: Digital tomosynthesis of the hip following intra-articular administration of contrast. Skelet. Radiol. 2011, 40, 1467–1471. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Boudabbous, S.; Arditi, D.; Paulin, E.; Syrogiannopoulou, A.; Becker, C.; Montet, X. Model-Based Iterative Reconstruction (MBIR) for the Reduction of Metal Artifacts on CT. Am. J. Roentgenol. 2015, 205, 380–385. [Google Scholar] [CrossRef]
- Wellenberg, R.; Hakvoort, E.; Slump, C.; Boomsma, M.; Maas, M.; Streekstra, G. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur. J. Radiol. 2018, 107, 60–69. [Google Scholar] [CrossRef]
- Choo, H.J.; Lee, S.J.; Kim, D.W.; Lee, Y.J.; Baek, J.W.; Han, J.-Y.; Heo, Y.J. Comparison of the Quality of Various Polychromatic and Monochromatic Dual-Energy CT Images with or without a Metal Artifact Reduction Algorithm to Evaluate Total Knee Arthroplasty. Korean J. Radiol. 2021, 22, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; Van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.; Liang, T.; Murphy, D.T.; Korzan, J.R.; Ouellette, H.; Munk, P. Dual-Energy CT: A Promising New Technique for Assessment of the Musculoskeletal System. Am. J. Roentgenol. 2012, 199, S78–S86. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and Avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, M.F.; Warringa, N.; Edens, M.A.; Mueller, D.; Ettema, H.B.; Verheyen, C.C.P.M.; Maas, M. Quantitative analysis of orthopedic metal artefact reduction in 64-slice computed tomography scans in large head metal-on-metal total hip replacement, a phantom study. Springerplus 2016, 5, 405. [Google Scholar] [CrossRef] [Green Version]
- Wellenberg, R.H.H.; Boomsma, M.F.; Osch van, J.A.C.; Milles, J.; Vlassenbroek, A.; Edens, M.A.; Streekstra, G.J.; Slump, C.H.; Maas, M. Computed tomography imaging of a hip prosthesis using iterative model-based reconstruction and orthopaedic metal artefact reduction: A quantitative analysis. J. Comput. Assist. Tomogr. 2016, 40, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Wellenberg, R.H.H.; Boomsma, M.F.; van Osch, J.A.C.; Vlassenbroek, A.; Milles, J.; Edens, M.A.; Streekstra, G.J.; Slump, C.H.; Maas, M. Low-dose CT imaging of a total hip arthroplasty phantom using model-based iterative reconstruction and orthopedic metal artifact reduction. Skelet. Radiol. 2017, 46, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Han, Q.; Xu, X.; Jiang, H.; Ma, L.; Zhang, Y.; Yang, K.; Chen, B.; Wang, J. Metal artifact reduction of orthopedics metal artifact reduction algorithm in total hip and knee arthroplasty. Medicine 2020, 99, e19268. [Google Scholar] [CrossRef]
- Hakvoort, E.; Wellenberg, R.; Streekstra, G. Quantifying near metal visibility using dual energy computed tomography and iterative metal artifact reduction in a fracture phantom. Phys. Medica 2020, 69, 9–18. [Google Scholar] [CrossRef]
- Neuhaus, V.; Hokamp, N.G.; Zopfs, D.; Laukamp, K.; Lennartz, S.; Abdullayev, N.; Maintz, D.; Borggrefe, J. Reducing artifacts from total hip replacements in dual layer detector CT: Combination of virtual monoenergetic images and orthopedic metal artifact reduction. Eur. J. Radiol. 2019, 111, 14–20. [Google Scholar] [CrossRef]
- Bolstad, K.; Flatabo, S.; Aadnevik, D.; Dalenhaug, I.; Vetti, N. Metal artifact reduction in CT, a phantom study: Subjective and objective evaluation of four commercial metal artifact reduction algorithms when used on three different orthopaedic metal implants. Acta Radiol. 2018, 59, 1110–1118. [Google Scholar] [CrossRef]
- Barreto, I.; Pepin, E.; Davis, I.; Dean, C.; Massini, T.; Rees, J.; Olguin, C.; Quails, N.; Correa, N.; Rill, L.; et al. Comparison of metal artifact reduction using single-energy CT and dual-energy CT with various metallic impants in cadavers. Eur. J. Radiol. 2020, 133, 109357. [Google Scholar] [CrossRef]
- Racine, D.; Ott, J.G.; Andreisek, G.; Omoumi, P.; Becce, F.; Verdun, F.R. Dual-Energy CT: Basic Principles, Technical Approaches, and Applications in Musculoskeletal Imaging (Part 1). Semin. Musculoskelet. Radiol. 2015, 19, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Carotti, M.; Salaffi, F.; Beci, G.; Giovagnoni, A. The application of dual-energy computed tomography in the diagnosis of musculoskeletal disorders: A review of current concepts and applications. La Radiol. Medica 2019, 124, 1175–1183. [Google Scholar] [CrossRef]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef]
- Coupal, T.M.; Mallinson, P.I.; McLaughlin, P.; Nicolaou, S.; Munk, P.L.; Ouellette, H. Peering through the glare: Using dual-energy CT to overcome the problem of metal artefacts in bone radiology. Skelet. Radiol. 2014, 43, 567–575. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, K.K.; Song, H.T.; Kim, S.; Suh, J.S. Metal artefact reduction in gemstone spectral imaging dual-energy CT with and without metal artefact reduction software. Eur. Radiol. 2012, 22, 1331–1340. [Google Scholar] [CrossRef]
- Kuchenbecker, S.; Faby, S.; Sawall, S.; Lell, M.; Kachelrieß, M. Dual energy CT: How well can pseudo-monochromatic imaging reduce metal artifacts? Med. Phys. 2015, 42, 1023–1036. [Google Scholar] [CrossRef]
- Horat, L.; Hamie, M.Q.; Huber, F.A.; Guggenberger, R. Optimization of Monoenergetic Extrapolations in Dual-Energy CT for Metal Artifact Reduction in Different Body Regions and Orthopedic Implants. Acad. Radiol. 2019, 26, e67–e74. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.-M.; Seo, J.S.; Kim, T.W.; Rhee, S.J.; Jeong, H.S. Metal Artifact Reduction Dual-Energy CT as an Accurate and Reliable Method for Measuring Total Knee Arthroplasty Femoral Component Rotation Compared to Conventional CT. J. Knee Surg. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Meinel, F.G.; Bischoff, B.; Zhang, Q.; Bamberg, F.; Reiser, M.F.; Johnson, T.R. Metal Artifact Reduction by Dual-Energy Computed Tomography Using Energetic Extrapolation: A systematically optimized protocol. Investig. Radiol. 2012, 47, 406–414. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Chung, B.M.; Moon, S.J.; Choi, J.-Y.; Chae, H.D.; Chang, M.-Y. Metal Artifact Reduction in Virtual Monoenergetic Spectral Dual-Energy CT of Patients with Metallic Orthopedic Implants in the Distal Radius. Am. J. Roentgenol. 2018, 211, 1083–1091. [Google Scholar] [CrossRef]
- Lee, K.Y.G.; Cheng, H.M.J.; Chu, C.Y.; Tam, C.W.A.; Kan, W.K. Metal artifact reduction by monoenergetic extrapolation of dual-energy CT in patients with metallic implants. J. Orthop. Surg. 2019, 27, 2309499019851176. [Google Scholar] [CrossRef] [Green Version]
- Donders, J.; Wellenberg, R.; Streekstra, G.; Maas, M.; Kloen, P. Improved diagnostic confidence in evaluating bone non-union using virtual monochromatic dual-energy CT. Eur. J. Radiol. 2020, 132, 109159. [Google Scholar] [CrossRef]
- Andersson, K.M.; Nowik, P.; Persliden, J.; Thunberg, P.; Norrman, E. Metal artefact reduction in CT imaging of hip prostheses-an evaluation of commercial techniques provided by four vendors. Br. J. Radiol. 2015, 88, 20140473. [Google Scholar] [CrossRef] [Green Version]
- Bongers, M.N.; Schabel, C.; Thomas, C.; Raupach, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Comparison and combination of dual-energy and iterative-based metal artefact reduction on hip prosthesis and dental implants. PLoS ONE 2015, 10, e0143584. [Google Scholar] [CrossRef]
- Long, Z.; Delone, D.R.; Kotsenas, A.L.; Lehman, V.T.; Nagelschneider, A.A.; Michalak, G.J.; Fletcher, J.G.; McCollough, C.H.; Yu, L. Clinical Assessment of Metal Artifact Reduction Methods in Dual-Energy CT Examinations of Instrumented Spines. Am. J. Roentgenol. 2019, 212, 395–401. [Google Scholar] [CrossRef]
- Yue, D.; Rong, C.F.; Ning, C.; Liang, H.; Lian, L.A.; Xin, W.R.; Hong, L.Y. Reduction of metal artifacts from unilateral hip arthroplasty on dual-energy CT with metal artifact reduction software. Acta Radiol. 2017, 59, 853–860. [Google Scholar] [CrossRef]
- Chae, H.-D.; Hong, S.H.; Shin, M.; Choi, J.-Y.; Yoo, H.J. Combined use of virtual monochromatic images and projection-based metal artifact reduction methods in evaluation of total knee arthroplasty. Eur. Radiol. 2020, 30, 5298–5307. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, S.M.; Kim, H.P.; Seo, J.K.; Chung, Y.E. CT sinogram-consistency learning for metal-induced beam hardening correction. Med. Phys. 2018, 45, 5376–5384. [Google Scholar] [CrossRef] [Green Version]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional network for biomedical image segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention, MICCAI 2015; Navab, N., Wells, W.M., Hornegger, J., Frangi, A.F., Eds.; Springer: Cham, Switzerland, 2015; Part 3; pp. 234–241. [Google Scholar]
- Zhang, Y.; Yu, H. Convolutional Neural Network Based Metal Artifact Reduction in X-ray Computed Tomography. IEEE Trans. Med. Imaging 2018, 37, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Noble, J.H.; Dawant, B.M. Conditional Generative Adversarial Networks for Metal Artifact Reduction in CT Images of the Ear. Med. Image Comput. Comput. Assist. Interv. 2018, 11070, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Talbot, B.S.; Weinberg, E.P. MR Imaging with Metal-suppression Sequences for Evaluation of Total Joint Arthroplasty. Radiographics 2016, 36, 209–225. [Google Scholar] [CrossRef]
- Olsen, R.V.; Munk, P.L.; Lee, M.J.; Janzen, A.L.; Xiang, Q.S.; Masri, B. Metal artifact reduction sequence: Early clinical application. Radiographics 2000, 20, 699–712. [Google Scholar] [CrossRef]
- Fritz, J.; Meshram, P.; Stern, S.E.; Fritz, B.; Srikumaran, U.; McFarland, E.G. Diagnostic Performance of Advanced Metal Artifact Reduction MRI for Periprosthetic Shoulder Infection. J. Bone Jt. Surg. 2022, 104, 1352–1361. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Ding, H.; Huang, Z.; Zhang, C.; Li, W.; Liu, X.; Tu, Z.; Zhang, W.; Fang, X. Metal Artifact Reduction Sequences MRI: A Useful Reference for Preoperative Diagnosis and Debridement Planning of Periprosthetic Joint Infection. J. Clin. Med. 2022, 11, 4371. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Ganter, C.; Schaeffeler, C.J.; Bauer, J.S.; Baum, T.; Meier, R.; Nittka, M.; Pohlig, F.; Rechl, H.; Von Eisenhart-Rothe, R.; et al. View-Angle Tilting and Slice-Encoding Metal Artifact Correction for Artifact Reduction in MRI: Experimental Sequence Optimization for Orthopaedic Tumor Endoprostheses and Clinical Application. PLoS ONE 2015, 10, e0124922. [Google Scholar] [CrossRef]
- Lu, W.; Pauly, K.B.; Gold, G.E.; Pauly, J.M.; Hargreaves, B.A. SEMAC: Slice encoding for metal artifact correction in MRI. Magn. Reson. Med. 2009, 62, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Hayter, C.L.; Koff, M.F.; Shah, P.; Koch, K.M.; Miller, T.T.; Potter, H.G. MRI after Arthroplasty: Comparison of MAVRIC and Conventional Fast Spin-Echo Techniques. Am. J. Roentgenol. 2011, 197, W405–W411. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.J.; Lee, S.; Yoon, D.; Lee, R.W.; Hong, J.U.; Ryu, D.-S.; Bae, J. Metallic Artifact Reduction of Multiacquisition with Variable Resonance Image Combination Selective–Short Tau Inversion Recovery for Postoperative Cervical Spine with Artificial Disk Replacement: A Preliminary Study. J. Comput. Assist. Tomogr. 2022, 46, 274–281. [Google Scholar] [CrossRef]
- Sutter, R.; Ulbrich, E.J.; Jellus, V.; Nittka, M.; Pfirrmann, C.W.A. Reduction of Metal Artifacts in Patients with Total Hip Arthroplasty with Slice-encoding Metal Artifact Correction and View-Angle Tilting MR Imaging. Radiology 2012, 265, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Sutter, R.; Hodek, R.; Fucentese, S.F.; Nittka, M.; Pfirrmann, C.W.A. Total Knee Arthroplasty MRI Featuring Slice-Encoding for Metal Artifact Correction: Reduction of Artifacts for STIR and Proton Density–Weighted Sequences. Am. J. Roentgenol. 2013, 201, 1315–1324. [Google Scholar] [CrossRef]
- Galley, J.; Sutter, R.; Stern, C.; Filli, L.; Rahm, S.; Pfirrmann, C.W.A. Diagnosis of Periprosthetic Hip Joint Infection Using MRI with Metal Artifact Reduction at 1.5 T. Radiology 2020, 296, 98–108. [Google Scholar] [CrossRef]
- Takahashi, T.; Thaker, S.; Lettieri, G.; Redmond, A.; Backhouse, M.R.; Stone, M.; Pandit, H.; O’Connor, P. Reliability of slice-encoding for metal artefact correction (SEMAC) MRI to identify prosthesis loosening in patients with painful total hip arthroplasty—A single centre, prospective, surgical validation study. Br. J. Radiol. 2022, 95, 20210940. [Google Scholar] [CrossRef]
- Zochowski, K.C.; Miranda, M.A.; Cheung, J.; Argentieri, E.C.; Lin, B.; Kaushik, S.S.; Burge, A.J.; Potter, H.G.; Koff, M.F. MRI of Hip Arthroplasties: Comparison of Isotropic Multiacquisition Variable-Resonance Image Combination Selective (MAVRIC SL) Acquisitions with a Conventional MAVRIC SL Acquisition. Am. J. Roentgenol. 2019, 213, W277–W286. [Google Scholar] [CrossRef]
- Fritz, J.; Guggenberger, R.; Del Grande, F. Rapid Musculoskeletal MRI in 2021: Clinical Application of Advanced Accelerated Techniques. Am. J. Roentgenol. 2021, 216, 718–733. [Google Scholar] [CrossRef]
- Khodarahmi, I.; Haroun, R.R.; Lee, M.; Fung, G.S.K.; Fuld, M.K.; Schon, L.C.; Fishman, E.K.; Fritz, J. Metal artifact reduction computed tomography of arthroplasty implants: Effects of combined modeled iterative reconstruction and dual-energy virtual monoenergetic extrapolation at higher photon energies. Investig. Radiol. 2018, 53, 728–735. [Google Scholar] [CrossRef]
- Khodarahmi, I.; Fritz, J. Advanced MR Imaging after Total Hip Arthroplasty: The Clinical Impact. Semin. Musculoskelet. Radiol. 2017, 21, 616–629. [Google Scholar] [CrossRef]
- Fritz, J.; Fritz, B.; Thawait, G.K.; Raithel, E.; Gilson, W.D.; Nittka, M.; Mont, M.A. Advanced metal artifact reduction MRI of metal-on-metal hip resurfacing arthroplasty implants: Compressed sensing acceleration enables the time-neutral use of SEMAC. Skelet. Radiol. 2016, 45, 1345–1356. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Bensler, S.; Zingg, P.; Fritz, B.; Pfirrmann, C.W.; Sutter, R. Improved Visualization of Juxtaprosthetic Tissue Using Metal Artifact Reduction Magnetic Resonance Imaging: Experimental and clinical optimization of compressed sensing SEMAC. Investig. Radiol. 2019, 54, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Filli, L.; Jungmann, P.M.; Zingg, P.O.; Rüdiger, H.A.; Galley, J.; Sutter, R.; Pfirrmann, C.W.A. MRI with state-of-the-art metal artifact reduction after total hip arthroplasty: Periprosthetic findings in asymptomatic and symptomatic patients. Eur. Radiol. 2020, 30, 2241–2252. [Google Scholar] [CrossRef]
- Fritz, J.; Ahlawat, S.; Demehri, S.; Thawait, G.K.; Raithel, E.; Gilson, W.D.; Nittka, M. Compressed Sensing SEMAC: 8-fold Accelerated High Resolution Metal Artifact Reduction MRI of Cobalt-Chromium Knee Arthroplasty Implants. Investig. Radiol. 2016, 51, 666–676. [Google Scholar] [CrossRef]
- Netto, C.D.C.; Schon, L.C.; Da Fonseca, L.F.; Chinanuvathana, A.; Stern, S.; Fritz, J. Metal artifact reduction MRI for total ankle replacement sagittal balance evaluation. Foot Ankle Surg. 2019, 25, 739–747. [Google Scholar] [CrossRef]
- Netto, C.D.C.; Fonseca, L.F.; Fritz, B.; Stern, S.E.; Raithel, E.; Nittka, M.; Schon, L.C.; Fritz, J. Metal artifact reduction MRI of total ankle arthroplasty implants. Eur. Radiol. 2018, 28, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, I.; Isaac, A.; Fishman, E.K.; Dalili, D.; Fritz, J. Metal about the Hip and Artifact Reduction Techniques: From Basic Concepts to Advanced Imaging. Semin. Musculoskelet. Radiol. 2019, 23, e68–e81. [Google Scholar] [CrossRef] [Green Version]
- Christoph, G.; Falkowski, A.L.; von Deuster, C.; Nanz, D.; Sutter, R. Basic and advanced metal-artifact reduction techniques at ultra-high field 7T magnetic resonance imaging-phantom study investigating feasibility and efficacy. Investig. Radiol. 2022, 57, 387–398. [Google Scholar]
- Kohyama, S.; Nishiura, Y.; Hara, Y.; Ogawa, T.; Ikumi, A.; Okano, E.; Totoki, Y.; Yamazaki, M. A novel three-dimensional MRI-CT image fusion technique for precise preoperative evaluation and treatment of capitellar osteochondritis dissecans. Eur. Radiol. 2021, 31, 5721–5733. [Google Scholar] [CrossRef]
- Kohyama, S.; Nishiura, Y.; Hara, Y.; Ogawa, T.; Ikumi, A.; Okano, E.; Totoki, Y.; Yoshii, Y.; Yamazaki, M. Preoperative Evaluation and Surgical Simulation for Osteochondritis Dissecans of the Elbow Using Three-Dimensional MRI-CT Image Fusion Images. Diagnostics 2021, 11, 2337. [Google Scholar] [CrossRef]

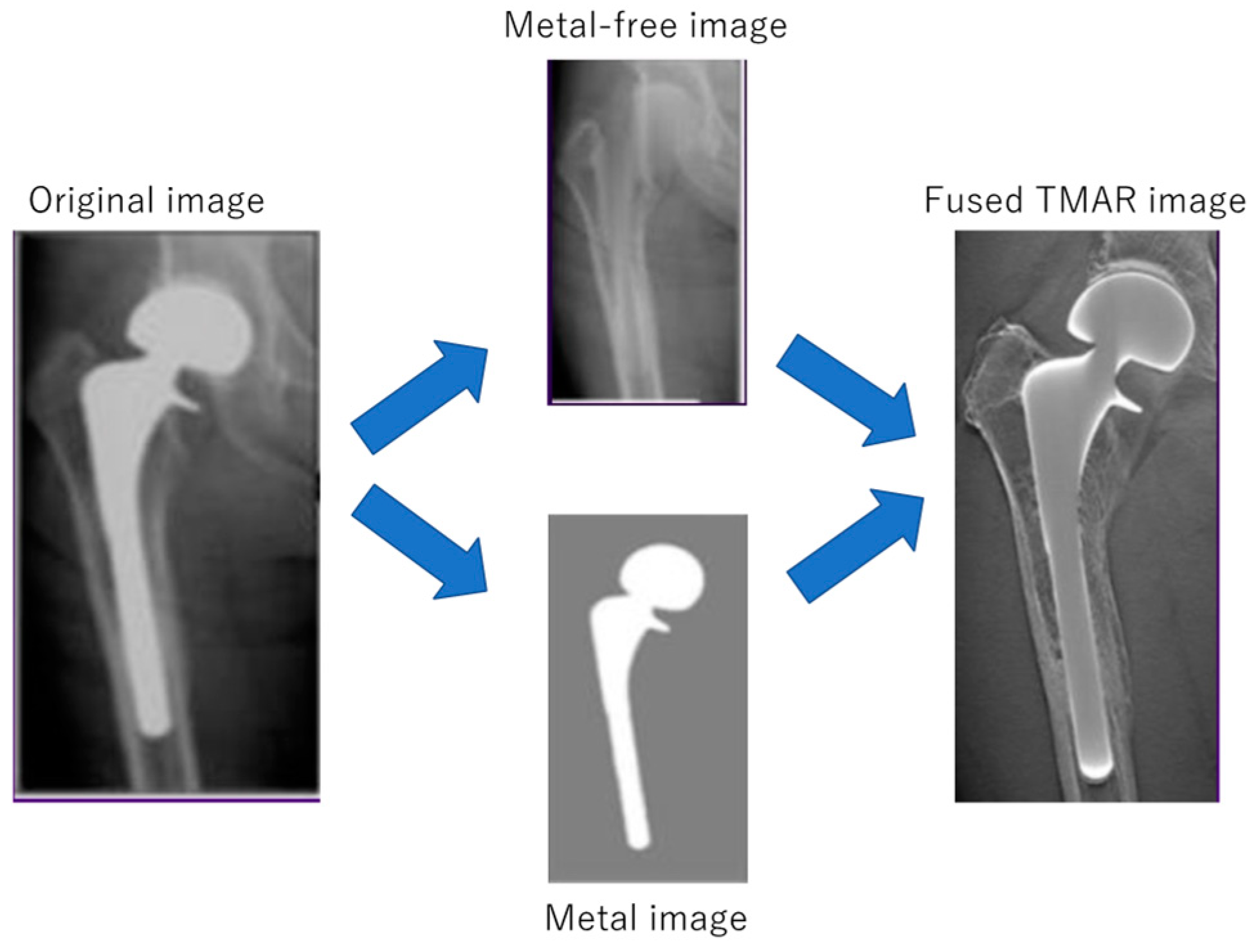
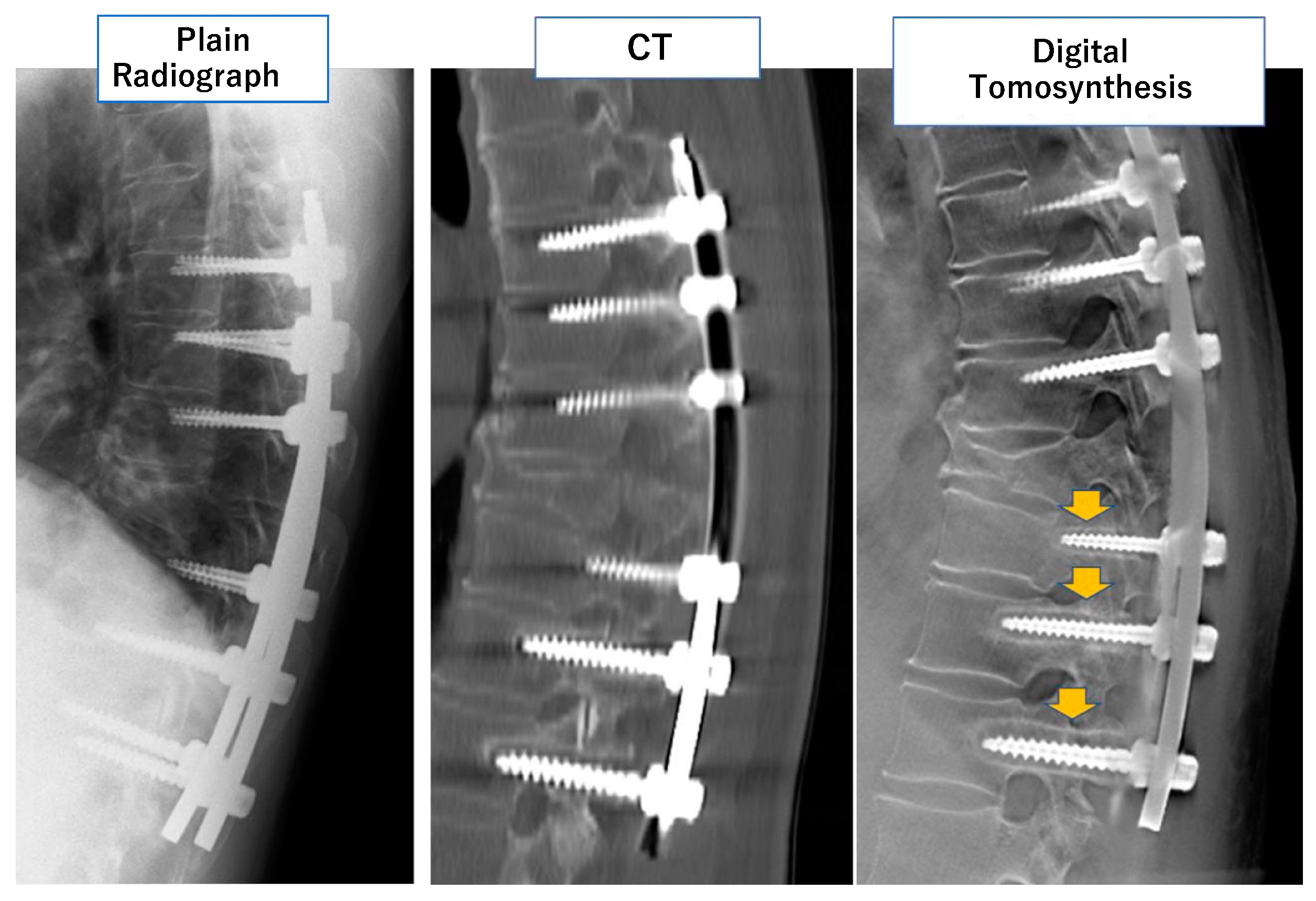
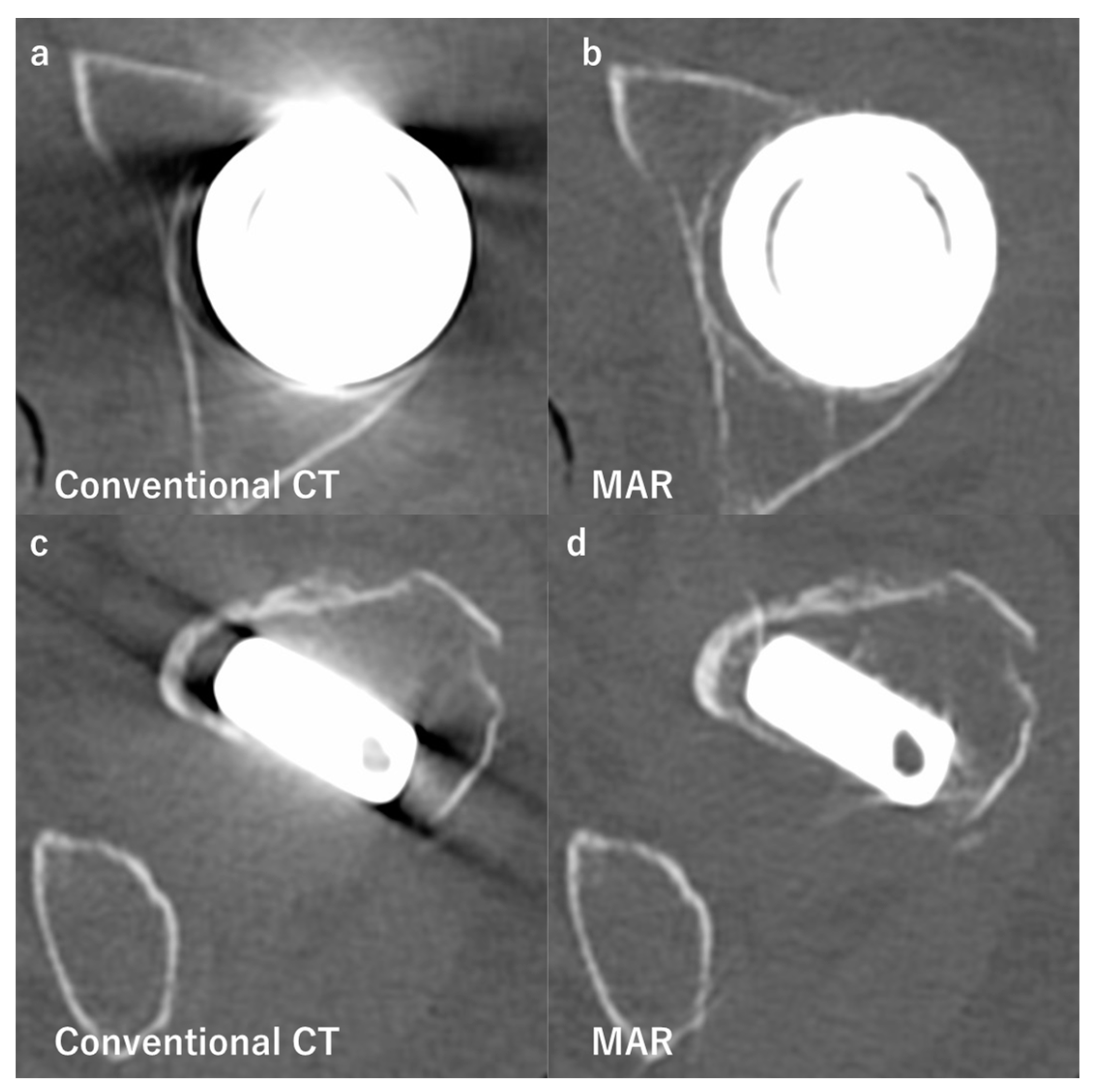
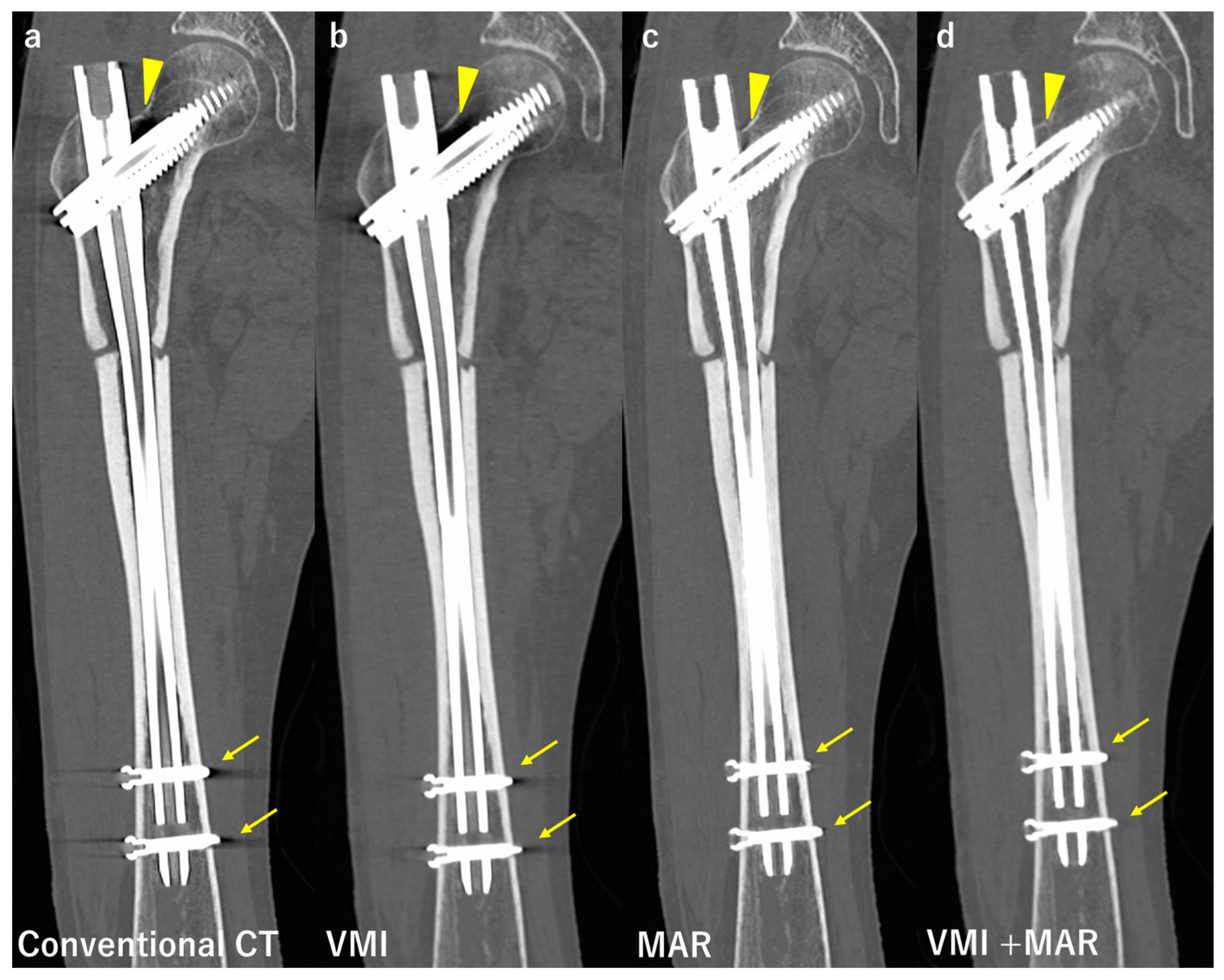
| Compared Modalities | Subject | Results | |
|---|---|---|---|
| Tang et al. [21] | Radiography, DT with TMAR, CT | 4 cadaveric femurs Femoral stem | Sensitivity Radiography 20.5% DT with TMAR 63.3% CT 50.2% Specificity Radiography 92.5% DT with TMAR 87.5% CT 82.5% |
| Ottenin et al. [18] | Radiography, DT CT | 100 patients with acute wrist trauma Carpal bones | Sensitivity Radiography 61–80% DT 77–87% CT 93–95% Specificity Radiography 65–83% DT 76–82% CT 86–95% |
| Tang et al. [1] | Radiography, DT with TMAR, CT | 48 patients with cementless THA (Femoral stem and acetabular cup) | Diagnostic accuracy Femoral stem Radiography 84.5% DT with TMAR 82.6% CT 44.6% Acetabular cup Radiography 39.6% DT with TMAR 67.3% CT 74.6% |
| Guo et al. [22] | Radiography, DT with TMAR, CT | 24 patients with cementless THA 13 femoral stems and 14 acetabular components were evaluated. | Sensitivity Femoral side Radiography 50.4% DT with TMAR 73.8% CT 36.4% Acetabular side Radiography 45.9% DT with TMAR 60.2% CT 45.1% Specificity Femoral side Radiography 87.8% DT with TMAR 94.3% CT 90.9% Acetabular side Radiography 66.4% DT with TMAR 86.4% CT 73.5% |
| Gillet et al. [23] | Radiography DT CT + MAR | 49 patients with painful hip prostheses. Evaluated prosthestic loosening. | Sensitivity Radiography 33.3–51.5% DT 39.9–45.4% CT + MAR 84.5% Specificity Radiography 96.9–100% DT 98.5–100% CT + MAR 95.4–96.9% |
| Toyooka et al. [24] | DT CT | Bone integration of 27 patients who underwent ACL reconstruction was evaluated | DT was equivalent to CT for the evaluation of bone plug integration within a 15% diagnostic error. Sensitivity 79–96% Specificity 64–100% Diagnostic accuracy 81–96% |
| Ishibashi et al. [25] | DT | Open Wedge High Tibial osteotomy Gap filling value (GFV) and modified van Hemert’s score (MVHS) | GFV had strong correlation with MVHS (r = 0.630, p < 0.001) ICC value for intraobserver reliability GFV 0.958 MVHS 0.978 ICC value for interobserver reliability GFV 0.975 MVHS 0.950 |
| Mataki et al. [27] | DT | Pedicle screw (PS) displacement angle Loosening group vs. group without PS loosening | The displacement angle was significantly greater in loosening group (5.7° vs. 0.6°) Sensitivity 100% Specificity 93% AUC = 0.98 |
| Compared Modalities | Subjects | Results | |
|---|---|---|---|
| Lee et al. [55] | Conventional CT VMI (70 and 150 kV) | 40 patients with metallic implants | VMI at high kV reduced metal artifacts, increased SNR, and improved image quality. |
| Donders et al. [56] | VMI Low (70) kV versus high (130–150) kV | 41 patients with a clinical suspected non-union with hardware in place. Likert scores were used. | Image quality 1.83 (high kV) > 0.88 (low kV) Number of false-negative non-unions; 5% reduced by high kV. Diagnostic confidence 2.37 (high kV) > 1.43 (low kV) |
| Barreto et al. [44] | Conventional CT, MAR, VMI | Cadavers with hip bipolar hemiarthroplasty, TKA, and an implant for anterior cervical disc fusion. | Rank of the original 5 points scale Hip; MAR > CT > VMI TKA; MAR > CT > VMI Spine; VMI > MAR > CT |
| Neuhaus et al. [42] | Conventional CT, MAR, VMI, VMI + MAR | 24 patients after THA | VMI + MAR reduced artifacts the most. VMI + MAR improved the assessment of adjacent structures the most. |
| Andersson et al. [57] | Conventional CT, MAR, VMI, VMI + MAR | Bilateral hip prosthesis phantom | Artifact reduction rate MAR 52–75% VMI 12–52% (in a certain region artifact increased up to 32%) VMI + MAR 75–77% |
| Bongers et al. [58] | Conventional CT, MAR, VMI, VMI + MAR | Hip prosthesis and dental implants. Qualitative and quantitative evaluation. | Artifact reduction rate (Hip, dental implant, respectively) VMI 33%, 8% MAR 56%, 71% VMI + MAR 76%, 76% |
| Long et al. [59] | MAR VMI VMI + MAR | 20 patients with instrumented spines. Artifact score (1 to 5) Image quality score (1 to 4) | VMI + MAR showed the best artifact and image quality scores. ICC 0.779 |
| Yue et al. [60] | VMI VMI + MAR (80, 100, 120 and 140 kV) | 35 patients with THA. Artifact index (AI) CT number Subjective scores | AI in VMI + MAR at 120 and 140 kV were significantly lower than others. Accuracy of CT numbers for the peroprosthetic region improved with VMI + MAR. VMI + MAR at 120 and 140 kV had higher subjective scores. |
| Chae et al. [61] | Conventional CT MAR VMI VMI + MAR | 57 patients with TKA Area of the artifacts Mean attenuation Artifact index (AI) Contrast-to-noise ratio (CNR) | VMI + MAR showed the best performance in artifact reduction and soft tissue depiction. MAR depicted bony structures the best. |
| Modalities, Sequences | Subjects | Results | |
|---|---|---|---|
| Galley et al. [77] | 1.5 T system STIR-SEMAC | 40 patients with periprosthetic infections after THA Periosteal reaction, capsule edema, and intramuscular edema were evaluated. | Sensitivities 78, 83, 95%, respectively, Specificities 90, 95, 86%, respectively, Accuracies 86, 91, 89%, respectively, Interobserver agreement ICC values 0.88–0.92 |
| Takahashi et al. [78] | 1.5 T system T1WI-SEMAC STIR-SEMAC PDW-SEMAC | 47 patients after THA Prosthesis loosening was evaluated. | T1WI-SEMAC Sensitivity 72.7% Specificity 64.3% PPV 44.4%, NPV 85.7% STIR-SEMAC Sensitivity 90.9%, Specificity 46.4%, PPV 40.0%, NPV 92.9% PDW-SEMAC Sensitivity 36.3% Specificity 78.5% PPV 40.0%, NPV 75.8% |
| Jungman et al. [71] | 1.5T system Conventional MRI VAT VAT + SEMAC (STIR, T1W, T2W were taken for each group) | 25 malignant bone tumor patients after surgery (metal implants used) with clinical suspicion of tumor recurrence. | VAT + SEMAC reduced artifact diameters and distortions (p < 0.001). VAT + SEMAC improved diagnostic confidence (p < 0.05). Two cases of tumor recurrence were diagnosed. |
| Zochowski et al. [79] | 1.5T system Conventional MAVRIC SL Isotropic MAVRIC SL Reduced TR MAVRIC SL | 84 patients after THA | Isotropic MAVRIC SL and reduced TR MAVRIC SL decreased blurring and improved visualization of the synovium and the periprosthetic bone (p < 0.001). Isotropic MAVRIC SL was more effective than reduced-TR MAVRIC SL (p < 0.032). ICC values 0.61–1.00 |
| Kim et al. [74] | 3T system MAVRIC SL STIR STIR | A cadaver 5 volunteers | Cadaveric study MAVRIC SL STIR better visualized anatomic structures, less distortion and pile-up. Fat suppression was better with STIR. Interobserver agreement κ = 0.7 Volunteer study MAVRIC SL STIR better visualized anatomic structures, less distortion. Spinal cord was better depicted by STIR. Interobserver agreement κ = 0.89 |
| Advantages | Disadvantages | |
|---|---|---|
| DT |
|
|
| CT |
|
|
| MRI |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohyama, S.; Yoshii, Y.; Okamoto, Y.; Nakajima, T. Advances in Bone Joint Imaging-Metal Artifact Reduction. Diagnostics 2022, 12, 3079. https://doi.org/10.3390/diagnostics12123079
Kohyama S, Yoshii Y, Okamoto Y, Nakajima T. Advances in Bone Joint Imaging-Metal Artifact Reduction. Diagnostics. 2022; 12(12):3079. https://doi.org/10.3390/diagnostics12123079
Chicago/Turabian StyleKohyama, Sho, Yuichi Yoshii, Yoshikazu Okamoto, and Takahito Nakajima. 2022. "Advances in Bone Joint Imaging-Metal Artifact Reduction" Diagnostics 12, no. 12: 3079. https://doi.org/10.3390/diagnostics12123079
APA StyleKohyama, S., Yoshii, Y., Okamoto, Y., & Nakajima, T. (2022). Advances in Bone Joint Imaging-Metal Artifact Reduction. Diagnostics, 12(12), 3079. https://doi.org/10.3390/diagnostics12123079







