An Unusual Case of Urachal Cyst Misdiagnosed as a Paraovarian Cyst: Ultrasound Assessment and Differential Diagnosis
Abstract
:1. Introduction
2. Case Presentation
2.1. Patient Information
2.2. Clinical History
2.3. Physical Examination and Paraclinical Tests
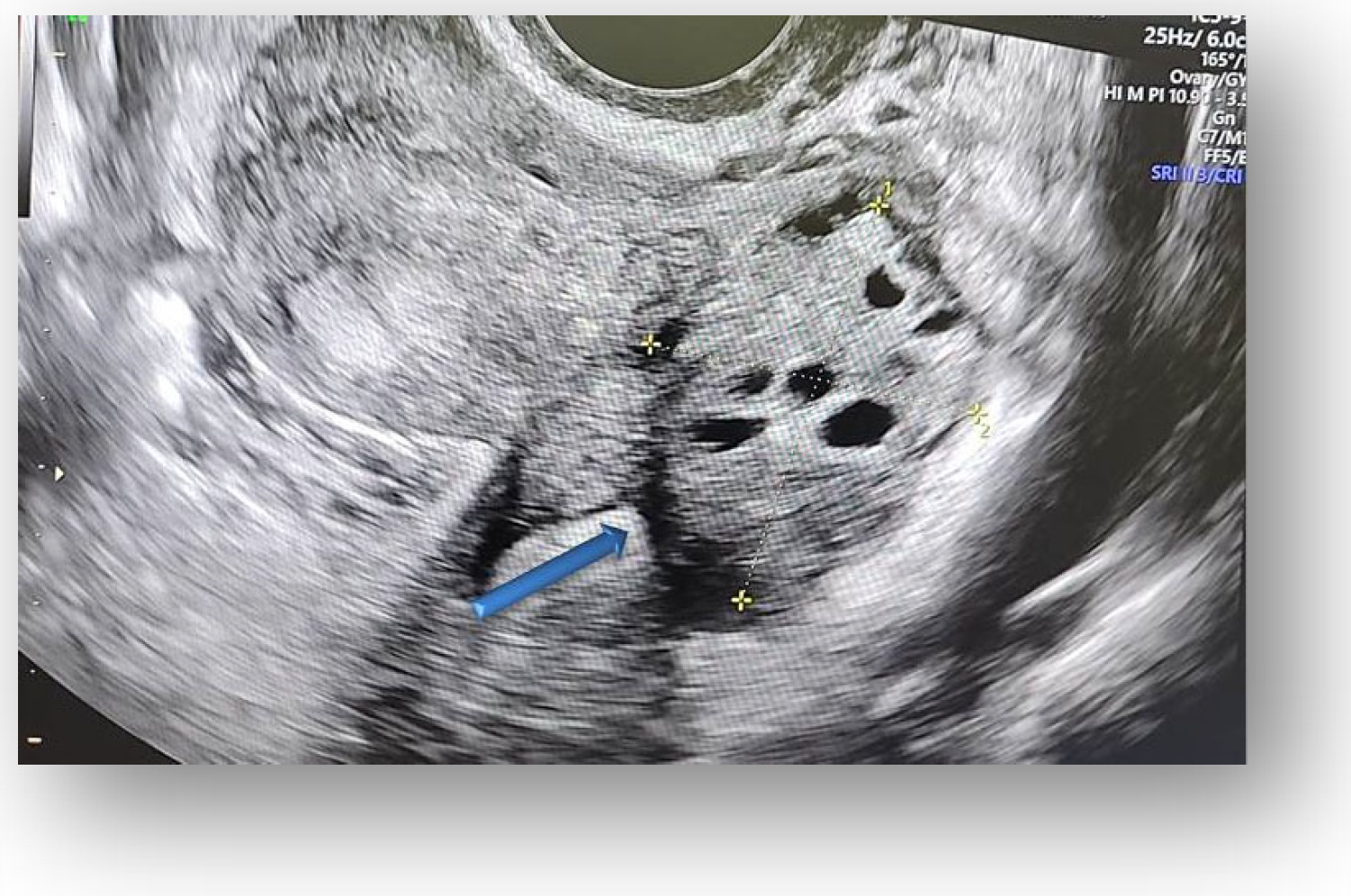
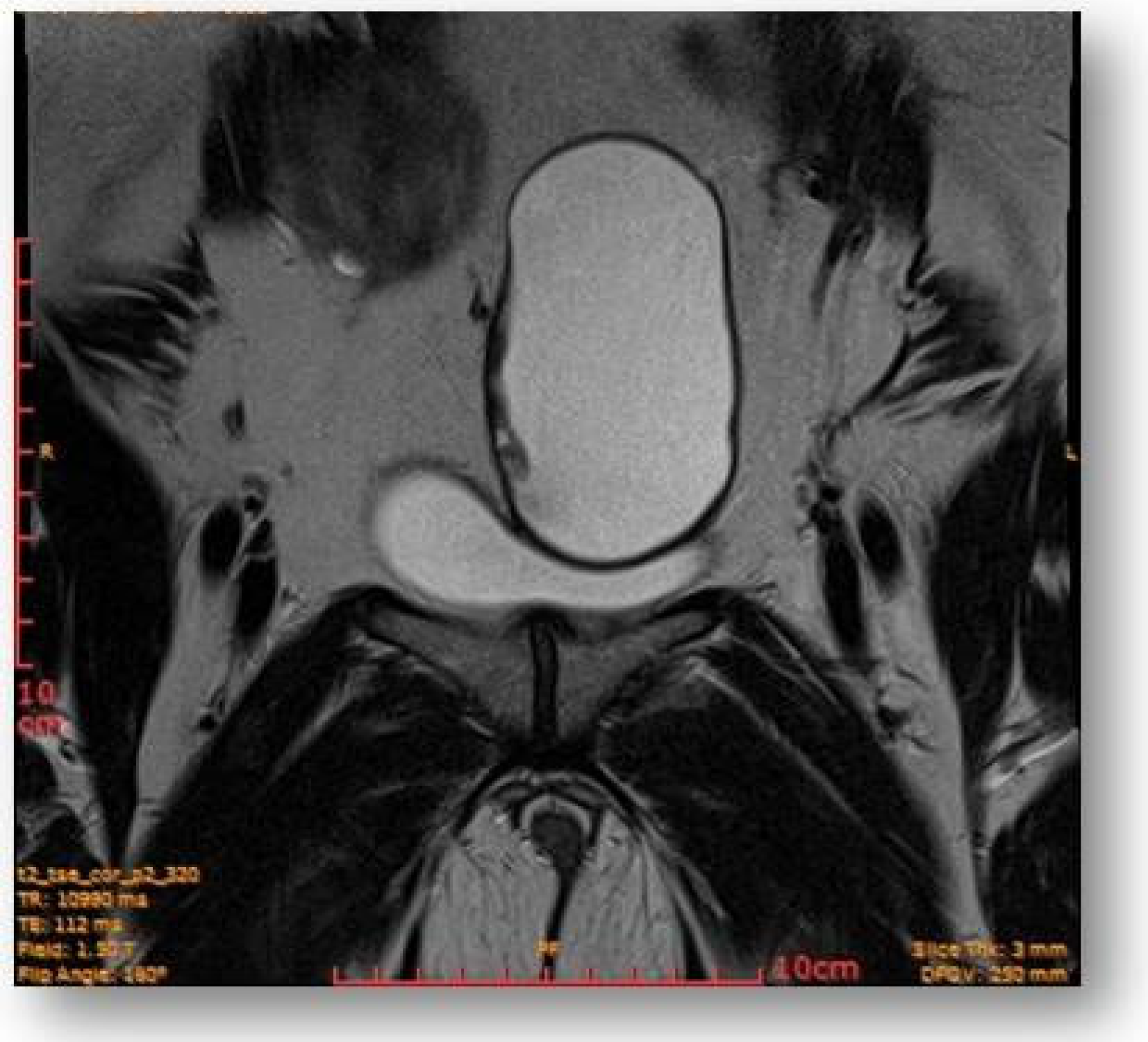
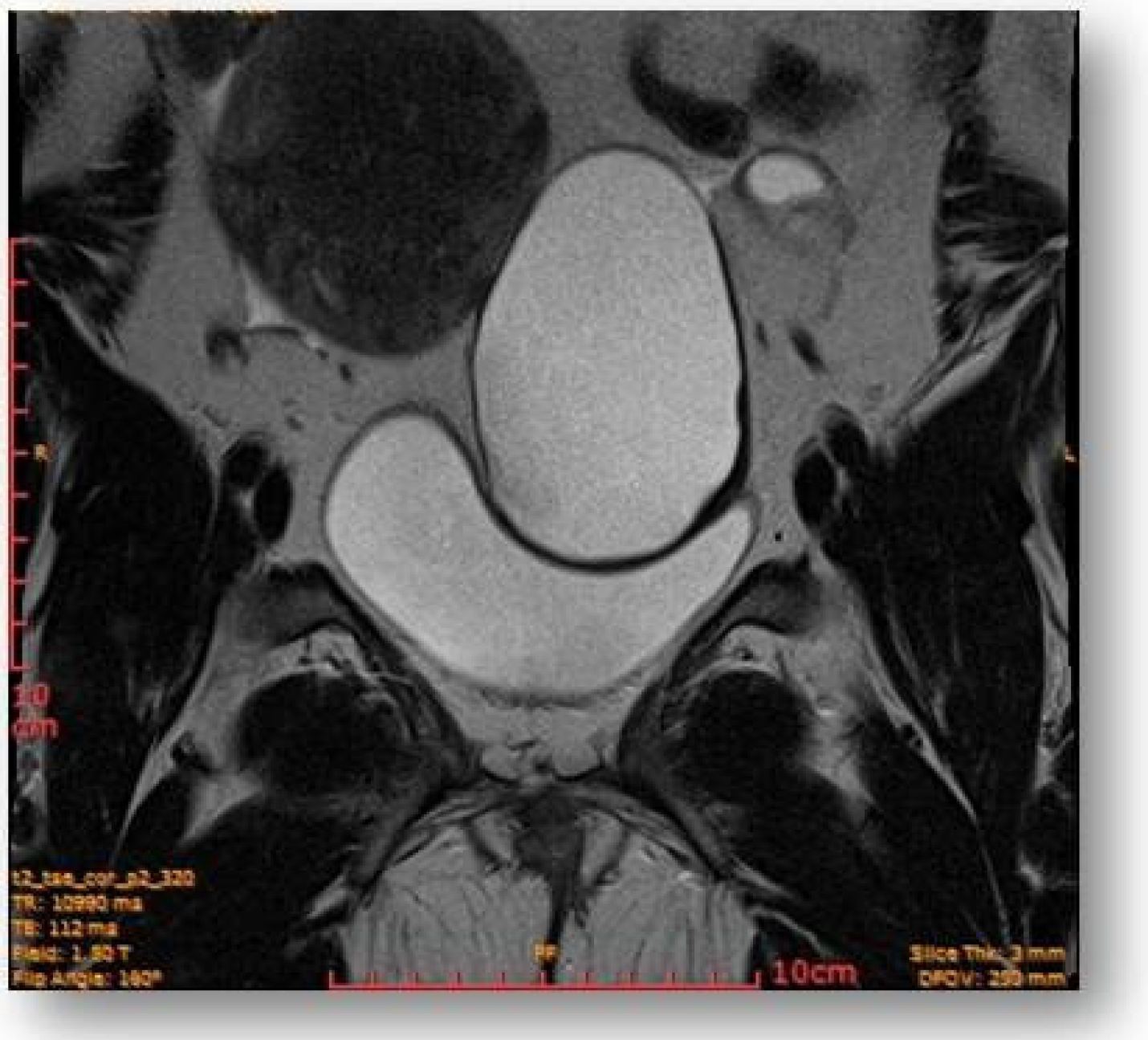
2.4. Diagnostic Assessment and Investigations
2.5. Surgical Treatment
2.6. Anatomopathological Result: Urachal Mucinous Cystadenoma
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahmy, M. (Ed.) Urachal Anomalies BT. In Umbilicus and Umbilical Cord; Springer International Publishing: Cham, Switzerland, 2018; pp. 229–252. ISBN 978-3-319-62383-2. [Google Scholar]
- Wilson, A.L.; Gandhi, J.; Seyam, O.; Rahmani, B.; Patel, S.; Joshi, G.; Smith, N.L.; Khan, S.A. Urachal anomalies: A review of pathological conditions, diagnosis, and management. Transl. Res. Anat. 2019, 16, 100041. [Google Scholar] [CrossRef]
- Yu, J.-S.; Kim, K.W.; Lee, H.-J.; Lee, Y.-J.; Yoon, C.-S.; Kim, M.-J. Urachal Remnant Diseases: Spectrum of CT and US Findings. RadioGraphics 2001, 21, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, J.L.; Porter, M.P.; Li, C.I.; Lange, P.H.; Lin, D.W. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer 2006, 107, 721–728. [Google Scholar] [CrossRef]
- Munichor, M.; Szvalb, S.; Cohen, H.; Bitterman, W. Mixed Adenocarcinoma and Neuroendocrine Carcinoma Arising in the Urachus. Eur. Urol. 1995, 28, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Elser, C.; Sweet, J.; Cheran, S.; Haider, M.; Jewett, M.; Sridhar, S. A case of metastatic urachal adenocarcinoma treated with several different chemotherapeutic regimens. Can. Urol. Assoc. J. 2012, 6, E27–E31. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Kinslow, K.; Ehrhardt, J.D.; Hai, S.; McKenney, M.; Boneva, D. Surgical management for an infected urachal cyst in an adult: Case report and literature review. Int. J. Surg. Case Rep. 2019, 57, 130–133. [Google Scholar] [CrossRef]
- Ueno, T.; Hashimoto, H.; Yokoyama, H.; Ito, M.; Kouda, K.; Kanamaru, H. Urachal anomalies: Ultrasonography and management. J. Pediatr. Surg. 2003, 38, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Severson, C.R. Enhancing nurse practitioner understanding of urachal anomalies. J. Am. Acad. Nurse Pract. 2011, 23, 2–7. [Google Scholar] [CrossRef]
- Yohannes, P.; Bruno, T.; Pathan, M.; Baltaro, R. Laparoscopic Radical Excision of Urachal Sinus. J. Endourol. 2003, 17, 475–479. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, J.M.; Ahn, S.Y.; Oh, J.-T.; Han, S.W.; Lee, J.S. Urachal Anomalies in Children: A Single Center Experience. Yonsei Med. J. 2006, 47, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Mesrobian, H.G.O.; Zacharias, A.; Balcom, A.H.; Cohen, R.D. Ten Years of Experience With Isolated Urachal Anomalies in Children. J. Urol. 1997, 158, 1316–1318. [Google Scholar] [CrossRef]
- Siow, S.L.; Mahendran, H.A.; Hardin, M. Laparoscopic management of symptomatic urachal remnants in adulthood. Asian J. Surg. 2015, 38, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Faye, P.M.; Gueye, M.L.; Thiam, O.; Niasse, A.; Ndong, A.; Ndiaye, M.; Seye, Y.; Sarr, I.S.S.; Seck, M.; Toure, A.O.; et al. Infected urachal cyst in an adult, report of two observations. Int. J. Surg. Case Rep. 2022, 97, 107394. [Google Scholar] [CrossRef]
- Calagna, G.; Rotolo, S.; Catinella, V.; Maranto, M.; Carlisi, B.; Bisso, C.; Venezia, R.; Mangione, D.; Cucinella, G. Unexpected finding of urachal remnant cyst. Tips for laparoscopic approach. Int. J. Surg. Case Rep. 2020, 77, S139–S142. [Google Scholar] [CrossRef]
- Agatstein, E.H.; Stabile, B.E. Peritonitis due to Intraperitoneal Perforation of Infected Urachal Cysts. Arch. Surg. 1984, 119, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.; Maskell, D.; McMillan, C.; Wijewardena, C. An infected urachal cyst presenting as an acute abdomen—A case report. Int. J. Surg. Case Rep. 2013, 4, 633–635. [Google Scholar] [CrossRef] [Green Version]
- Gleason, J.M.; Bowlin, P.R.; Bagli, D.J.; Lorenzo, A.J.; Hassouna, T.; Koyle, M.A.; Farhat, W.A. A Comprehensive Review of Pediatric Urachal Anomalies and Predictive Analysis for Adult Urachal Adenocarcinoma. J. Urol. 2015, 193, 632–636. [Google Scholar] [CrossRef]
- Muthiah, M.; Jayakumari, S.; Prabhu, K.; Kavimani, M. A rare case of urachal cyst in a 35 year old male—A case report. J. Anat. Soc. India 2017, 66, S22–S24. [Google Scholar] [CrossRef]
- MacNeily, A.E.; Koleilat, N.; Kiruluta, H.G.; Homsy, Y.L. Urachal abscesses: Protean manifestations, their recognition, and management. Urology 1992, 40, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Bacanakgıl, B.H.; Soyman, Z.; Kerımova, R.; Battal Havare, S.; Kaya, B. An Infected Urachal Cyst in an Adult Woman. Case Rep. Obstet. Gynecol. 2015, 2015, 791408. [Google Scholar] [CrossRef] [PubMed]
- Ashley, R.A.; Inman, B.A.; Routh, J.C.; Rohlinger, A.L.; Husmann, D.A.; Kramer, S.A. Urachal Anomalies: A Longitudinal Study of Urachal Remnants in Children and Adults. J. Urol. 2007, 178, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Lee, S.-J.; Chang, S.-G. Treatment of Infected Urachal Cysts. Yonsei Med. J. 2006, 47, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueras-Ocaña, M.; Rodríguez-Belmonte, R.; Uberos-Fernández, J.; Jiménez-Pacheco, A.; Merino-Salas, S.; Zuluaga-Gómez, A. Urachal anomalies in children: Surgical or conservative treatment? J. Pediatr. Urol. 2014, 10, 522–526. [Google Scholar] [CrossRef]
- Pujari, B.D.; Phansopkar, M.M.; Deodhare, S.G. Squamous Cell Carcinoma of the Urachus with Vesical Calculus. Br. J. Urol. 1977, 49, 292. [Google Scholar] [CrossRef]
- Shaw, R. Squamous-Cell carcinoma in a cyst of the urachus. Br. J. Urol. 1958, 30, 87–89. [Google Scholar] [CrossRef]
- Lin, R.Y.; Rappoport, A.E.; Deppisch, L.M.; Natividad, N.S.; Katz, W. Squamous cell carcinoma of the urachus. J. Urol. 1977, 118, 1066–1067. [Google Scholar] [CrossRef]
- Fujiyama, C.; Nakashima, N.; Tokuda, Y.; Uozumi, J. Squamous cell carcinoma of the urachus. Int. J. Urol. 2007, 14, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.-C.; Lin, W.-C.; Tzen, C.-Y.; Chow, Y.-K.; Lo, K.-Y. Squamous Cell Carcinoma Of The Urachus. J. Urol. 2000, 163, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Sekita, N.; Suga, K.; Saito, H.; Kamiya, N. A case of squamous cell carcinoma in urachus. Hinyokika 2004, 58, 311–313. [Google Scholar]
- Chiarenza, S.F.; Bleve, C. Laparoscopic management of urachal cysts. Transl. Pediatr. 2016, 5, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Hamilou, Z.; North, S.; Canil, C.; Wood, L.; Hotte, S.; Sridhar, S.S.; Soulières, D.; Latour, M.; Taussky, D.; Kassouf, W.; et al. Management of urachal cancer: A consensus statement by the Canadian Urological Association and Genitourinary Medical Oncologists of Canada. Can. Urol. Assoc. J. 2019, 14, E57–E64. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.A.; Clayman, R.V.; Gonzalez, R.; Williams, R.D.; Fraley, E.E. Malignant urachal lesions. J. Urol. 1984, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, F.K.; Thomson, R.V.; Dean, A.L.J. Mucous adenocarcinoma of the urinary bladder. Cancer 1955, 8, 741–758. [Google Scholar] [CrossRef]
- Parada Villavicencio, C.; Adam, S.Z.; Nikolaidis, P.; Yaghmai, V.; Miller, F.H. Imaging of the Urachus: Anomalies, Complications, and Mimics. RadioGraphics 2016, 36, 2049–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naiditch, J.A.; Radhakrishnan, J.; Chin, A.C. Current diagnosis and management of urachal remnants. J. Pediatr. Surg. 2013, 48, 2148–2152. [Google Scholar] [CrossRef]
- Lipskar, A.M.; Glick, R.D.; Rosen, N.G.; Layliev, J.; Hong, A.R.; Dolgin, S.E.; Soffer, S.Z. Nonoperative management of symptomatic urachal anomalies. J. Pediatr. Surg. 2010, 45, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Furuta, S.; Tsuji, S.; Kawase, H.; Kitagawa, H. The current strategy for urachal remnants. Pediatr. Surg. Int. 2015, 31, 581–587. [Google Scholar] [CrossRef]
- Galati, V.; Donovan, B.; Ramji, F.; Campbell, J.; Kropp, B.P.; Frimberger, D. Management of Urachal Remnants in Early Childhood. J. Urol. 2008, 180, 1824–1827. [Google Scholar] [CrossRef]
- Minevich, E.; Wacksman, J.; Lewis, A.G.; Bukowski, T.P.; Sheldon, C.A. The infected urachal cyst: Primary excision versus a staged approach. J. Urol. 1997, 157, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Nakayama, H.; Aoki, N.; Kusafuka, T.; Takayama, T. Staged approach to the urachal cyst with infected omphalitis. Int. Surg. 2006, 91, 52–56. [Google Scholar]
- Reis, H.; Szarvas, T. Urachal cancer—Current concepts of a rare cancer. Pathologe 2019, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tazi, F.; Ahsaini, M.; Khalouk, A.; Mellas, S.; Stuurman-Wieringa, R.E.; Elfassi, M.J.; Farih, M.H. Abscess of urachal remnants presenting with acute abdomen: A case series. J. Med. Case Rep. 2012, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.E.; Tan, A.; Baker, D.A.; Sue, H.K. Urachal cyst in the adult: Ultrasound diagnosis. Am. J. Roentgenol. 1979, 132, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Sarno, R.C.; Klauber, G.; Carter, B.L. Computer Assisted Tomography of Urachal Abnormalities. J. Comput. Assist. Tomogr. 1983, 7, 674–676. [Google Scholar] [CrossRef]






| Age | Sex | Symptoms | Size (cm) | Treatment | Histology | Reference |
|---|---|---|---|---|---|---|
| Thirty-five | Male | Dysuria, perineal pain | 11.5 × 6 × 3 | Partial cystectomy * | Squamous cell carcinoma of the urachus | [25] |
| Thirty-eight | Female | Lower abdominal pain | 14 × 7 × 6.5 | Laparoscopy * | Tubo-ovarian abscess and Cystic urachal remnant | [15] |
| Forty-three | Female | Lower abdominal pain | 9.7 × 7.5 | Partial cystectomy and Tumorectomy | Urachal Mucinous Cystadenoma | Present case |
| Fifty-nine | Male | Hematuria, lower abdominal pain | 7 × 4 | Partial cystectomy * | Squamous cell carcinoma of the urachus | [26] |
| Sixty-two | Female | Suprapubic pain | 16 × 14 × 11 | Radiotherapy and Chemotherapy | Squamous cell carcinoma of the urachus | [27] |
| Sixty-four | Male | Dysuria, lower abdominal pain | 9 × 6 | Partial cystectomy * | Squamous cell carcinoma of the urachus | [28] |
| Seventy | Male | Dysuria, hematuria | 8 × 6 × 6 | Partial cystectomy * | Squamous cell carcinoma of the urachus | [29] |
| Eighty-four | Male | Dysuria, lower abdominal pain | 8 × 7 × 6 | Partial cystectomy * | Squamous cell carcinoma of the urachus | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilea, C.; Ilie, O.-D.; Stoian, I.-L.; Scripcariu, I.-S.; Doroftei, B. An Unusual Case of Urachal Cyst Misdiagnosed as a Paraovarian Cyst: Ultrasound Assessment and Differential Diagnosis. Diagnostics 2022, 12, 3166. https://doi.org/10.3390/diagnostics12123166
Ilea C, Ilie O-D, Stoian I-L, Scripcariu I-S, Doroftei B. An Unusual Case of Urachal Cyst Misdiagnosed as a Paraovarian Cyst: Ultrasound Assessment and Differential Diagnosis. Diagnostics. 2022; 12(12):3166. https://doi.org/10.3390/diagnostics12123166
Chicago/Turabian StyleIlea, Ciprian, Ovidiu-Dumitru Ilie, Irina-Liviana Stoian, Ioana-Sadyie Scripcariu, and Bogdan Doroftei. 2022. "An Unusual Case of Urachal Cyst Misdiagnosed as a Paraovarian Cyst: Ultrasound Assessment and Differential Diagnosis" Diagnostics 12, no. 12: 3166. https://doi.org/10.3390/diagnostics12123166
APA StyleIlea, C., Ilie, O.-D., Stoian, I.-L., Scripcariu, I.-S., & Doroftei, B. (2022). An Unusual Case of Urachal Cyst Misdiagnosed as a Paraovarian Cyst: Ultrasound Assessment and Differential Diagnosis. Diagnostics, 12(12), 3166. https://doi.org/10.3390/diagnostics12123166







