Serum Growth Differentiation Factor-15/Albumin Ratio as a 2-Year Survival Marker of End-Stage Renal Disease Patients Initiating Maintenance Hemodialysis
Abstract
:1. Introduction
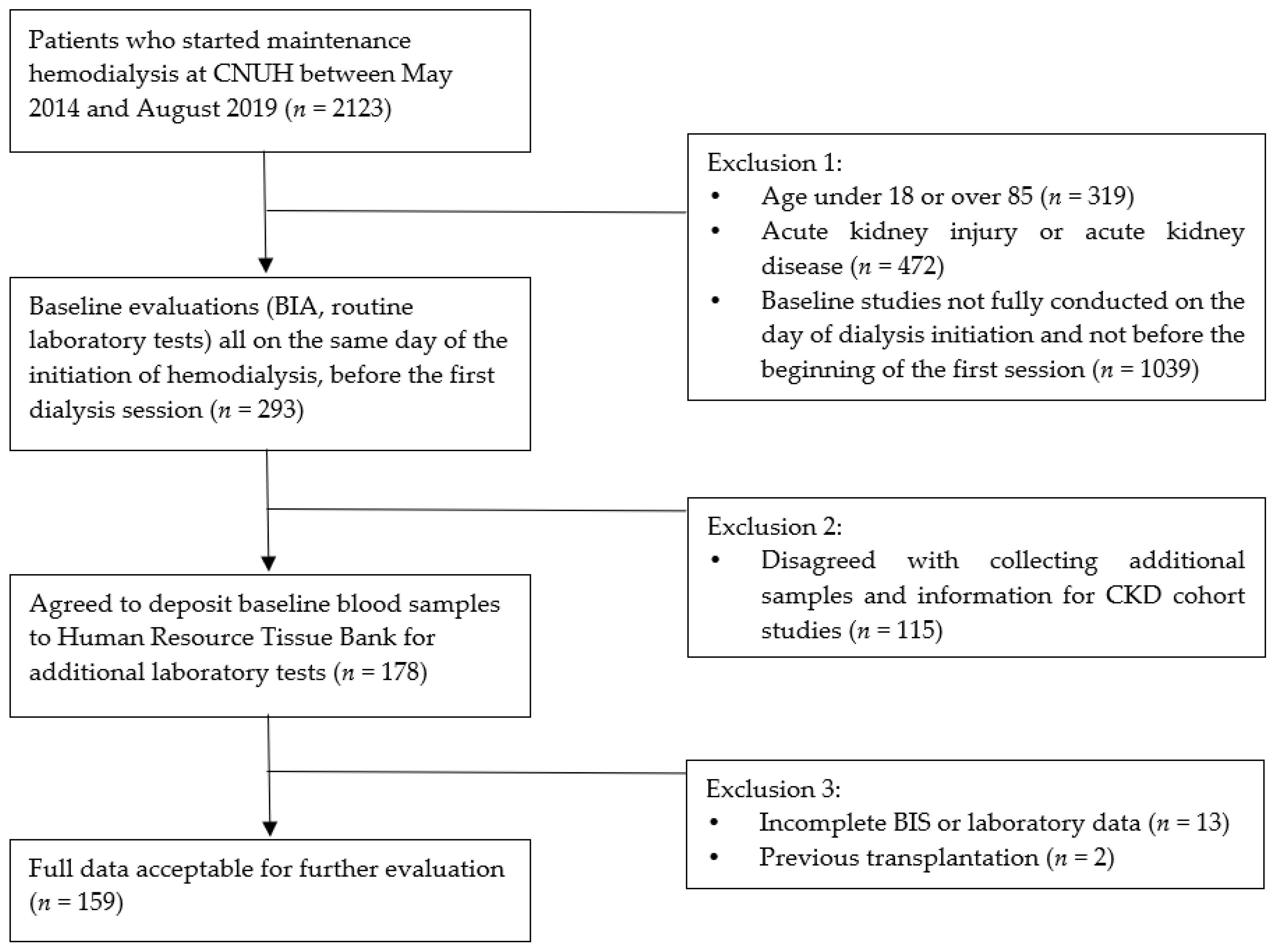
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Body Composition and Overhydration State
2.3. Assessment of Biomarker (GDF-15)
2.4. Assessment of Clinical Parameters and Comorbidities
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Association of GDF-15/Albumin with All-Cause Mortality
3.3. Association of GDF-15/Albumin with Clinical and Biochemical Variables
3.4. Increased Risk of All-Cause Mortality in Patients with Higher GDF-15/Albumin
3.5. Potential Value of GDF-15/Albumin for Prediction of All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Owolabi, M.O. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.M.; Akizawa, T.; Jager, K.J.; Kerr, P.G.; Saran, R.; Pisoni, R.L. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: Differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016, 388, 294–306. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Nordio, M.; Limido, A.; Maggiore, U.; Nichelatti, M.; Postorino, M.; Quintaliani, G. Survival in patients treated by long-term dialysis compared with the general population. Am. J. Kidney Dis. 2012, 59, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Robinson, B.M.; Zhang, J.; Morgenstern, H.; Bradbury, B.D.; Ng, L.J.; McCullough, K.P.; Gillespie, B.W.; Hakim, R.; Rayner, H.; Fort, J.; et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014, 85, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, A.; Charra, B.; Sherrard, D.J.; Scribner, B.H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N. Engl. J. Med. 1974, 290, 697–701. [Google Scholar] [CrossRef]
- de Jager, D.J.; Grootendorst, D.C.; Jager, K.J.; van Dijk, P.C.; Tomas, L.M.; Ansell, D.; Collart, F.; Finne, P.; Heaf, J.G.; De Meester, J.; et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009, 302, 1782–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldwasser, P.; Mittman, N.; Antignani, A.; Burrell, D.; Michel, M.A.; Collier, J.; Avram, M.M. Predictors of mortality in hemodialysis patients. J. Am. Soc. Nephrol. 1993, 3, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Jeon, H.J.; Kim, Y.H.; Jeon, J.; Ham, Y.R.; Chung, S.; Choi, D.E.; Na, K.R.; Lee, K.W. Overhydration measured by bioimpedance analysis and the survival of patients on maintenance hemodialysis: A single-center study. Kidney Res. Clin. Pract. 2015, 34, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Hung, S.C.; Kuo, K.L.; Peng, C.H.; Wu, C.H.; Lien, Y.C.; Wang, Y.C.; Tarng, D.C. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014, 85, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Cigarran, S.; Barril, G.; Cirugeda, A.; Bernis, C.; Aguilera, A.; Sanz, P.; Herraez, I.; Alegre, L.; Selgas, R. Hypoalbuminemia is also a marker of fluid excess determined by bioelectrical impedance parameters in dialysis patients. Ther. Apher Dial. 2007, 11, 114–120. [Google Scholar] [CrossRef]
- Korevaar, J.C.; van Manen, J.G.; Dekker, F.W.; de Waart, D.R.; Boeschoten, E.W.; Krediet, R.T. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J. Am. Soc. Nephrol. 2004, 15, 2916–2922. [Google Scholar] [CrossRef] [Green Version]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; de Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef] [Green Version]
- Tentori, F.; Blayney, M.J.; Albert, J.M.; Gillespie, B.W.; Kerr, P.G.; Bommer, J.; Young, E.W.; Akizawa, T.; Akiba, T.; Pisoni, R.L.; et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2008, 52, 519–530. [Google Scholar] [CrossRef]
- Madsen, L.H.; Ladefoged, S.; Corell, P.; Schou, M.; Hildebrandt, P.R.; Atar, D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007, 71, 548–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The TGF-β superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, F.E.; Bennet, A.M.; Magnusson, P.K.; Eriksson, U.K.; Lindmark, F.; Wu, L.; Yaghoutyfam, N.; Marquis, C.P.; Stattin, P.; Pedersen, N.L.; et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): A new marker of all-cause mortality. Aging Cell 2010, 9, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Sugulle, M.; Dechend, R.; Herse, F.; Weedon-Fekjaer, M.S.; Johnsen, G.M.; Brosnihan, K.B.; Anton, L.; Luft, F.C.; Wollert, K.C.; Kempf, T.; et al. Circulating and placental growth-differentiation factor 15 in preeclampsia and in pregnancy complicated by diabetes mellitus. Hypertension 2009, 54, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.A.; Lindmark, F.; Stattin, P.; Bälter, K.; Adami, H.O.; Zheng, S.L.; Xu, J.; Isaacs, W.B.; Grönberg, H.; Breit, S.N.; et al. Macrophage inhibitory cytokine 1: A new prognostic marker in prostate cancer. Clin. Cancer Res. 2009, 15, 6658–6664. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kimball, T.R.; Lorenz, J.N.; Brown, D.A.; Bauskin, A.R.; Klevitsky, R.; Hewett, T.E.; Breit, S.N.; Molkentin, J.D. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 2006, 98, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; de Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth Differentiation Factor-15 and Risk of CKD Progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef] [Green Version]
- Koopmann, J.; Buckhaults, P.; Brown, D.A.; Zahurak, M.L.; Sato, N.; Fukushima, N.; Sokoll, L.J.; Chan, D.W.; Yeo, C.J.; Hruban, R.H.; et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin. Cancer Res. 2004, 10, 2386–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS ONE 2015, 10, e0127518. [Google Scholar] [CrossRef]
- Wallin, U.; Glimelius, B.; Jirström, K.; Darmanis, S.; Nong, R.Y.; Pontén, F.; Johansson, C.; Påhlman, L.; Birgisson, H. Growth differentiation factor 15: A prognostic marker for recurrence in colorectal cancer. Br. J. Cancer 2011, 104, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF-15, Galectin 3, Soluble ST2, and Risk of Mortality and Cardiovascular Events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef]
- You, A.S.; Kalantar-Zadeh, K.; Lerner, L.; Nakata, T.; Lopez, N.; Lou, L.; Veliz, M.; Soohoo, M.; Jing, J.; Zaldivar, F.; et al. Association of Growth Differentiation Factor 15 with Mortality in a Prospective Hemodialysis Cohort. Cardiorenal Med. 2017, 7, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.F.; Chen, P.C.; Hsieh, C.Y.; Liou, J.C. A Growth Differentiation Factor 15-Based Risk Score Model to Predict Mortality in Hemodialysis Patients. Diagnostics 2021, 11, 286. [Google Scholar] [CrossRef]
- Yilmaz, H.; Çelik, H.T.; Gurel, O.M.; Bilgic, M.A.; Namuslu, M.; Bozkurt, H.; Ayyildiz, A.; Inan, O.; Bavbek, N.; Akcay, A. Increased serum levels of GDF-15 associated with mortality and subclinical atherosclerosis in patients on maintenance hemodialysis. Herz 2015, 40 (Suppl. 3), 305–312. [Google Scholar] [CrossRef]
- Breit, S.N.; Carrero, J.J.; Tsai, V.W.; Yagoutifam, N.; Luo, W.; Kuffner, T.; Bauskin, A.R.; Wu, L.; Jiang, L.; Barany, P.; et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol. Dial. Transplant. 2012, 27, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarkun, P.; Birtas Atesoglu, E.; Mehtap, O.; Musul, M.M.; Hacihanefioglu, A. Serum growth differentiation factor 15 levels in newly diagnosed multiple myeloma patients. Acta Haematol. 2014, 131, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chazot, C.; Wabel, P.; Chamney, P.; Moissl, U.; Wieskotten, S.; Wizemann, V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol. Dial. Transplant. 2012, 27, 2404–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wabel, P.; Moissl, U.; Chamney, P.; Jirka, T.; Machek, P.; Ponce, P.; Taborsky, P.; Tetta, C.; Velasco, N.; Vlasak, J.; et al. Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol. Dial. Transplant. 2008, 23, 2965–2971. [Google Scholar] [CrossRef]
- Ham, Y.R.; Song, C.H.; Bae, H.J.; Jeong, J.Y.; Yeo, M.K.; Choi, D.E.; Na, K.R.; Lee, K.W. Growth Differentiation Factor-15 as a Predictor of Idiopathic Membranous Nephropathy Progression: A Retrospective Study. Dis. Markers 2018, 2018, 1463940. [Google Scholar] [CrossRef] [PubMed]
- Na, K.R.; Kim, Y.H.; Chung, H.K.; Yeo, M.K.; Ham, Y.R.; Jeong, J.Y.; Kim, K.S.; Lee, K.W.; Choi, D.E. Growth differentiation factor 15 as a predictor of adverse renal outcomes in patients with immunoglobulin A nephropathy. Intern. Med. J. 2017, 47, 1393–1399. [Google Scholar] [CrossRef]
- Bao, X.; Xu, B.; Borné, Y.; Orho-Melander, M.; Melander, O.; Nilsson, J.; Christensson, A.; Engström, G. Growth differentiation factor-15 and incident chronic kidney disease: A population-based cohort study. BMC Nephrol. 2021, 22, 351. [Google Scholar] [CrossRef]
- Mazagova, M.; Buikema, H.; van Buiten, A.; Duin, M.; Goris, M.; Sandovici, M.; Henning, R.H.; Deelman, L.E. Genetic deletion of growth differentiation factor 15 augments renal damage in both type 1 and type 2 models of diabetes. Am. J. Physiol. Renal Physiol. 2013, 305, F1249–F1264. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, M.M.; Santhanakrishnan, R.; Chong, J.P.; Chen, Z.; Tai, B.C.; Liew, O.W.; Ng, T.P.; Ling, L.H.; Sim, D.; Leong, K.T.; et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail 2016, 18, 81–88. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Jena, A.; Srivatsan, V.; Muthukumar, R.; Dhandapani, V.E. GDF 15—A Novel Biomarker in the Offing for Heart Failure. Curr. Cardiol. Rev. 2016, 12, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claus, R.; Berliner, D.; Bavendiek, U.; Vodovar, N.; Lichtinghagen, R.; David, S.; Patecki, M.; Launay, J.M.; Bauersachs, J.; Haller, H.; et al. Soluble neprilysin, NT-proBNP, and growth differentiation factor-15 as biomarkers for heart failure in dialysis patients (SONGBIRD). Clin. Res. Cardiol. 2020, 109, 1035–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Streja, E.; Kovesdy, C.P.; Oreopoulos, A.; Noori, N.; Jing, J.; Nissenson, A.R.; Krishnan, M.; Kopple, J.D.; Mehrotra, R.; et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2010; Volume 85, pp. 991–1001. [Google Scholar]
- Agarwal, R. Body mass index-mortality paradox in hemodialysis: Can it be explained by blood pressure? Hypertension 2011, 58, 1014–1020. [Google Scholar] [CrossRef] [PubMed]


| Overall (n = 159) | Quartile 1 (n = 40) | Quartile 2 (n = 40) | Quartile 3 (n = 39) | Quartile 4 (n = 40) | p Value | |
|---|---|---|---|---|---|---|
| Age at hemodialysis initiation (years) | 61.78 ± 12.52 | 56.38 ± 14.34 | 61.20 ± 10.92 | 63.078 ± 12.38 | 66.50 ± 10.29 | 0.003 |
| Male (n, %) | 100 (62.9%) | 19 (47.5%) | 24 (60.0%) | 27 (69.2%) | 30 (75.0%) | 0.061 |
| Median survival period (months) | 20.03 ± 7.73 | 20.38 ± 7.77 | 22.73 ± 4.30 | 20.46 ± 6.99 | 16.58 ± 7.79 | 0.004 |
| Number of deaths (%) b | 17 (10.7%) | 2 (5.0%) | 0 (0.0%) | 3 (7.7%) | 12 (30.0%) | <0.001 |
| Etiology | ||||||
| DM (%) | 83 (52.2%) | 12 (30.0%) | 21 (52.5%) | 25 (64.1%) | 25 (62.5%) | 0.008 |
| HTN (%) | 132 (83.0%) | 34 (85.0%) | 32 (80.0%) | 31 (79.5%) | 35 (87.5%) | 0.733 |
| GN, Nephrotic syndrome (%) b | 10 (6.3%) | 4 (10.0%) | 3 (7.5%) | 1 (2.6%) | 2 (5.0%) | 0.261 |
| PCKD (%) b | 8 (5.0%) | 6 (15.0%) | 1 (2.5%) | 1 (2.6%) | 0 (0.0%) | 0.013 |
| Others (%) | 27 (17.0%) | 8 (20.0%) | 6 (15.0%) | 5 (12.8%) | 8 (20.0%) | 0.775 |
| Comorbidities (%) | ||||||
| Coronary artery disease (%) b | 18 (11.3%) | 3 (7.5%) | 5 (12.5%) | 5 (12.8%) | 5 (12.5%) | 0.867 |
| Heart failure (%) b | 12 (7.5%) | 2 (5.0%) | 4 (10.0%) | 3 (7.7%) | 3 (7.5%) | 0.920 |
| Cerebrovascular disease (%) b | 18 (11.3%) | 6 (15.0%) | 3 (7.5%) | 3 (7.7%) | 6 (15.0%) | 0.584 |
| Malignancy (%) b | 11 (6.9%) | 1 (2.5%) | 4 (10.0%) | 2 (5.1%) | 4 (10.0%) | 0.486 |
| Height (cm) | 162.31 ± 9.33 | 162.02 ± 9.47 | 161.43 ± 10.02 | 162.95 ± 8.91 | 162.86 ± 9.13 | 0.871 |
| Weight (kg) | 65.23 ± 14.29 | 64.19 ± 13.66 | 66.18 ± 14.01 | 66.56 ± 14.23 | 64.03 ± 15.53 | 0.803 |
| Systolic blood pressure (mmHg) | 147.15 ± 22.79 | 140.32 ± 16.83 | 143.82 ± 20.36 | 151.82 ± 23.59 | 152.14 ± 27.23 | 0.079 |
| Diastolic blood pressure (mmHg) a | 78.00 (18) | 80.00 (14) | 70.00 (23) | 77.50 (18) | 80.00 (64) | 0.237 |
| BMI (kg/m2) a | 24.20 (5.50) | 23.30 (5.82) | 25.10(4.85) | 25.70 (5.70) | 23.45 (4.82) | 0.306 |
| OH (L) a | 2.50 (3.9) | 1.55 (3.3) | 1.25 (1.9) | 3.50 (4.1) | 4.45 (4.2) | <0.001 |
| TBW (L) a | 35.60 (13.30) | 33.00 (11.88) | 34.70 (14.53) | 37.30 (13.60) | 37.15 (13.03) | 0.289 |
| ECW (L) a | 17.10 (6.50) | 15.70 (5.85) | 16.40 (6.65) | 18.50 (7.10) | 18.75 (7.28) | 0.025 |
| ICW (L) a | 18.40 (6.9) | 17.70 (5.8) | 17.80 (7.9) | 19.00 (5.7) | 18.75 (7.5) | 0.918 |
| E/I ratio (ECW/ICW) a | 0.94 (0.20) | 0.88 (0.178) | 0.90 (0.150) | 0.98 (0.210) | 1.03 (0.267) | <0.001 |
| OH/ECW (%) | 15.54 ± 12.49 | 10.43 ± 10.06 | 10.30 ± 9.39 | 18.21 ± 11.93 | 23.27 ± 13.42 | <0.001 |
| LTI (kg/m2) | 15.25 ± 3.38 | 15.00 ± 2.87 | 15.25 ± 3.69 | 15.46 ± 3.00 | 15.31 ± 3.99 | 0.944 |
| FTI (kg/m2) a | 7.50 (5.9) | 8.10 (6.2) | 7.95 (6.3) | 7.20 (5.5) | 6.90 (6.3) | 0.095 |
| LTM (kg) | 40.64 ± 11.50 | 39.73 ± 9.85 | 10.46 ± 12.70 | 41.61 ± 10.75 | 40.81 ± 12.76 | 0.911 |
| Fat (kg) a | 14.60 (10.6) | 14.60 (12.2) | 15.40 (11.2) | 12.60 (11.7) | 13.40 (10.2) | 0.113 |
| BCM (kg) | 23.35 ± 7.70 | 22.69 ± 6.50 | 23.24 ± 8.53 | 23.93 ± 7.16 | 23.54 ± 8.62 | 0.909 |
| Dry weight (kg) a | 61.54 (17.50) | 61.35 (19.25) | 64.30 (19.20) | 62.50 (14.80) | 57.48 (14.17) | 0.101 |
| Hemoglobin (g/dL) | 9.56 ± 1.33 | 9.84 ± 1.28 | 9.67 ± 1.22 | 9.53 ± 1.37 | 9.20 ± 1.41 | 0.163 |
| Total protein (g/dL) a | 6.30 (1.0) | 6.70 (0.7) | 6.30 (1.0) | 6.20 (1.1) | 6.20 (1.1) | <0.001 |
| Albumin (g/dL) a | 3.50 (0.7) | 3.80 (0.5) | 3.50 (0.7) | 3.50 (0.9) | 3.15 (1.0) | <0.001 |
| A/G ratio | 1.21 ± 0.29 | 1.38 ± 0.23 | 1.33 ± 0.25 | 1.16 ± 0.16 | 1.00 ± 0.29 | <0.001 |
| Total cholesterol (mg/dL) a | 148.0 (53) | 141.0 (35) | 144.5 (52) | 157.0 (64) | 158.0 (55) | 0.116 |
| Blood urea nitrogen (mg/dL) | 89.63 ± 45.57 | 102.29 ± 75.69 | 90.68 ± 31.37 | 85.94 ± 27.82 | 79.52 ± 24.76 | 0.148 |
| Creatinine (mg/dL) | 8.93 ± 3.77 | 8.82 ± 3.43 | 9.09 ± 3.31 | 9.2 ± 4.13 | 8.42 ± 4.19 | 0.687 |
| eGFR (mL/min/1.73m2) a | 6.30 (3.60) | 6.35 (3.17) | 6.05 (4.20) | 6.20 (3.70) | 6.65 (4.47) | 0.521 |
| Total calcium (mg/dL) a | 7.90 (1.3) | 8.30 (1.0) | 8.00 (1.2) | 7.70 (1.7) | 7.70 (1.2) | 0.017 |
| Corrected calcium (mg/dL) a | 8.40 (1.26) | 8.48 (1.06) | 8.27 (1.21) | 8.22 (1.66) | 8.58 (1.40) | 0.091 |
| Phosphate (mg/dL) a | 5.20 (1.92) | 5.20 (1.70) | 4.80 (2.80) | 5.60 (1.80) | 5.20 (1.88) | 0.599 |
| Potassium (mEq/L) | 5.11 ± 0.88 | 5.13 ± 0.92 | 5.19 ± 0.73 | 5.10 ± 0.82 | 5.01 ± 1.05 | 0.842 |
| CRP (mg/dL) a | 0.20 (0.70) | 0.10 (0.40) | 0.20 (0.60) | 0.20 (0.70) | 0.60 (2.3) | 0.006 |
| HbA1c (%) a | 5.90 (1) | 5.30 (1) | 5.60 (2) | 6.00 (2) | 6.30 (2) | 0.014 |
| Ferritin (ng/mL) a | 235.00 (272) | 214.00 (182) | 251.00 (290) | 251.00 (342) | 208.00 (274) | 0.134 |
| Total CO2 (mEq/L) | 18.91 ± 4.39 | 18.67 ± 4.52 | 18.35 ± 4.87 | 18.54 ± 4.24 | 19.98 ± 3.90 | 0.354 |
| PTH (pg/mL) a | 223.27 (218) | 257.79 (277) | 265.22 (307) | 200.80 (155) | 179.78 (241) | 0.015 |
| GDF-15/Albumin (ng/g) a | 156.71 (119.41) | 89.23 (24.72) | 133.09 (19.67) | 187.01 (30.32) | 318.02 (180.42) | <0.001 |
| GDF-15 (ng/mL) a | 5.22 (3.034) | 3.44 (1.172) | 4.86 (0.632) | 5.89 (1.654) | 8.62 (4.668) | <0.001 |
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Age | 2.527 (0.744, 4.310) | 0.006 | 2.732 (1.038, 4.427) | 0.002 | 2.537 (0.726, 4.349) | 0.006 |
| OH/ECW (%) | 3.541 (1.796, 5.286) | <0.001 | 3.694 (1.994, 5.393) | <0.001 | 3.023 (1.165, 4.881) | 0.002 |
| BMI | −1.262 (−6.954, 4.430) | 0.662 | ||||
| LTI | −3.462 (−10.207, 3.283) | 0.312 | ||||
| FTI | −1.254 (−6.549, 4.041) | 0.640 | ||||
| Total cholesterol | 0.601 (0.071, 1.131) | 0.026 | 0.252 (−0.276, 0.780) | 0.347 | ||
| Creatinine | −5.528 (−11.540, 0.484) | 0.071 | ||||
| eGFR | 3.777 (0.280, 7.273) | 0.034 | 1.755 (−1.806, 5.315) | 0.332 | ||
| CRP | 6.319 (−0.762, 13.400) | 0.080 | ||||
| HbA1c | 2.443 (−3.015, 7.900) | 0.377 | ||||
| Variables | Reference (GDF-15/Albumin Quartile 1, 2, 3) | GDF-15/Albumin Quartile 4 (>75 Percentile) | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Model 1 | 1 | N/A | 8.468 (2.981, 24.054) | <0.001 |
| Model 2 | 1 | N/A | 5.507 (1.774, 17.096) | 0.003 |
| Model 3 | 1 | N/A | 5.510 (1.774, 17.144) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-J.; Hwang, H.-B.; Han, S.-H.; Ham, Y.-R.; Shin, J.-A.; Lee, K.-W.; Na, K.-R.; Choi, D.-E. Serum Growth Differentiation Factor-15/Albumin Ratio as a 2-Year Survival Marker of End-Stage Renal Disease Patients Initiating Maintenance Hemodialysis. Diagnostics 2022, 12, 257. https://doi.org/10.3390/diagnostics12020257
Lee E-J, Hwang H-B, Han S-H, Ham Y-R, Shin J-A, Lee K-W, Na K-R, Choi D-E. Serum Growth Differentiation Factor-15/Albumin Ratio as a 2-Year Survival Marker of End-Stage Renal Disease Patients Initiating Maintenance Hemodialysis. Diagnostics. 2022; 12(2):257. https://doi.org/10.3390/diagnostics12020257
Chicago/Turabian StyleLee, Eu-Jin, Haet-Bit Hwang, Soo-Hyun Han, Young-Rok Ham, Jin-Ah Shin, Kang-Wook Lee, Ki-Ryang Na, and Dae-Eun Choi. 2022. "Serum Growth Differentiation Factor-15/Albumin Ratio as a 2-Year Survival Marker of End-Stage Renal Disease Patients Initiating Maintenance Hemodialysis" Diagnostics 12, no. 2: 257. https://doi.org/10.3390/diagnostics12020257
APA StyleLee, E.-J., Hwang, H.-B., Han, S.-H., Ham, Y.-R., Shin, J.-A., Lee, K.-W., Na, K.-R., & Choi, D.-E. (2022). Serum Growth Differentiation Factor-15/Albumin Ratio as a 2-Year Survival Marker of End-Stage Renal Disease Patients Initiating Maintenance Hemodialysis. Diagnostics, 12(2), 257. https://doi.org/10.3390/diagnostics12020257






