Multiplex Immunofluorescence Assay of Infiltrating Mononu-Clear Cell Subsets in Acute T-Cell-Mediated Rejection and BK Virus-Associated Nephropathy in the Allograft Kidney
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection Criteria and Tissue Pathology Grading
2.3. Multiplex Immunofluorescence (IF) Assay
2.4. Image Acquisition and Quantitative Data Analysis
2.5. Region of Interest (ROI)
2.6. Statistical Analysis
3. Results
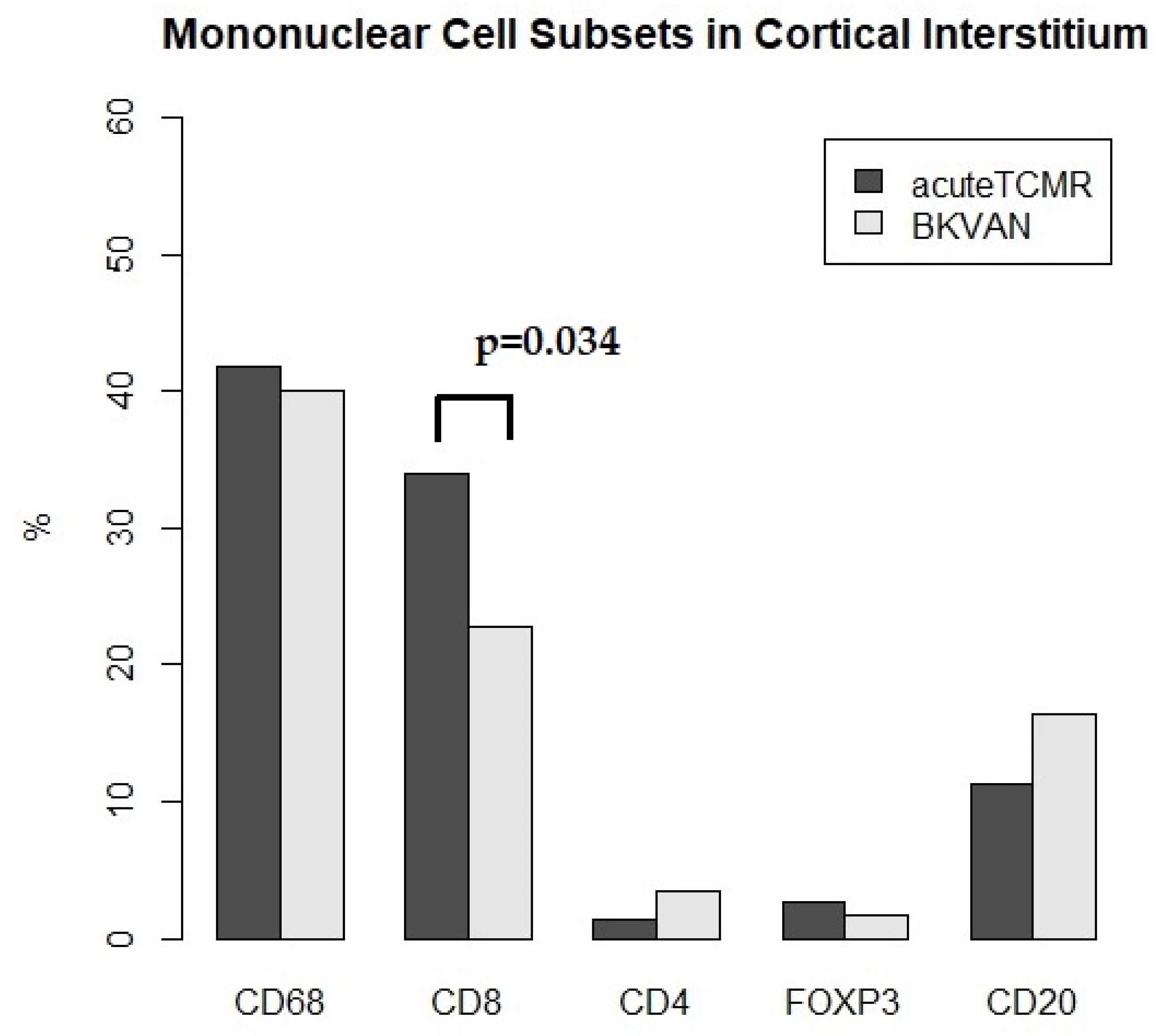
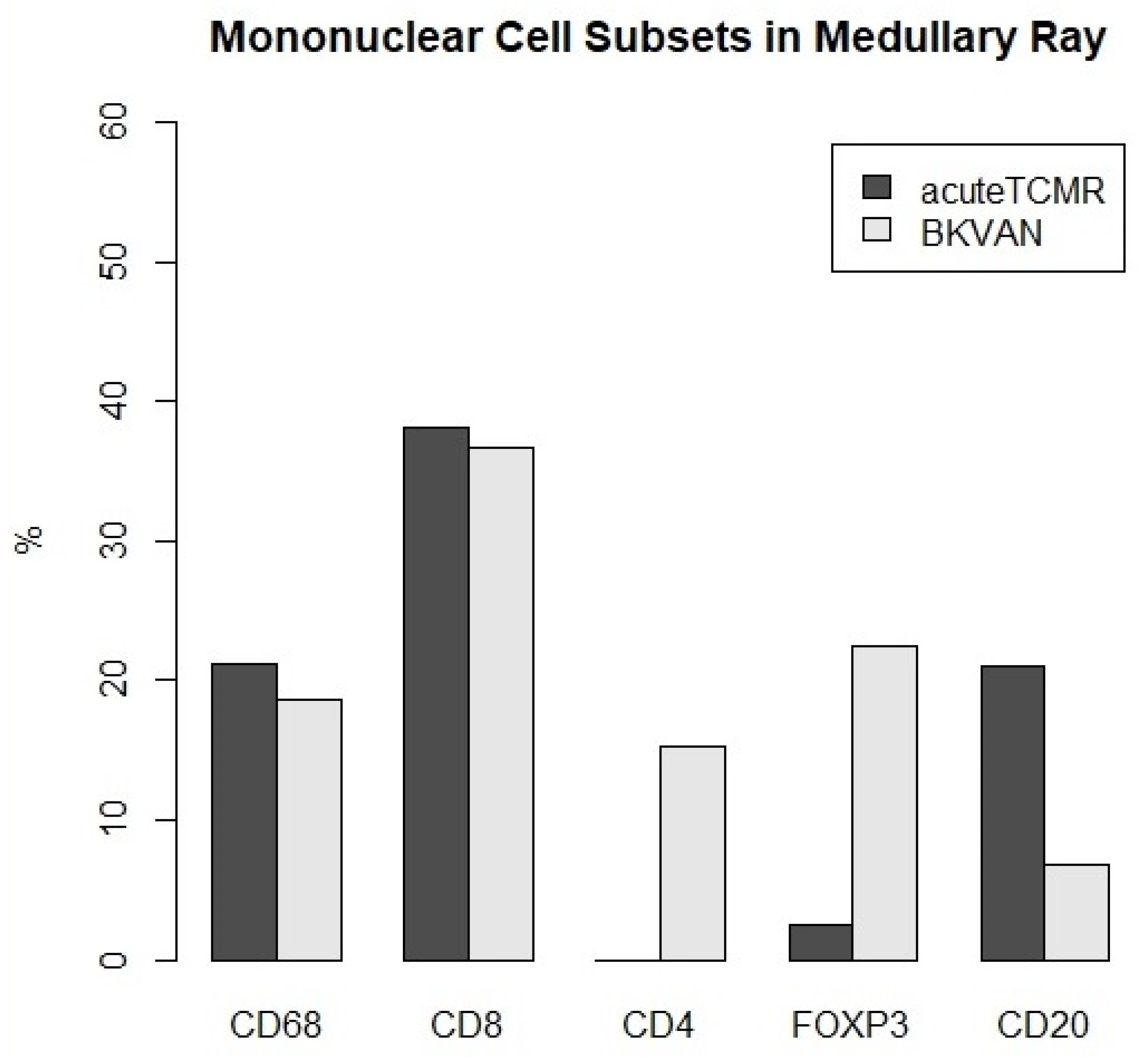
3.1. The Proportions of Mononuclear Cell Subsets in Acute TCMR Cases in Relation to Different Regions of Interest (ROIs)
3.2. The Proportions of Mononuclear Cell Subsets in BKVAN Cases in Relation to Different Regions of Interest (ROIs)
3.3. The Proportions of Mononuclear Cell Subsets in Acute TCMR versus BKVAN cases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Racusen, L.C.; Solez, K. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, H.H.; Brennan, D.C. Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 2005, 79, 1277–1286. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Platt, J.L.; LeBien, T.W. Interstitial mononuclear cell populations in renal graft rejection. Identification by monoclonal antibodies in tissue sections. J. Exp. Med. 1982, 155, 17–30. [Google Scholar] [CrossRef]
- Kolbeck, P.C.; Tatum, A.H. Relationships among the histologic pattern, intensity, and phenotypes of T cells infiltrating renal allografts. Transplantation 1984, 38, 709–713. [Google Scholar] [CrossRef]
- Sako, H.; Nakane, Y. Immunohistochemical study of the cells infiltrating human renal allografts by the ABC and the IGSS method using monoclonal antibodies. Transplantation 1987, 44, 43–50. [Google Scholar]
- Waltzer, W.C.; Miller, F. Immunohistologic analysis of human renal allograft dysfunction. Transplantation 1987, 43, 100–105. [Google Scholar] [CrossRef]
- Hofman, P.; Badoual, C. Multiplexed Immunohistochemistry for Molecular and Immune Profiling in Lung Cancer—Just About Ready for Prime-Time? Cancers 2019, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Mani, N.L.; Schalper, K.A. Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer. Breast Cancer Res. 2016, 18, 78. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.; Loupy, A. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Nickeleit, V.; Singh, H.K. The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: Morphologic Definitions and Clinical Correlations. J. Am. Soc. Nephrol. 2018, 29, 680–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponticelli, C. The mechanisms of acute transplant rejection revisited. J. Nephrol. 2012, 25, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Whitelegg, A.; Barber, L.D. The structural basis of T-cell allorecognition. Tissue Antigens 2004, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef] [Green Version]
- von Willebrand, E.; Häyry, P. Composition and in vitro cytotoxicity of cellular infiltrates in rejecting human kidney allografts. Cell Immunol. 1978, 41, 358–372. [Google Scholar] [CrossRef]
- Dy, M.; Debray-Sachs, M. Role of macrophages in allograft rejection. Transplant. Proc. 1979, 11, 811–815. [Google Scholar] [PubMed]
- Hancock, W.W.; Thomson, N.M. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation 1983, 35, 458–463. [Google Scholar] [CrossRef]
- Girlanda, R.; Kleiner, D.E. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am. J. Transplant. 2008, 8, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Magil, A.B. Monocytes/macrophages in renal allograft rejection. Transplant. Rev. 2009, 23, 199–208. [Google Scholar] [CrossRef]
- Sablik, K.A.; Jordanova, E.S. Immune Cell Infiltrate in Chronic-Active Antibody-Mediated Rejection. Front. Immunol. 2020, 10, 3106. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, C. The Evolving Roles of Macrophages in Organ Transplantation. J. Immunol. Res. 2019, 2019, 5763430. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, B.J.; Frye, C. Role of donor macrophages after heart and lung transplantation. Am. J. Transplant. 2020, 20, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Sis, B.; Sarioglu, S. Renal medullary changes in renal allograft recipients with raised serum creatinine. J. Clin. Pathol. 2006, 59, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsib, S.M.; Reznicek, M.J. Renal medulla in the diagnosis of acute cellular rejection. Transplantation 1989, 48, 690–692. [Google Scholar] [PubMed]
- Wang, H.; Nanra, R.S. The renal medulla in acute renal allograft rejection: Comparison with renal cortex. Nephrol. Dial. Transplant. 1995, 10, 1428–1431. [Google Scholar]
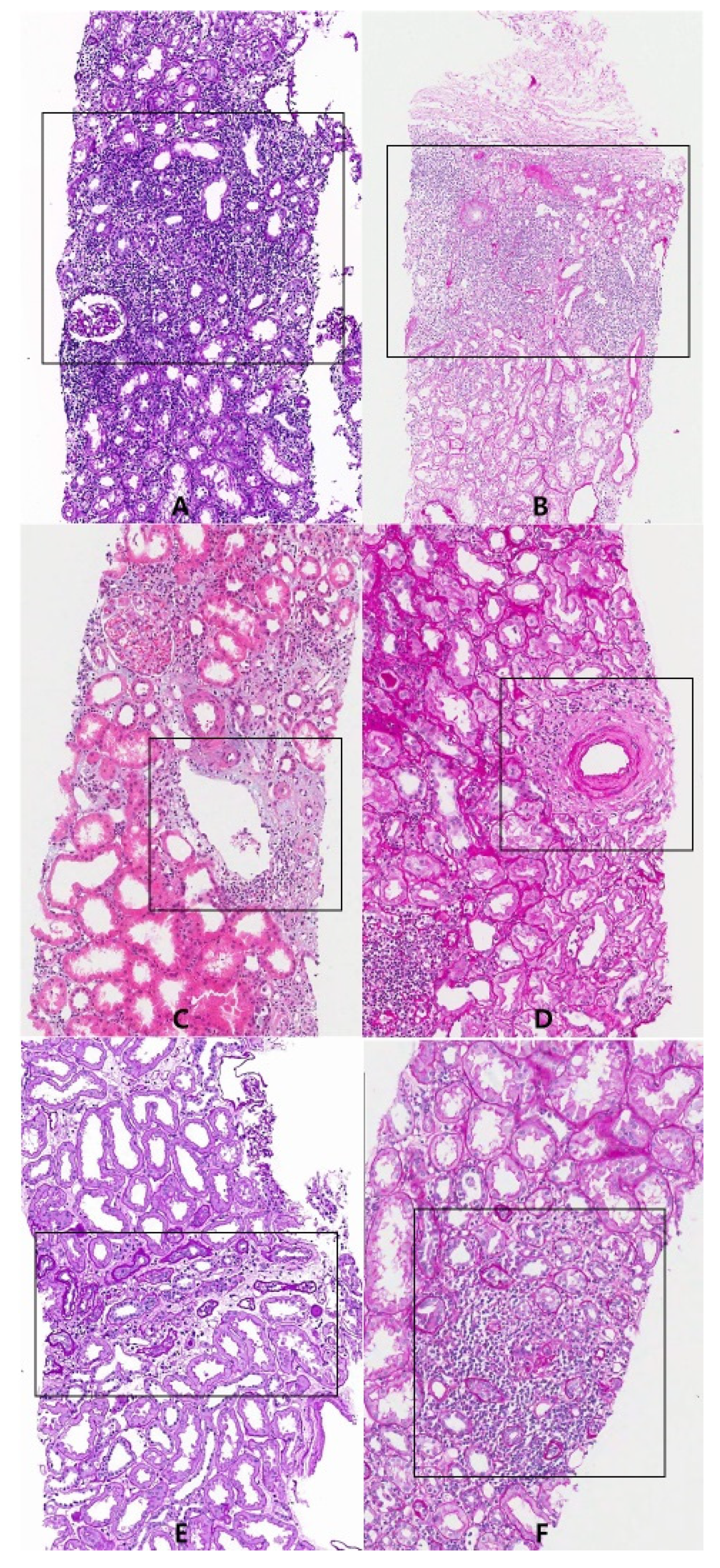
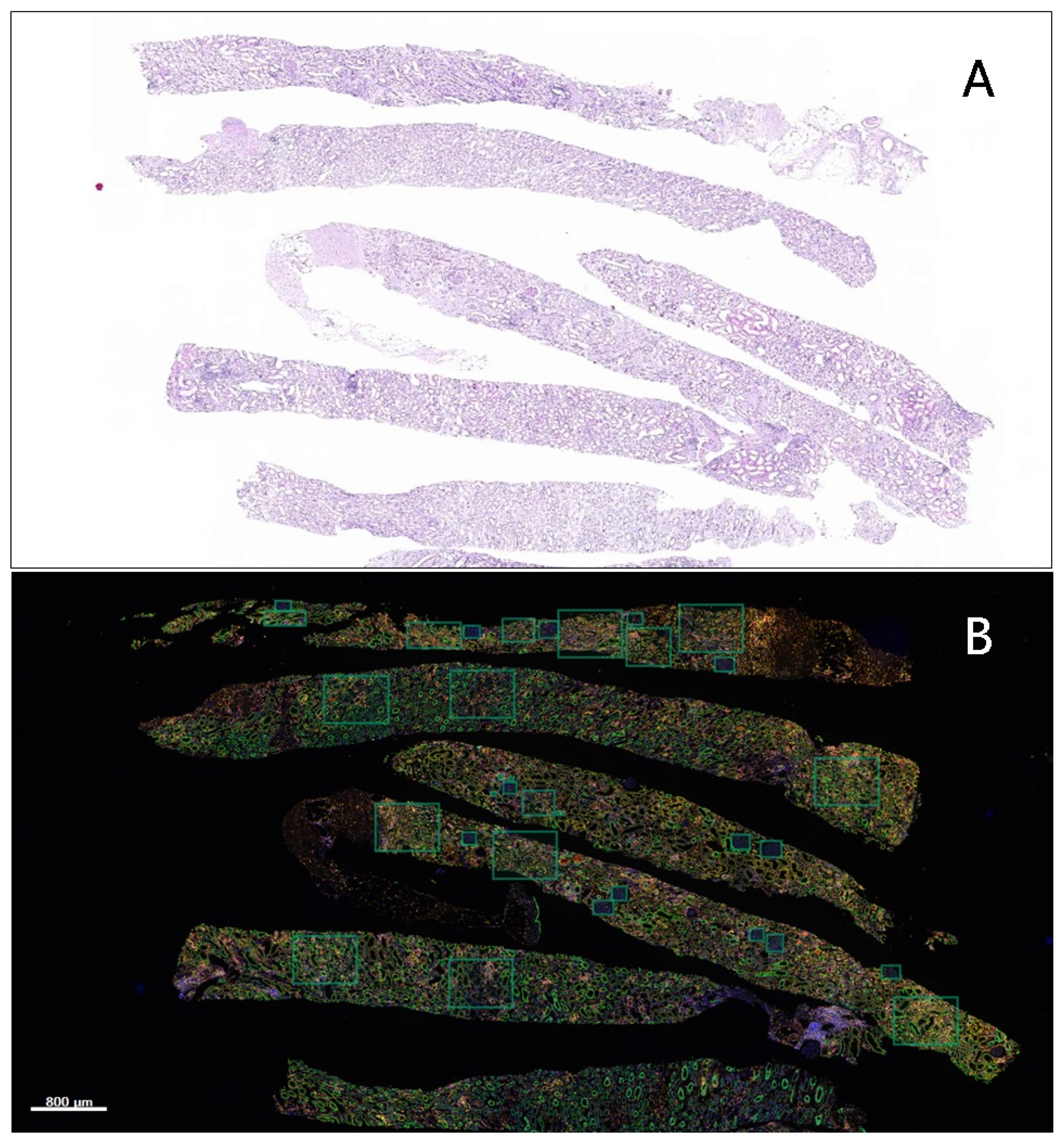



| Case | Age | Sex | Graft Age | Creatinine | Banff Score | Grade |
|---|---|---|---|---|---|---|
| Months | mg/dL | |||||
| 1 | 42 | F | 3 | 1.8 | i2, t3, ptc1, ti2 | 1A |
| 2 | 33 | M | 15 | 4.3 | i2, t3, ptc1, ci2, ct2 | 1B |
| 3 | 40 | M | 9 | 4.4 | i3, t3, v2 | 2B |
| 4 | 33 | M | 8 | 2.7 | i2, t2, ptc1 | 1A |
| 5 | 55 | M | 1 | 1.2 | i2, t2, v2, ptc1 | 2B |
| 6 | 56 | F | 10 | 2.4 | i2, t3, cv1 | 1B |
| 7 | 58 | M | 4 | 2.8 | i3, t2, ptc1, ti3 | 1A |
| 8 | 62 | M | 8 | 2.4 | t1, v2, ptc1, ci1, ct1, ti2, i-IFTA3 | 2B |
| 9 | 42 | F | 7 | 4.5 | i3, t3, g1, ci2, ct2, cv1, ah1, ti3 | 1B |
| Case | Age | Sex | Graft Age | Creatinine | Banff Score | Stage |
|---|---|---|---|---|---|---|
| Months | mg/dL | |||||
| 1 | 62 | M | 5.5 | 2.2 | ci1, ct1, cv1, i-IFTA1 | B |
| 2 | 58 | M | 2.5 | 1.8 | i3, t1, ptc1, ti3 | B |
| 3 | 30 | M | 7 | 1.8 | ci1, ct1, ti3 | A |
| 4 | 25 | M | 4 | 3.3 | I2,ci1,ct1,ti3 | B |
| 5 | 62 | M | 6.5 | 3.1 | i2, ptc1, ci2, ct3, ti3, i-IFTA3 | C |
| Acute TCMR | Acute TCMR | Acute TCMR | Acute TCMR | BKVAN | BKVAN | BKVAN | BKVAN | |
|---|---|---|---|---|---|---|---|---|
| Cortical Interstitium | Medullary Ray | Medulla | Area Surrounding Large Vessel | Cortical Interstitium | Medullary Ray | Medulla | Area Surrounding Large Vessel | |
| (n = 16) | (n = 8) | (n = 10) | (n = 13) | (n = 12) | (n = 2) | (n = 3) | (n = 3) | |
| Count | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) |
| CD68 | 345.5 | 284 | 365.5 | 283 | 552 | 275 | 913 | 335 |
| (111.0–868.8) | (58.5–404.5) | (125.5–1242.0) | (102.0–501.0) | (82.0–955.0) | (151.5–398.5) | (776.5–1538.0) | (223.5–695.5) | |
| CD8 | 316.5 | 252 | 410 | 322 | 321.5 | 486 | 552 | 1522 |
| (80.5–600.3) | (69.5–496.5) | (195.0–1919.0) | (132.0–691.5) | (42.5–1041.0) | (329–643) | (468.5–1990.5) | (762–1604.5) | |
| CD4 | 8 | 0 | 43 | 2 | 61 | 126 | 24 | 226 |
| (1.0–30.8) | (0–160.3) | (5.0–147.3) | (0–51.5) | (2.3–697.5) | (69.5–182.5) | (12–240) | (113.5–504.5) | |
| FOXP3 | 39.5 | 32.5 | 27.5 | 49 | 21 | 190.5 | 68 | 197 |
| (4.8–69.8) | (11.0–55.3) | (7.5–285.8) | (19.5–109.5) | (3.5–623.3) | (117.75–263.25) | (43–175) | (99–1860) | |
| CD20 | 137 | 370 | 209.5 | 210 | 329.5 | 93 | 1423 | 900 |
| (27.5–476.0) | (11.5–488.0) | (26.8–423.8) | (69.5–872.0) | (39.5–689.8) | (59.5–126.5) | (1391.5–1430.5) | (501–1524) | |
| Total | 902.5 | 1172 | 1127 | 1111 | 1753 | 1170.5 | 2739 | 4066 |
| (276.8–2117.5) | (179.0–1906.3) | (304.3–4583.8) | (374.5–2171.0) | (205.8–4286.5) | (985.75–1355.25) | (2730.5–5214.5) | (2142–6188.5) | |
| Ratio | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) |
| CD68 (%) | 41.8 | 21.2 | 36.8 | 26.9 | 40.1 | 18.7 | 28.1 | 26 |
| (31.7–46.9) | (19.3–30.9) | (21.0–42.8) | (18.1–34.2) | (23.4–48.7) | (11.1–26.3) | (25.8–30.7) | (15–38.7) | |
| CD8 (%) | 34 | 38.2 | 41.2 | 31.6 | 22.8 | 36.7 | 20.3 | 18.3 |
| (24.7–45.1) | (18.3–41.8) | (30.3–46.2) | (18.5–39.2) | (13.6–30.3) | (29.1–44.3) | (17.2–32.4) | (9.6–29.9) | |
| CD4 (%) | 1.3 | 0 | 1.7 | 0.4 | 3.4 | 15.3 | 0.9 | 5.6 |
| (0.2–6.6) | (0–14.5) | (0.7–21.7) | (0–1.8) | (0.7–32.3) | (8.1–22.6) | (0.4–3.4) | (3–7.5) | |
| FOXP3 (%) | 2.7 | 2.5 | 4 | 5.7 | 1.7 | 22.4 | 2.5 | 4.8 |
| (1.5–6.8) | (1.3–20.1) | (1.1–6.1) | (3.4–15.2) | (1.2–15.7) | (12.7–32.2) | (1.6–3.1) | (2.7–23.6) | |
| CD20 (%) | 11.3 | 21 | 11.3 | 32.6 | 16.4 | 6.8 | 51.9 | 25.8 |
| (3.7–23.1) | (8.5–34.3) | (5.2–16.8) | (18.8–41.8) | (7.2–25.3) | (5–8.6) | (34.8–52.4) | (24–36.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Lim, J.-H.; Han, M.-H.; Kim, S.-Y.; Kim, Y.J.; Kim, Y.-J. Multiplex Immunofluorescence Assay of Infiltrating Mononu-Clear Cell Subsets in Acute T-Cell-Mediated Rejection and BK Virus-Associated Nephropathy in the Allograft Kidney. Diagnostics 2022, 12, 268. https://doi.org/10.3390/diagnostics12020268
Kim M-S, Lim J-H, Han M-H, Kim S-Y, Kim YJ, Kim Y-J. Multiplex Immunofluorescence Assay of Infiltrating Mononu-Clear Cell Subsets in Acute T-Cell-Mediated Rejection and BK Virus-Associated Nephropathy in the Allograft Kidney. Diagnostics. 2022; 12(2):268. https://doi.org/10.3390/diagnostics12020268
Chicago/Turabian StyleKim, Mee-Seon, Jeong-Hoon Lim, Man-Hoon Han, Sang-Yeob Kim, Yun Jae Kim, and Yong-Jin Kim. 2022. "Multiplex Immunofluorescence Assay of Infiltrating Mononu-Clear Cell Subsets in Acute T-Cell-Mediated Rejection and BK Virus-Associated Nephropathy in the Allograft Kidney" Diagnostics 12, no. 2: 268. https://doi.org/10.3390/diagnostics12020268
APA StyleKim, M.-S., Lim, J.-H., Han, M.-H., Kim, S.-Y., Kim, Y. J., & Kim, Y.-J. (2022). Multiplex Immunofluorescence Assay of Infiltrating Mononu-Clear Cell Subsets in Acute T-Cell-Mediated Rejection and BK Virus-Associated Nephropathy in the Allograft Kidney. Diagnostics, 12(2), 268. https://doi.org/10.3390/diagnostics12020268







