A Challenging Diagnosis: Placental Mesenchymal Dysplasia—Literature Review and Case Report
Abstract
:1. Introduction
2. Materials and Method
3. Case Report
4. Results
5. Discussions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guenot, C.; Kingdom, J.; De Rham, M.; Osterheld, M.; Keating, S.; Vial, Y.; Van Mieghem, T.; Jastrow, N.; Raio, L.; Spinelli, M.; et al. Placental mesenchymal dysplasia: An underdiagnosed placental pathology with various clinical outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, G.; Jauniaux, E.; Hustin, J. Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia. A new clinico-pathological entity? Pathol. Res. Pract. 1991, 187, 324–328. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Tang, X.; Yang, F.; Yang, K.-X. Placental mesenchymal dysplasia: A case of a normal-appearing fetus with intrauterine growth restriction. Int. J. Clin. Exp. Pathol. 2014, 7, 5302–5307. [Google Scholar] [PubMed]
- Cohen, M.C.; Roper, E.C.; Sebire, N.; Stanek, J.; Anumba, D.O.C. Placental mesenchymal dysplasia associated with fetal aneuploidy. Prenat. Diagn. 2005, 25, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, J.; Jiang, Z.; Zhang, L.; Ding, S.; Liu, X. Placental mesenchymal dysplasia in a normal female infant: A rare case report with follow-up. Int. J. Clin. Exp. Pathol. 2020, 13, 896–900. [Google Scholar] [PubMed]
- Toru, H.S.; Aytekin, E.C.; Sanhal, C.Y.; Yakut, S.; Cetin, Z.; Mendilcioglu, I.I.; Pestereli, H.E. We can diagnose it if we consider it. diagnostic pitfall for placenta: Placental mesenchymal dysplasia. Turk. J. Pathol. 2018, 34, 100–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavanier, D.; Allias, F.; Huissoud, C.; Hajri, T.; Golfer, F.; Massardier, J. Ultrasound findings and clinical outcomes in 23 cases of placental mesenchymal dysplasia. J. Repr. Med. 2017, 62, 366–375. [Google Scholar]
- Rosner-Tenerowicz, A.; Pomorski, M.; Fuchs, T.; Sliwa, J.; Zimmer-Stelmach, A.; Bek, W.; Zimmer, M. Placental mesenchymal dysplasia and hepatic cyst. Ginekol. Polska 2020, 91, 779–780. [Google Scholar] [CrossRef]
- Ishikawa, S.; Morikawa, M.; Umazume, T.; Yamada, T.; Kanno, H.; Takakuwa, E.; Minakami, H. Anemia in a neonate with placental mesenchymal dysplasia. Clin. Case Rep. 2016, 4, 463–465. [Google Scholar] [CrossRef]
- Pham, T.; Stayboldt, C.; Steele, J.; Chan, L.; Benirschke, K. Placental Mesenchymal Dysplasia Is Associated With High Rates of Intrauterine Growth Restriction and Fetal Demise: A Report of 11 New Cases and a Review of the Literature. Am. J. Clin. Pathol. 2006, 126, 67–78. [Google Scholar] [CrossRef]
- Arizawa, M.; Nakayama, M. Suspected involvement of the X chromosome in placental mesenchymal dysplasia. Congenit. Anom. 2002, 42, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; Malloy, J. Cystic placentomegaly on a second-trimester ultrasound. J. Am. Acad. Physician Assist. 2018, 31, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Himoto, Y.; Kido, A.; Minamiguchi, S.; Moribata, Y.; Okumura, R.; Mogami, H.; Nagano, T.; Konishi, I.; Togashi, K. Prenatal differential diagnosis of complete hydatidiform mole with a twin live fetus and placental mesenchymal dysplasia by magnetic resonance imaging. J. Obstet. Gynaecol. Res. 2014, 40, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.F.; Sampson, A. Placental mesenchymal dysplasia: A report of four cases with differentiation from partial hydatidiform mole. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Woo, G.W.; Rocha, F.G.; Gaspar-Oishi, M.; Bartholomew, M.L.; Thompson, K.S. Placental mesenchymal dysplasia. Am. J. Obstet. Gynecol. 2011, 205, e3–e5. [Google Scholar] [CrossRef]
- Gheysen, W.; Strybol, D.; Moerman, P.; Steylemans, A.; Corveleyn, A.; De Catte, L.; Couck, I.; Lewi, L. Discordance for placental mesenchymal dysplasia in a monochorionic diamniotic twin pregnancy: A case report. Clin. Case Rep. 2018, 6, 1557–1560. [Google Scholar] [CrossRef] [Green Version]
- Jitsumori, S.; Shiro, M.; Kojima, F.; Ota, N.; Minami, S.; Ino, K. Placental mesenchymal dysplasia with severe fetal growth restriction in one placenta of a dichorionic diamniotic twin pregnancy. J. Obstet. Gynaecol. Res. 2018, 44, 951–954. [Google Scholar] [CrossRef]
- Sander, C.M. Angiomatous Malformation of Placental Chronic Stem Vessels and Pseudo-Partial Molar Placentas: Report of Five Cases. Fetal Pediatr. Pathol. 1993, 13, 621–633. [Google Scholar] [CrossRef]
- Matsui, H.; Iitsuka, Y.; Yamazawa, K.; Tanaka, N.; Mitsuhashi, A.; Seki, K.; Sekiya, S. Placental mesenchymal dysplasia initially diagnosed as partial mole. Pathol. Int. 2003, 53, 810–813. [Google Scholar] [CrossRef]
- Højberg, K.-E.; Aagaard, J.; Henriques, U.; Sunde, L. Placental Vascular Malformation with Mesenchymal Hyperplasia and a Localized Chorioangioma: A Rarity Simulating Partial Mole. Pathol-Res. Pr. 1994, 190, 808–813. [Google Scholar] [CrossRef]
- Ohira, S.; Ookubo, N.; Tanaka, K.; Takatsu, A.; Kobara, H.; Kikuchi, N.; Ohya, A.; Kanai, M.; Shiozawa, T. Placental mesenchymal dysplasia: Chronological observation of placental images dur-inggestation and review of the literature. Gynecol. Obstet. Invest. 2013, 75, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-P.; Chern, S.-R.; Wang, T.-Y.; Huang, Z.-D.; Huang, M.-C.; Chuang, C.-Y. Pregnancy with concomitant chorangioma and placental vascular malformation with mesenchymal hyperplasia. Hum. Reprod. 1997, 12, 2553–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauniaux, E.; Nicolaides, K.; Hustin, J. Perinatal features associated with placental mesenchymal dysplasia. Placenta 1997, 18, 701–706. [Google Scholar] [CrossRef]
- Huang, T.-C.; Chang, K.-C.; Chang, J.-Y.; Tsai, Y.-S.; Yang, Y.-J.; Chang, W.-C.; Mo, C.-F.; Yu, P.-H.; Chiang, C.-T.; Lin, S.-P.; et al. Variants in Maternal Effect Genes and Relaxed Imprinting Control in a Special Placental Mesenchymal Dysplasia Case with Mild Trophoblast Hyperplasia. Biomedicine 2021, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Heazell, A.; Sahasrabudhe, N.; Grossmith, A.; Martindale, E.; Bhatia, K. A Case of Intrauterine Growth Restriction in Association with Placental Mesenchymal Dysplasia with Abnormal Placental Lymphatic Development. Placenta 2009, 30, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.K.; Chi, M.J.G.; Cha, M.K.S. An Unusual Venous Anomaly of the Placenta. Am. J. Clin. Pathol. 1991, 95, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kaiser-Rogers, K..; McFadden, D.E.; Livasy, C..; Dansereau, J.; Jiang, R.; Knops, J.F.; Lefebvre, L.; Rao, K.W.; Robinson, W.P. Androgenetic/biparental mosaicism causes placental mesenchymal dysplasia. J. Med. Genet. 2006, 43, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Psarris, A.; Sindos, M.; Kourtis, P.; Pampanos, A.; Antsaklis, P.; Theodora, M.; Chondrogianni, M.E.; Morphopoulos, G.; Loutradis, D.; Daskalakis, G. Placental Mesenchymal Dysplasia: Ultrasound Characteristics and Diagnostic Pitfalls. Ultrasound Int. Open 2020, 6, E2–E3. [Google Scholar] [CrossRef]
- Pal, S.; Bose, K.; Ch Mondal, P.; Chakrabarti, S.; Sikder, M. Placental Mesenchymal Dysplasia With Normal Fetus: A Rare Case Report. Iran J. Pathol. 2017, 12, 307–310. [Google Scholar] [CrossRef]
- Toscano, M.P.; Schultz, R. Placental mesenchymal dysplasia: Case report with gross and histological findings. Autops. Case Rep. 2014, 4, 51–56. [Google Scholar] [CrossRef]
- Gizzo, S.; Di Gangi, S.; Patrelli, T.S.; Saccardi, C.; D’antona, D.; Nardelli, G.B. Placental mesenchymal dysplasia: Can early diagnosis ensure a good materno-foetal outcome? A case report. Arch. Gynecol. Obstet. 2012, 286, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Srinish, M.; Balan, P.; Sadasivan, S.; Nair, K.B. Mesenchymal dysplasia of placenta. Indian J. Pathol. Microbiol. 2015, 58, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Taga, S.; Haraga, J.; Sawada, M.; Nagai, A.; Yamamoto, D.; Hayase, R. A Case of Placental Mesenchymal Dysplasia. Case Rep. Obstet. Gynecol. 2013, 2013, 265159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qichang, W.; Wenbo, W.; Liangkai, Z.; Hui, K.; Xiaoqin, H.; Li, S.; Yasong, X. Pregnancy with Concomitant Chorioangioma and Placental Mesenchymal Dysplasia: A Rare Placental Abnormality. Case Rep. Obstet. Gynecol. 2013, 2013, 591956. [Google Scholar] [CrossRef] [Green Version]
- Koga, H.; Makimura, M.; Tanaka, H.; Sumioki, H. Placental Mesenchymal Dysplasia and Fetal Hematologic Disorder. J. Pediatr. Hematol. 2014, 36, e389–e391. [Google Scholar] [CrossRef]
- Gibson, B.; Muir-Padilla, J.; Champeaux, A.; Suarez, E. Mesenchymal dysplasia of the placenta. Placenta 2004, 25, 671–672. [Google Scholar] [CrossRef]
- Kinoshita, T.; Fukaya, S.; Yasuda, Y.; Itoh, M. Placental mesenchymal dysplasia. J. Obstet. Gynaecol. Res. 2007, 33, 83–86. [Google Scholar] [CrossRef]
- Gurram, D.; Joung, S.J.S.; Ryder, L.; Nayyar, R. Late diagnosis of hepatic mesenchymal hamartoma and placental mesenchymal dysplasia. Australas. J. Ultrasound Med. 2016, 19, 123–125. [Google Scholar] [CrossRef]
- Mulch, A.D.; Stallings, S.P.; Salafia, C.M. Elevated maternal serum alpha-fetoprotein, umbilical vein varix, and mesenchymal dysplasia: Are they related? Prenat. Diagn. 2006, 26, 659–661. [Google Scholar] [CrossRef]
- Surti, U.; Hill, L.M.; Dunn, J.; Prosen, T.; Hoffner, L. Twin pregnancy with a chimeric androgenetic and biparental placenta in one twin displaying placental mesenchymal dysplasia phenotype. Prenat. Diagn. 2005, 25, 1048–1056. [Google Scholar] [CrossRef]
- Rohilla, M.; Siwatch, S.; Jain, V.; Nijhawan, R. Placentomegaly and placental mesenchymal dysplasia. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, L.; Rabban, J.T.; Poder, L.; Chetty, S.; Ueda, S.; Chen, L.-M. Differentiating complete hydatidiform mole and coexistent fetus and placental mesenchymal dysplasia: A series of 9 cases and review of the literature. Gynecol. Oncol. Rep. 2021, 37, 100811. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Beischel, L.; Schoof, J.; Johnson, J.; Raff, M.L.; Kapur, R.P. Androgenetic/Biparental Mosaicism in an Infant with Hepatic Mesenchymal Hamartoma and Placental Mesenchymal Dysplasia. Pediatr. Dev. Pathol. 2008, 11, 377–383. [Google Scholar] [CrossRef]
- Colpaert, R.M.; Ramseyer, A.M.; Luu, T.; Quick, C.M.; Frye, L.T.; Magann, E.F. Diagnosis and Management of Placental Mesenchymal Disease. A Review of the Literature. Obstet. Gynecol. Surv. 2019, 74, 611–622. [Google Scholar] [CrossRef]
- Oide, S.; Kuwata, T.; Wang, L.; Imai, K.; Chikazawa, K.; Takagi, K. Placental mesenchymal dysplasia with a good outcome: A case report. J. Obstet. Gynaecol. Res. 2019, 45, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Kodera, C.; Aoki, S.; Ohba, T.; Higashimoto, K.; Mikami, Y.; Fukunaga, M.; Soejima, H.; Katabuchi, H. Clinical manifestations of placental mesenchymal dysplasia in Japan: A multicenter case series. J. Obstet. Gynaecol. Res. 2021, 47, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Umazume, T.; Kataoka, S.; Kamamuta, K.; Tanuma, F.; Sumie, A.; Shirogane, T.; Kudou, T.; Ikeda, H. Placental mesenchymal dysplasia, a case of intrauterine sudden death of fetus with rupture of cirsoid periumbilical chorionic vessels. Diagn. Pathol. 2011, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Lazebnik, N. Placental Mesenchymal Dysplasia; Current Understanding of the Sonographic, Histologic, and Molecular Findings of a Rare and Challengin Disorder. J. Gynecol. Womens Health 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Cubal, A.; Carvalho, J.; Faria, B.; Rodrigues, G.; Carmo, O. Placental mesenchymal dysplasia. Acta Obstet. Ginecol. Port. 2015, 9, 235–240. [Google Scholar]
- Kuwata, T.; Takahashi, H.; Matsubara, S. ‘Stained-glass’ sign for placental mesenchymal dysplasia. Ultrasound Obstet. Gynecol. 2014, 43, 355. [Google Scholar] [CrossRef]
- Parveen, Z.; Tongson-Ignacio, J.E.; Fraser, C.R.; Killeen, J.L.; Thompso, K.S. Placental Mesenchymal Dysplasia. Arch. Pathol. Lab. Med. 2007, 131, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, H.; Miyahara, Y.; Ebina, Y.; Yamada, H. Ultrasound and MRI Findings of Twin Pregnancies with Complete Hydatidiform Mole and Coexisting Normal Fetus: Two Case Reports. Kobe J. Med Sci. 2018, 64, E1–E5. [Google Scholar] [PubMed]
- Tanuma, A.; Kawaguchi, R.T.; Yanagisawa, H.; Tanaka, T.; Yanaihara, N.; Okamoto, A. Prenatal imaging and pathology of placental mesenchymal dysplasia: A report of three cases. Case Rep. Périnat. Med. 2016, 5, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.; Carreon, C.K.; Vohra, N.; Williamson, A.; Dolgin, S.; Rochelson, B. Placental Mesenchymal Dysplasia with Hepatic Mesenchymal Hamartoma: A Case Report and Literature Review. Fetal Pediatr. Pathol. 2013, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Repnikova, E.; Roberts, J.; Kats, A.; Habeebu, S.; Schwager, C.; Joyce, J.; Manalang, M.; Amudhavalli, S. Biparental/androgenetic mosaicism in a male with features of overgrowth and placental mesenchymal dysplasia. Clin. Genet. 2018, 94, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Ulker, V.; Aslan, H.; Gedikbasi, A.; Yararbas, K.; Yildirim, G.; Yavuz, E. Placental mesenchymal dysplasia: A rare clinicopathologic entity confused with molar pregnancy. J. Obstet. Gynaecol. 2013, 33, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Armes, J.E.; McGown, I.; Williams, M.; Broomfield, A.; Gough, K.; Lehane, F.; Lourie, R. The placenta in Beckwith-Wiedemann syndrome: Genotype-phenotype associations, excessive extravillous trophoblast and placental mesenchymal dysplasia. Pathol. 2012, 44, 519–527. [Google Scholar] [CrossRef]
- Jimbo, T.; Fujita, Y.; Yumoto, Y.; Fukushima, K.; Kato, K. Rare fetal complications associated with placental mesenchymal dysplasia: A report of two cases. J. Obstet. Gynaecol. Res. 2015, 41, 304–308. [Google Scholar] [CrossRef]
- Robinson, W.; Slee, J.; Smith, N.; Murch, A.; Watson, S.K.; Lam, W.; McFadden, D. Placental mesenchymal dysplasia associated with fetal overgrowth and mosaic deletion of the maternal copy of 11p15.5. Am. J. Med Genet. Part A 2007, 143A, 1752–1759. [Google Scholar] [CrossRef]
- Brătilă, E.; Ionescu, C.A.; Vlădescu, C.T.; Cîrstoiu, M.M.; Berceanu, C. Gestational choriocarcinoma after term pregnancy: A case report. Rom. J. Morphol. Embryol. 2015, 56, 267–271. [Google Scholar]
- Nisa Abbasi, A.U.; Fawad, A.; Islam, A.; Ismail, A.; Ullah, O. Choriocarcinoma After Normal Vaginal Delivery; A Rare Entity. J. Ayub Med. Coll. Abbottabad. 2019, 31, 629–630. [Google Scholar] [PubMed]
- Iloki, L.H.; Gbala-Sapoulou, M.V.; Kocko, I. Choriocarcinome après un accouchement normal. A propos d’un cas observé à Brazzaville [Choriocarcinoma after a normal pregnancy. A case report observed in Brazzaville]. Med. Trop. 1996, 56, 170–172. (In French) [Google Scholar]
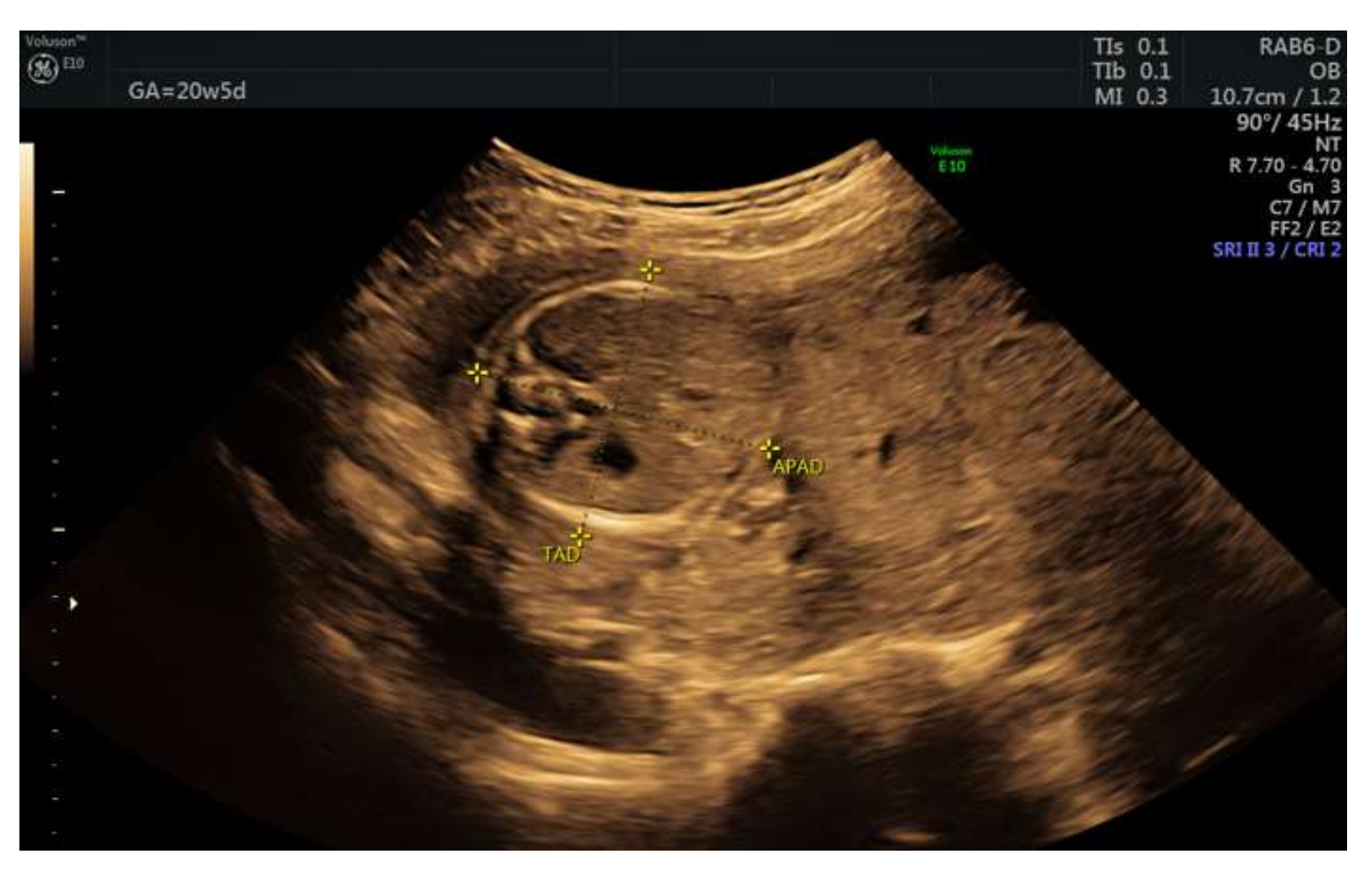


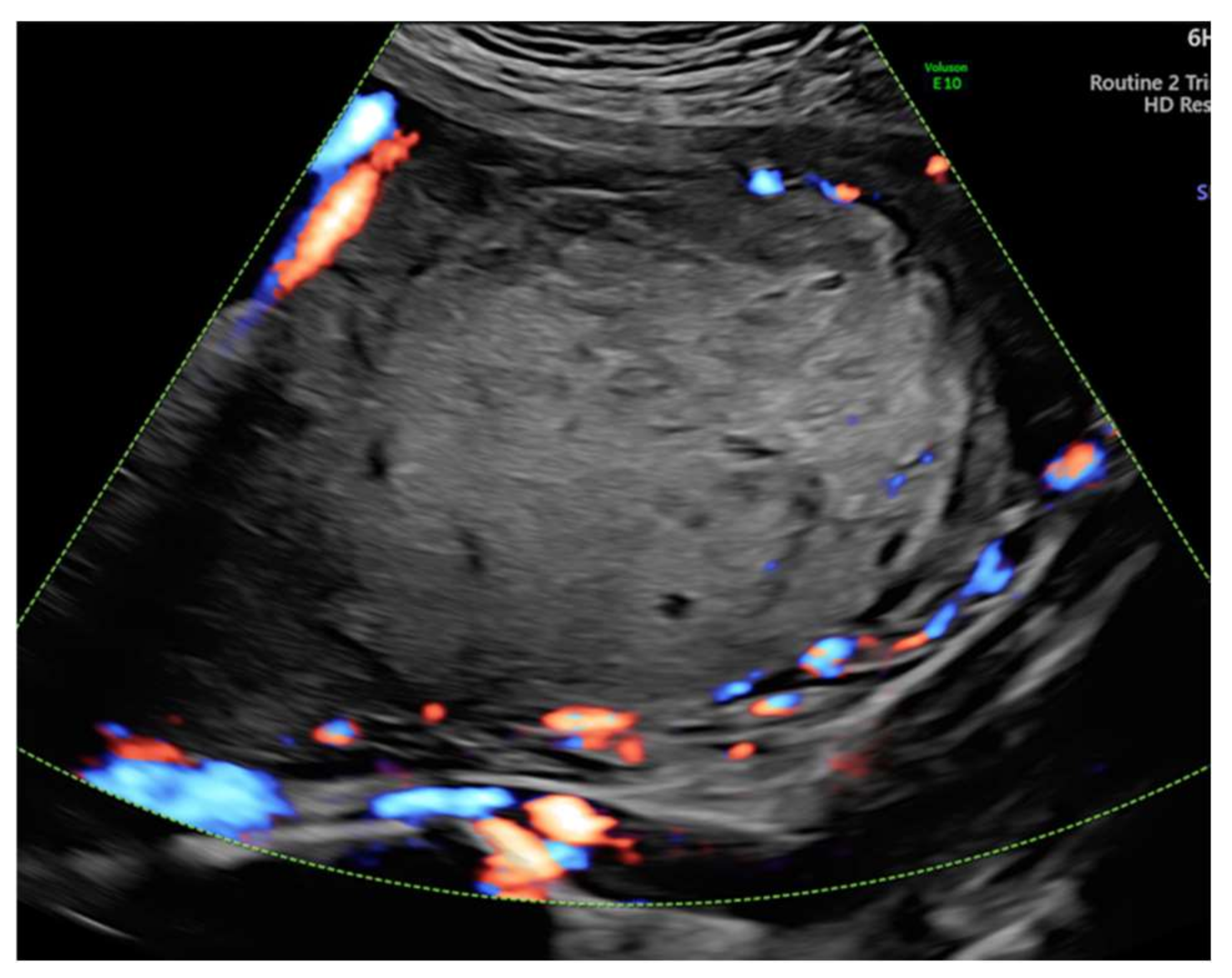



| Nr. | Author | Year | Number of Cases | Karyotype | Elevated Alpha-Fetoprotein | Elevated Human Chorionic Gonadotrophin | Placenta Weight | Immunohistochemical Staining |
|---|---|---|---|---|---|---|---|---|
| 1. | Guenot et al. [1] | 2019 | 5/22 * | Chromosomal evaluation of the 6 infants revealed no SA | 5 (23%) | 3 (14%) | NA | NA |
| 2. | Moscoso et al. [2] | 1991 | 2 | 46XX, 46XX No SA | >2.5 MoM | Normal | 1200 g 3.03 MoM 3280 g 7.92 MoM | AFP and factor VIII were negative |
| 3 | Li et al. [3] | 2014 | 1 | 46XX Chromosomal evaluation of the infant revealed no SA | Elevated (6.22 U/mL) | 16 GW normal level 4335 mUI/mL 3 days postpartum 7.5 mUI/mL 3 weeks after delivery | 760 g | negative for CD34 and D2-40 vimentin labeled p57kip2 negative low detectable Ki-67 expression |
| 4. | Sun et al. [5] | 2020 | 1 | 46XX No SA | NA | NA | 520 g | positive p57 in all cytotrophoblast cells of PMD low detectable Ki-67 expression in PMD |
| 5. | Toru et al. [6] | 2014 | 1 | NA No SA | NA | NA | 487 g | negative for CD34 and D2-40 |
| 6. | Rosner–Tenerowicz et al. [8] | 2020 | 1 | 46XX No SA | Elevated | Normal | 500 g (90th percentile for GW) | NA |
| 7. | Ishikawa et al. [9] | 2016 | 1 | 46XX No SA | 22 MoM (7261 ng/mL)- 23 GW 33 MoM (10,786 ng/mL)-30 GW 4990 ng/mL–3rd day postpartum | NA | 575 g | NA |
| 8. | Pham et al. [10] | 2006 | 5/11 * | 5 of 46XX No SA | NA | 10.300 IU/L | 686 g 2.38 MoM 670 g 1.76 MoM 450 g 1.14MoM 440 g 1.06 MoM 1000 g 2.42 MoM | No detectable Ki-67 or Flk-1 protein expression in either tissue |
| 9 | Arizawa and Nakayama [11] | 2002 | 6/15 * | 6 of 46XX No SA | NA | NA | 685 g 940 g 440 g 950 g 860 g 930 g | NA |
| 10. | Adams et al. [12] | 2018 | 1 | 46XY No SA | 3,8 MoM | 48.000 IU/L | NA | NA |
| 11. | Himoto et al. [13] | 2014 | 3 | 3 of 46XX No SA | NA | 90.436 mIU/mL, 98.171 mUI/mL, 151.370 mUI/mL | 1100 g 930 g 838 g | NA |
| 12. | Chan et al. [14] | 2003 | 1 | 46XY No SA | NA | NA | 513 g | NA |
| 13 | Woo et al. [15] | 2011 | 1 | 46XX No SA | NA | Elevated-167402 mUI/mL, 6.95 MoM | 1380 g | P57 stain decreased, but not absent diploidy |
| 14 | Gheysen et al. [16] | 2018 | 2/1 ** | Monochorionic diamniotic twin pregnancy 46XY/46XY No SA | NA | NA | NA | Stem villi: the stromal fibroblasts were p57 negative whereas the trophoblastic cells were p57 positive |
| 15 | Jitsumori et al. [17] | 2018 | 2/1 ** | Dichorionic diamniotic twin pregnancy 46XX/ 46XX No SA | NA | 24 GW – 44,084 mIU/mL | Both placentas: 1066 g (above the 90th percentile) | p57kip2 was lost in the PMD lesions |
| 16 | Sander et al. [18] | 1993 | 3 | 46XY, 46XX,46XX No SA | NA | NA | 600 g (1.45 MoM) 815 g (2.52 MoM) 829 g (2.09 MoM) | NA |
| 17. | Matsui et al. [19] | 2003 | 1 | 46XX No SA | NA | 65,960 mUI/mL 27 GW | 465 g (>90% of the normal range for GW) | NA |
| 18. | Hojberg et al. [20] | 1994 | 1 | 46XX No SA | 3.03 MoM 15 GW | 47,000 mUI/mL 15 GW | 1500 g | NA |
| 19. | Ohira et al. [21] | 2013 | 1 | 46XX No SA | NA | 241,270 mIU/mL at 12 GW, and then gradually decreased | 1200 g | NA |
| 20. | Chen et al. [22] | 1997 | 2 | 46XX, 46XX No SA | NA | NA | 1150 g 1100 g | NA |
| 21. | Jauniaux et al. [23] | 1997 | 3 | 46XX, 46XX, 46XX No SA | NA | NA | 1535 g 1430 g 1250 g | NA |
| 22. | Huang et al. [24] | 2021 | 1 | 46XX No SA | 4.57 MoM 15 GW | 12 MoM 15 GW 60,000–160,000 mUI/mL | 1350 g | NA |
| 23. | Heazell et al. [25] | 2009 | 1 | 46XX No SA | N | 90,376 mUI/L 13 GW | NA | Mib-1 (Clone Ki-67) anti-CD34 anti-cytokeratin 7 and anti-E-cadherin |
| 24 | Lee et al. [26] | 1990 | 1 | 46XX No SA | NA | NA | 1490 g | NA |
| 25 | Kaiser-Rogers et al. [27] | 2006 | 3/2 ** | Case 1: Dichorionic twin placenta Case2: Dichorionic twin placenta 46XY, 46XX No SA | Case 1: NA Case 2: 1.64 MoM in 15 GW | Case 1: NA Case 2: Elevated 5.09 MoM in 15 GW | Case 1: 1900 g Case 2: 690 g | NA |
| 26 | Psarris et al. [28] | 2020 | 1 | 46XX No SA | NA | free β hCG was 33.77 IU/L (0.83 MoM) serum PAPP-A was 3.790IU/L (1.587 ΜοΜ) | 720 g | NA |
| 27 | Pal et al. [29] | 2017 | 1 | 46XX No SA | 485 ng/mL | 25,780 mIU/L | 950 g (20 × 20 × 3 cm) | NA |
| 28 | Toscano M.P. and Schultz R. [30] | 2014 | 1 | 46XX No SA | NA | NA | 1415 g (28.0 × 25.0 × 7.0 cm) | NA |
| 29 | Gizzo et al. [31] | 2012 | 1 | 46XX 11GW chorionic villus sampling normal female karyotype | NA | Normal values | 1100 g Increased thickness (6 cm) | NA |
| 30 | Balachandran et al. [32] | 2015 | 1 | NA No SA | NA | NA | 600 g | NA |
| 31 | Taga et al. [33] | 2013 | 1 | 46XX No SA | NA | 20124.97 U/L at 20 GW (normal) | 720 g 20 × 16 × 2 cm | NA |
| 32 | Qichang et al. [34] | 2013 | 1 | 46XX No SA | NA | 4611 mUI/mL at 2 days postpartum undetectable at 3 weeks postpartum | 1370 g 30 × 25 × 4.5 cm the largest tumor measured 11 × 8 × 4.5 cm | Expression of p57KIP2 in the villous cytotrophoblast |
| 33 | Koga et al. [35] | 2014 | 1 | 46XX No SA | NA | NA | 1690 g 25 cm in diameter | positive for vimentin and desmin, loss of p57 |
| 34 | Gibson et al. [36] | 2004 | 1 | 46XY No SA | NA | NA | 1258.0 g 23.0 × 18.0 × 3.5 cm | NA |
| 35 | Kinoshita et al. [37] | 2007 | 1 | 46XX No SA | NA | 67,500 mIU/mL at 19 GW(normal) | 930 g 21 × 19 × 5 cm | NA |
| 36 | Gurram et al. [38] | 2016 | 1 | 46XX No SA | NA | NA | 1970 g (>95th percentile) | NA |
| 37 | Mulch et al. [39] | 2006 | 1 | 46XX No SA | Elevated MSAFP (2.9 MoM at 18 GW) | NA | 480-g placenta, 3.5 × 1.8 × 1.9 cm | NA |
| 38 | Surti et al. [40] | 2005 | 2/1 ** | Twin gestation Twin A 46XX Twin B 46XY No SA | NA | NA | Diamniotic dichorionic 1325 g twin placenta Placenta A:370 g Placenta B: 955 g | NA |
| 39 | Rohilla et al. [41] | 2012 | 1 | 46XX No SA | NA | Postpartum after 3-weeks-0.01 IU/dl | 1700 g | NA |
| 40 | McNally et al. [42] | 2021 | 3 | 46XX 46XY 46XX No SA | amniocentesis: abnormal secondary to elevated AFP | The third case-the highest value of 72,786 IU/L | NA | NA |
| 41 | Reed et al. [43] | 2008 | 1 | 46XX No SA | NA | NA | 893 g; (expected weight 316 g) | p57KIP2 immunoreactivity |
| Nr. | Author | Year | Number of Cases | Preterm Delivery | Complications of the Mother: Preeclampsia/Gestational Hypertension, Gestational Diabetes, etc. | Fetal Outcome | Uncomplicated Pregnancy |
|---|---|---|---|---|---|---|---|
| 1. | Guenot et al. [1] | 2019 | 5/22 * | 9/14 (64%) | 6 (27%) | 11 (50%) IUGR | 3 (14%) |
| 2. | Moscoso et al. [2] | 1991 | 2 | 36 GW CS 37 GW CS | NO | 2200 g 2940 g | YES |
| 3 | Li et al. [3] | 2014 | 1 | 35 GW VD pPROM | NO | 1800 g APGAR 10/10 Pathological jaundice 30 GW–IUGR (<10th percentile) Follow-up of neonate and mother-uneventful at 10 months | YES |
| 4. | Sun et al. [5] | 2020 | 1 | 37 GW CS for IUGR | IUGR | 2290 g APGAR 10/10, jaundice Follow-up results-trophoblastic dysplasia in the uterine scar 5 months after cesarean section; the morphology was consistent with choriocarcinoma. | NO |
| 5. | Toru et al. [6] | 2014 | 1 | 32GW CS for intractable maternal tachycardia | atrial-mitral valve replacement operation used warfarin from the first trimester, no history of fever (with or without rash) | 2550 g; healthy baby First-trimester screening for aneuploidy had revealed a risk of 1:780 for Down syndrome The baby and mother were discharged in good condition 2 weeks later | NO |
| 6. | Rosner-Tenerowicz et al. [8] | 2020 | 1 | 29 GW pPROM ECS | NO | 1320 g APGAR 5/6 (74th percentile) Pathological CTG 80 mm multifocal liver cyst- surgery in the 4th day -simple cyst of the liver Good outcome | YES |
| 7. | Ishikawa et al. [9] | 2016 | 1 | 30 GW | NA | Transient tachypnea Neonate anemia 8.3 g/dL Normal findings on brain MRI at 93 days | YES |
| 8. | Pham et al. [10] | 2006 | 5/11 * | 30–37 GW | NA | 2-IUGR 1 Severe pallor and hypotonia; neonatal anemia and thrombocytopenia 2 normal newborns | 2 |
| 9 | Arizawa and Nakayama [11] | 2002 | 6/15 * | 24–38 GW | NA | 6 normal newborns | NA |
| 10. | Adams et al. [12] | 2018 | 1 | 33 GW CS | NA | AP 5/8, 1600 g (7th percentile), IUGR Decreased FHR reactivity and late decelerations | NA |
| 11. | Himoto et al. [13] | 2014 | 3 | 39 GW VD 40 GW VD 33 GW ECS | 1 gestational diabetes | 2- IUGR 1- OLIGOAMNION 1- FETAL DISTRESS | 1 |
| 12. | Chan et al. [14] | 2003 | 1 | 36 GW iVD | Mild preeclampsia | 2195 g | NO |
| 13 | Woo et al. [15] | 2011 | 1 | 33 GW PPROM VD | Preeclampsia | 1802 g, APGAR 4/7 | NO |
| 14 | Gheysen et al. [44] | 2018 | 2/1 ** | 34 GW CS | Hyperthyroidism (Treatment: propothiouracil) | Twin 1 2130 g APGAR 8/9 Twin 2970 g APGAR 7/9, severe IUGR, severe oligoamnios | NA |
| 15 | Jitsumori et al. [17] | 2018 | 2/1 ** | 32 GW +5 days CS | NO | Twin 1–1799 g APGAR 7/8 Twin 2–1215 g, APGAR 8/9, IUGR) | YES |
| 16 | Sander et al. [18] | 1993 | 3 | NA | NA | 2183 g (5th percentile)—IUGR 1985 g (60th percentile)—thrombocytopenia 2356 g (25th percentile) Normal | NA |
| 17. | Matsui et al. [19] | 2003 | 1 | 27 GW CS | Placenta praevia with massive bleeding | 820 g (within 50% of the normal range for gestational age) APGAR 1/2 | NO |
| 18. | Hojberg et al. [20] | 1994 | 1 | At term VD | NO | 2860g | YES |
| 19. | Ohira et al. [21] | 2013 | 1 | 39 GW ECS | NO | 1998 g (<3rd percentile) APGAR 8/9 Prolonged decelerations in FHR monitoring | YES |
| 20. | Chen et al. [22] | 1997 | 2 | 37 GW VD 27 GW VD pPROM | Polyhydramnios (both cases) | 1500 g, APGAR 5/9; hemangiomatosis (face, left year auricle, left arm, both palms, hepatic hemangioma-surgical removed) and hepatic cyst 976 g, APGAR 6/6, anemia (7,9g/dL) Both cases: follow-up 1 year–good outcome | NO |
| 21. | Jauniaux et al. [23] | 1997 | 3 | 39 GW VD 40 GW VD 37 GW VD | NO | 2400 g 3650 g 3320 g Normal findings at 1-year follow-up | YES |
| 22. | Huang et al. [24] | 2021 | 1 | 36 GW V | NO | 2626 g APGAR 9/10 | YES |
| 23. | Heazell et al. [25] | 2009 | 1 | 38 GW CS | Oligohydramnios | 2700 g (8th percentile) | YES |
| 24 | Lee et al. [26] | 1990 | 1 | 36 GW ECS | Partial placenta praevia | 2001 g, respiratory distress, anemia 5.6 g/dL Good outcome for mother and infant | NO |
| 25 | Kaiser-Rogers et al. [27] | 2006 | 3/2 ** | Case 1: 34 GW, Case 2: 37 GW iVD for IUGR | Case 1: Twin 1 IUFD Case 2: IUGR | Case 1: Twin 1 severe IUGR, IUFD, liver cyst Twin 2: normal growth and development (58thpercentile) Case 2: Twin 1 normal boy 2942 g APGAR 10/10 Twin 2 normal girl breech extraction 2210 g APGAR 10/10, IUGR (<5th percentile) | NO |
| 26 | Psarris et al. [28] | 2020 | 1 | 36 GW CS severe IUGR | Severe IUGR | 2210 g APGAR 9/10 | NO |
| 27 | Pal et al. [29] | 2017 | 1 | 37 GW VD | NO | 2450 g APGAR 8/9 | NO |
| 28 | Toscano M.P. and Schultz R. [30] | 2014 | 1 | 36 GW VD | NO | 2230 g (16thpercentile, −0,98 z score, adequate for gestational age), APGAR 9/9, Jaundice Good outcome for mother and infant | YES |
| 29 | Gizzo et al. [31] | 2012 | 1 | 36 GW iVD for severe IUGR coexistent with itching and cholestasis of pregnancy | increased factor IX and factor XI (thrombosis prophylaxis) hypothyroidism pulmonary embolism during contraceptive therapy itching and cholestasis of pregnancy | 2100 g APGAR 7/8/9 The baby was in good health, with no external dysmorphic features. She had transient physiological neonatal jaundice without other complications. | NO |
| 30 | Balachandran et al. [32] | 2015 | 1 | 40 GW VD | At 8GW—vaginal bleeding | 2250 g Post-natal follow-up was normal up to 12 weeks | YES |
| 31 | Taga et al. [33] | 2013 | 1 | 37 GW CS for previous CS | NA | 2520 g APGAR 8/9 Postoperative course was uneventful | YES |
| 32 | Qichang et al. [34] | 2013 | 1 | 27+3 GW (preterm labor) VG—unsuccessful tocolysis | Polyhydramnios AFI- 37.5 cm | 740 g APGAR 5/8 | NO |
| 33 | Koga et al. [35] | 2014 | 1 | 37 GW ECS non-reassuring fetal status and fetal growth restriction | NA | 1812 g APGAR 1/6, IUGR Anemic, with a bleeding tendency (Hemoglobin, 6.4 g/dL); Blood film showed a leukoerythroblastic picture with circulating myelocytes, nucleated red cells, schizocytes at 1 week of age, no bleeding tendency no other underlying disease and hematologic complications did not recur | NO |
| 34 | Gibson et al. [36] | 2004 | 1 | 39 GW VD | NA | 3250 g vascular hamartoma of the face and abnormal eye movements (left eye ptosis) which was repaired | YES |
| 35 | Kinoshita et al. [37] | 2007 | 1 | 39 GW CS due to non-reassuring fetal state | NA | 1452 g female (<10th centile) severe IUGR | NO |
| 36 | Gurram et al. [38] | 2016 | 1 | 38 GW VD | NA | 2700 g infant, born in good condition, discharged home day 2 postpartum liver harmartoma - planned to be operated in 1 year | YES |
| 37 | Mulch et al. [39] | 2006 | 1 | 36 GW VD induced labor after amniocentesis for fetal lung maturity | NA | 2800 g APGAR 8/9 No abnormalities have been found in the infant during a follow-up period of 1 year | YES |
| 38 | Surti et al. [40] | 2005 | 2/1 ** | 35 GW CS discordant twin growth reversal diastolic flow within the umbilical artery of Twin B | NA | Twin A—a female, weighed 2312 g Twin B—a male, weighed 1603 g At 18 months of age, both the twins showed normal development, although Twin B continues to be slightly smaller than his twin sister A | NO |
| 39 | Rohilla et al. [41] | 2012 | 1 | 36 GW VD pPROM tachycardia of the fetus 180 beats/min mild oligohydramnios | febrile (38.5°C) MPR 94/min | 2450 g APGAR 8/9, IUGR At 3-years old, the child had normal development | NO |
| 40 | McNally et al. [42] | 2021 | 3 | 39 GW 36 GW 29 GW ECS HELLP | HELLP in the third case | No fetal anomalies | YES YES NO |
| 41 | Reed et al. [43] | 2008 | 1 | 30 GW CS fetal distress | NA | 1110 g (expected: 1280 ± 350 g) At 11 months of age, cystic liver mass-resection. | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehedintu, C.; Frincu, F.; Ionescu, O.-M.; Cirstoiu, M.M.; Sajin, M.; Olinca, M.; Bratila, E.; Petca, A.; Carp-Veliscu, A. A Challenging Diagnosis: Placental Mesenchymal Dysplasia—Literature Review and Case Report. Diagnostics 2022, 12, 293. https://doi.org/10.3390/diagnostics12020293
Mehedintu C, Frincu F, Ionescu O-M, Cirstoiu MM, Sajin M, Olinca M, Bratila E, Petca A, Carp-Veliscu A. A Challenging Diagnosis: Placental Mesenchymal Dysplasia—Literature Review and Case Report. Diagnostics. 2022; 12(2):293. https://doi.org/10.3390/diagnostics12020293
Chicago/Turabian StyleMehedintu, Claudia, Francesca Frincu, Oana-Maria Ionescu, Monica Mihaela Cirstoiu, Maria Sajin, Maria Olinca, Elvira Bratila, Aida Petca, and Andreea Carp-Veliscu. 2022. "A Challenging Diagnosis: Placental Mesenchymal Dysplasia—Literature Review and Case Report" Diagnostics 12, no. 2: 293. https://doi.org/10.3390/diagnostics12020293
APA StyleMehedintu, C., Frincu, F., Ionescu, O.-M., Cirstoiu, M. M., Sajin, M., Olinca, M., Bratila, E., Petca, A., & Carp-Veliscu, A. (2022). A Challenging Diagnosis: Placental Mesenchymal Dysplasia—Literature Review and Case Report. Diagnostics, 12(2), 293. https://doi.org/10.3390/diagnostics12020293






