OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives
Abstract
:1. Introduction
- Determination of contrast agents accumulating in the tumor vascular net (CT, MRI with contrast);
- Determination of metabolic changes in tissues (5-ALA fluorescence, laser spectroscopy);
- Determination of brain areas with altered blood–brain barriers with fluorescein;
- Determination of changes in tissue density (ultrasound).
2. Materials and Methods
3. OCT Multimodality and Multitasking
3.1. OCT Multimodality
3.2. OCT Application in Neurosurgery
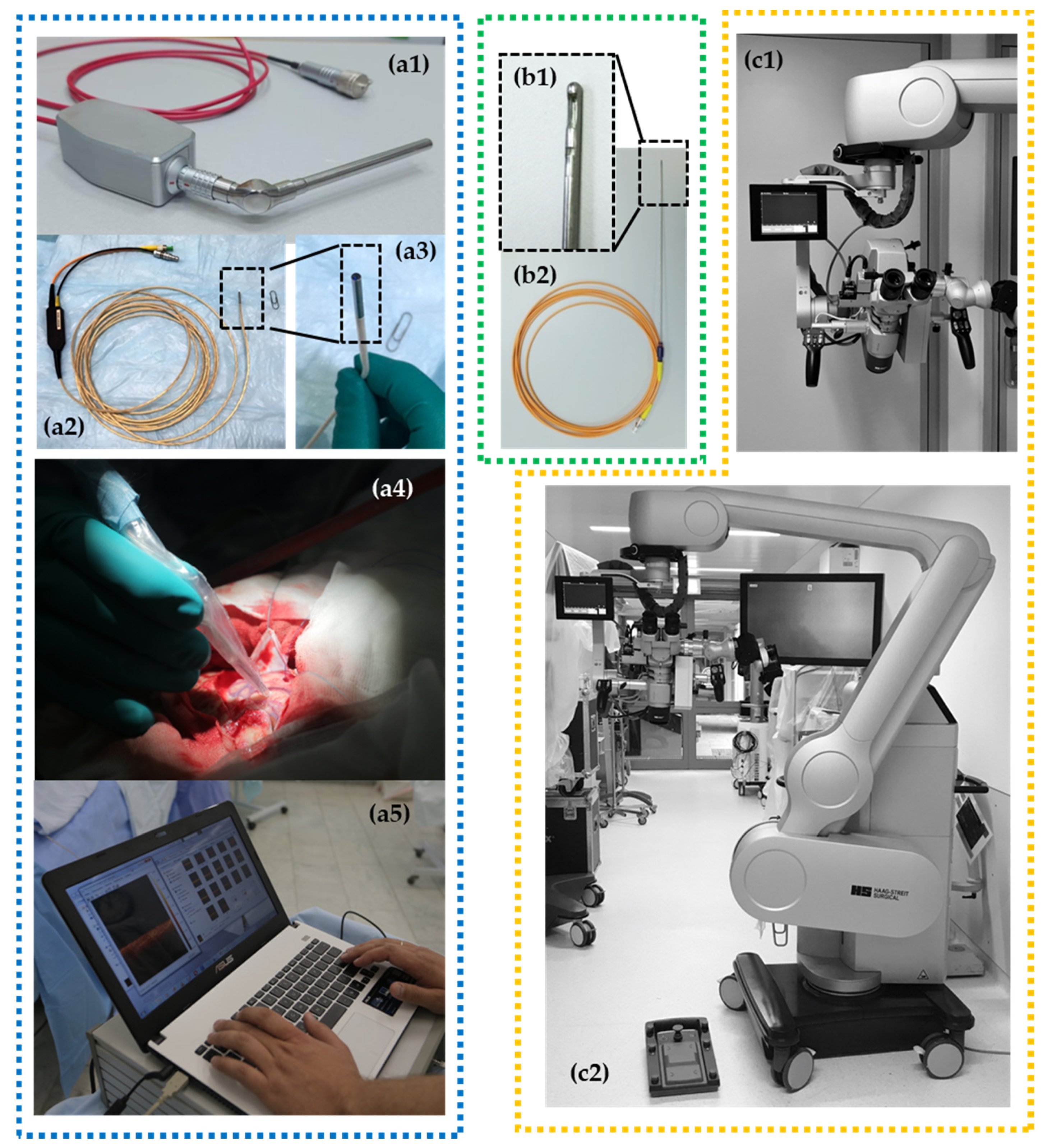
4. Clinical OCT Devices
5. Evaluation of OCT Data Obtained in Brain Tumor Surgery
6. Basics of OCT Signal Forming in Nervous Tissue
7. Clarifying the Boundaries of the Infiltrative Glioma Growth
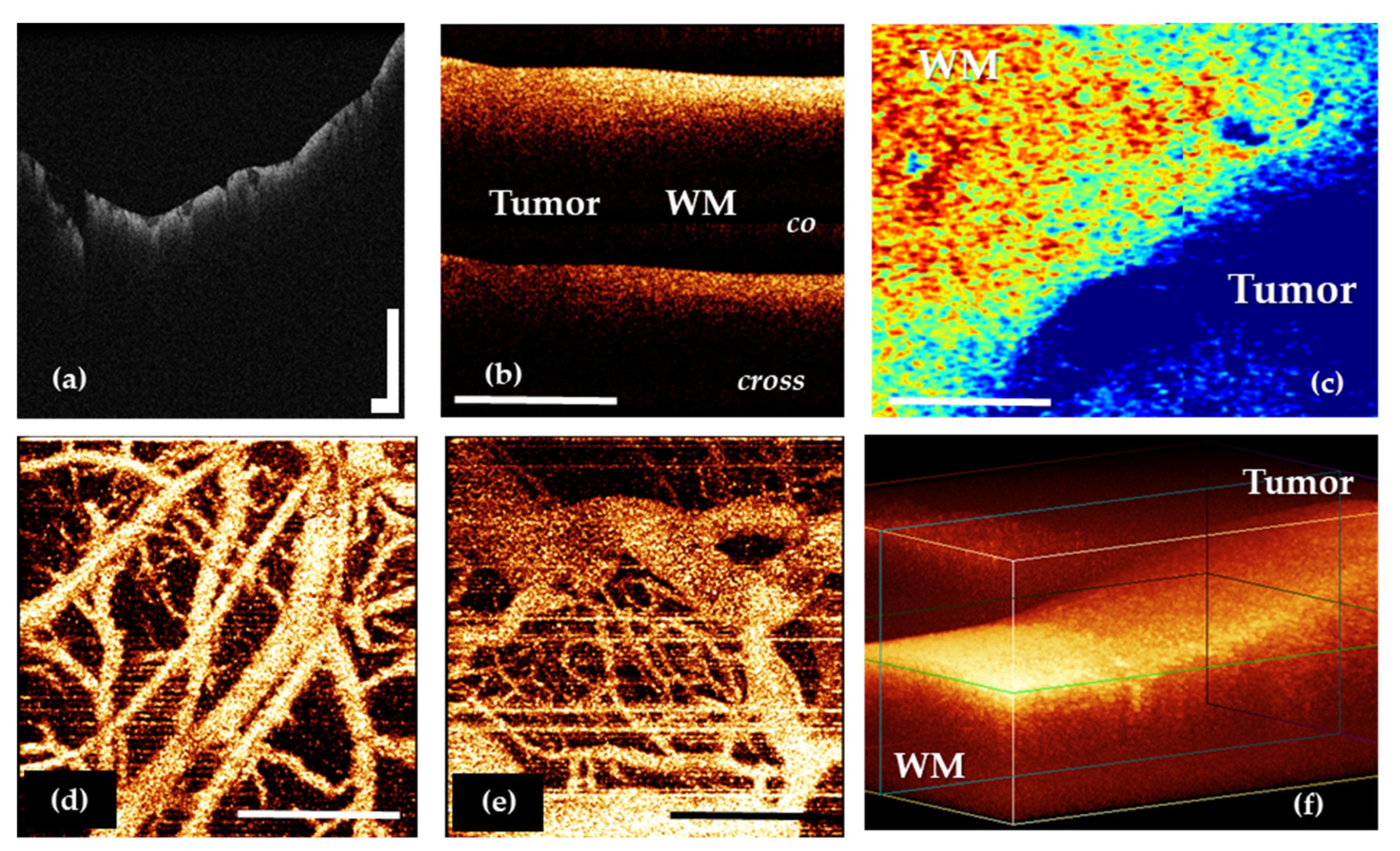
7.1. Using OCT for White Matter and Tumor Differentiation
7.2. Using OCT for Grey Matter and Tumor Differentiation
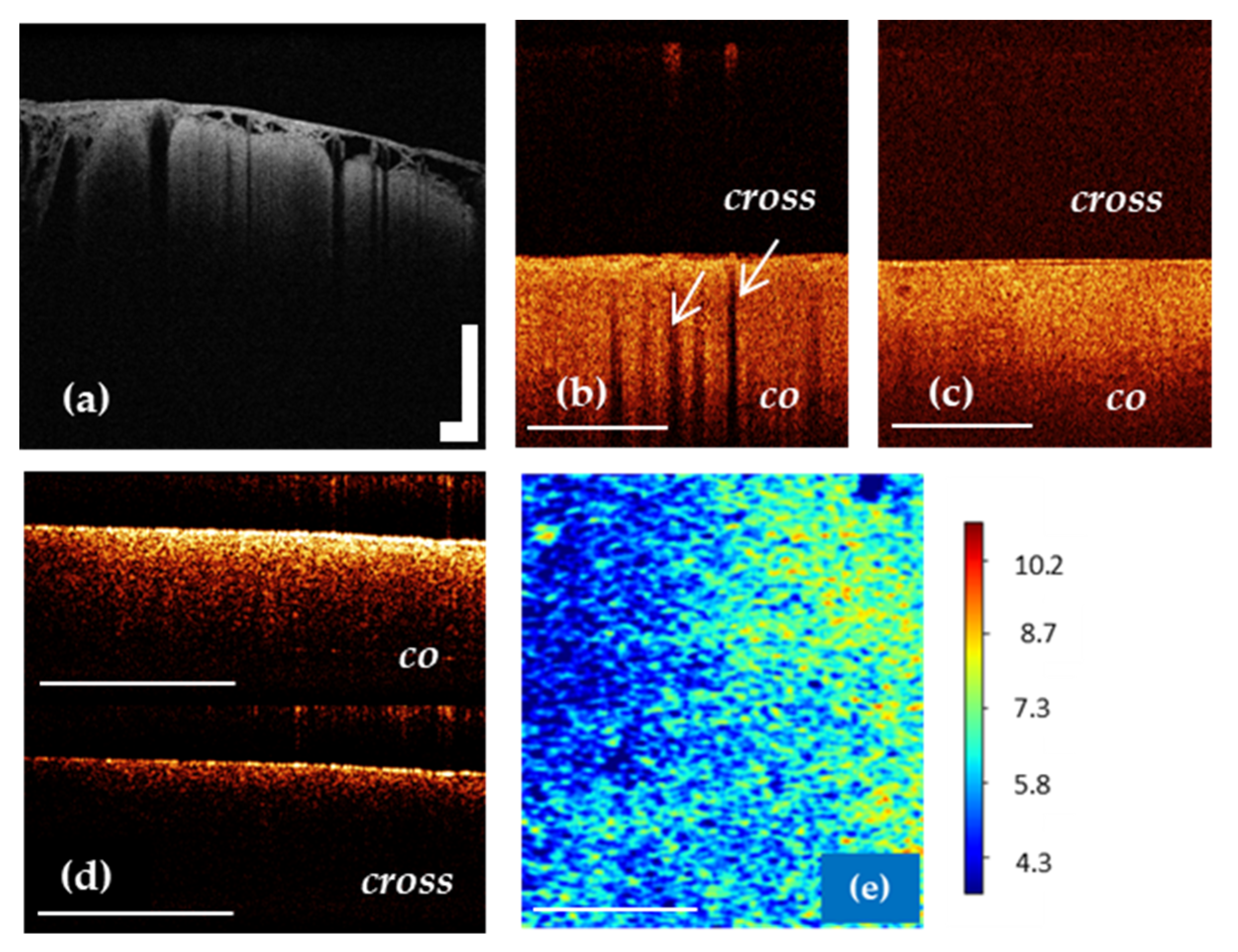
7.3. OCT for White Matter State Evaluation
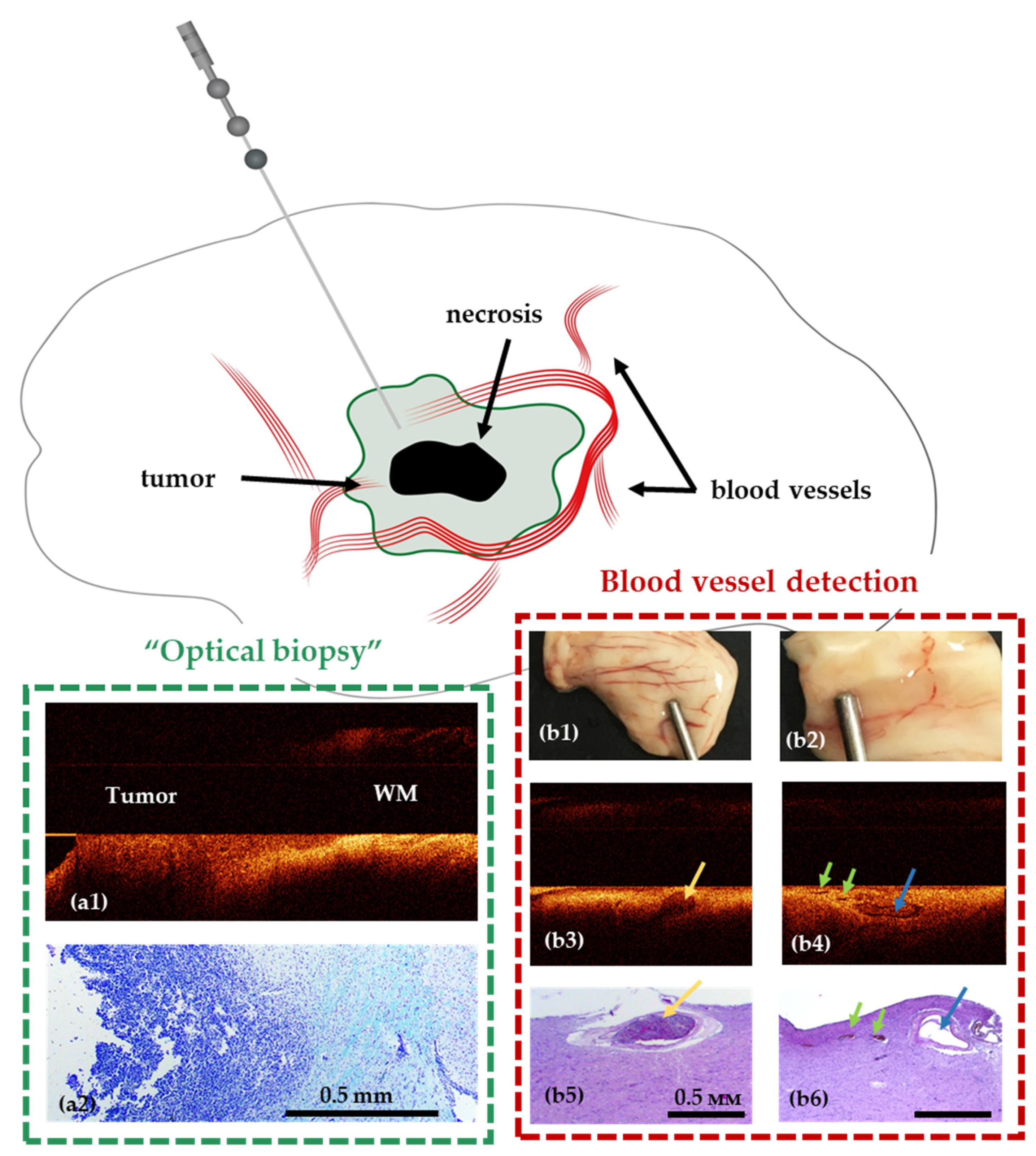
8. OCT for Stereotactic Biopsy
9. The Place of OCT among Other Intraoperative Imaging Techniques
10. OCT Future Perspectives in Glioma Surgery
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Crocetti, E.; Trama, A.; Stiller, C.; Caldarella, A.; Soffietti, R.; Jaal, J.; Weber, D.C.; Ricardi, U.; Slowinski, J.; Brandes, A.; et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur. J. Cancer 2012, 48, 1532–1542. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neuro-Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.A.; Wolf, A.B.; Oppenlander, M.E.; Bina, R.; Wilson, J.R.; Ashby, L.; Brachman, D.; Coons, S.W.; Spetzler, R.F.; Sanai, N. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J. Neurosurg. 2014, 120, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764, discussion 264–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576, discussion 564–576. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quinones-Hinojosa, A.R. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology 2011, 13, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.P.; Chaichana, K.L.; Rincon-Torroella, J.; Quinones-Hinojosa, A. The value of extent of resection of glioblastomas: Clinical evidence and current approach. Curr. Neurol. Neurosci. Rep. 2015, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Anton, K.; Baehring, J.M.; Mayer, T. Glioblastoma multiforme: Overview of current treatment and future perspectives. Hematol./Oncol. Clin. N. Am. 2012, 26, 825–853. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.; Okamoto, M.; Guiou, M.; Shirai, K.; Errett, A.; Chakravarti, A. Novel therapies in glioblastoma. Neurol. Res. Int. 2012, 2012, 428565. [Google Scholar] [CrossRef]
- Wolbers, J.G. Novel strategies in glioblastoma surgery aim at safe, supra-maximum resection in conjunction with local therapies. Chin. J. Cancer 2014, 33, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Colditz, M.J.; Jeffree, R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J. Clin. Neurosci. 2012, 19, 1471–1474. [Google Scholar] [CrossRef]
- Diez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; Garcia de Eulate, R.; Dominguez Echavarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdes, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Valdes, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, T.D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. δ-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: The need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro-Oncology 2011, 13, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Raheja, A.; Tandon, V.; Suri, A.; Sarat Chandra, P.; Kale, S.S.; Garg, A.; Pandey, R.M.; Kalaivani, M.; Mahapatra, A.K.; Sharma, B.S. Initial experience of using high field strength intraoperative MRI for neurosurgical procedures. J. Clin. Neurosci. 2015, 22, 1326–1331. [Google Scholar] [CrossRef]
- Bohringer, H.J.; Lankenau, E.; Stellmacher, F.; Reusche, E.; Huttmann, G.; Giese, A. Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir. 2009, 151, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, K.; Stein, K.-P.; Neyazi, B.; Sandalcioglu, I.E. Optical coherence tomography of cranial dura mater: Microstructural visualization in vivo. Clin. Neurol. Neurosurg. 2021, 200, 106370. [Google Scholar] [CrossRef] [PubMed]
- Yashin, K.S.; Kiseleva, E.B.; Gubarkova, E.V.; Moiseev, A.A.; Kuznetsov, S.S.; Shilyagin, P.A.; Gelikonov, G.V.; Medyanik, I.A.; Kravets, L.Y.; Potapov, A.A.; et al. Cross-Polarization Optical Coherence Tomography for Brain Tumor Imaging. Front. Oncol. 2019, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarez-Chambi, R.M.; Kut, C.; Rico-Jimenez, J.J.; Chaichana, K.L.; Xi, J.; Campos-Delgado, D.U.; Rodriguez, F.J.; Quinones-Hinojosa, A.; Li, X.; Jo, J.A. AI-Assisted In Situ Detection of Human Glioma Infiltration Using a Novel Computational Method for Optical Coherence Tomography. Clin. Cancer Res. 2019, 25, 6329–6338. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quinones-Hinojosa, A.; Li, X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 292ra100. [Google Scholar] [CrossRef] [Green Version]
- Yashin, К.S.; Karabut, M.M.; Fedoseeva, V.V.; Khalansky, A.S.; Matveev, L.A.; Elagin, V.V.; Kuznetsov, S.S.; Kiseleva, E.B.; Kravets, L.Y.; Medyanik, I.А.; et al. Multimodal Optical Coherence Tomography in Visualization of Brain Tissue Structure at Glioblastoma (Experimental Study). Sovrem. Tehnol. Med. 2016, 8, 73–81. [Google Scholar] [CrossRef]
- Böhringer, H.J.; Boller, D.; Leppert, J.; Knopp, U.; Lankenau, E.; Reusche, E.; Hüttmann, G.; Giese, A. Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers Surg. Med. 2006, 38, 588–597. [Google Scholar] [CrossRef]
- Hartmann, K.; Stein, K.P.; Neyazi, B.; Sandalcioglu, I.E. Theranostic applications of optical coherence tomography in neurosurgery? Neurosurg. Rev. 2021. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Fukumura, D.; Jain, R.K.; Bouma, B.E. Cancer imaging by optical coherence tomography: Preclinical progress and clinical potential. Nat. Rev. Cancer 2012, 12, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Henry, F.P.; Wang, Y.; Rodriguez, C.L.R.; Randolph, M.A.; Rust, E.A.Z.; Winograd, J.M.; de Boer, J.F.; Park, B.H. In vivo optical microscopy of peripheral nerve myelination with polarization sensitive-optical coherence tomography. J. Biomed. Opt. 2015, 20, 046002. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Jeon, S.H.; Park, Y.H.; Jang, W.H.; Lee, J.Y.; Kim, K.H. Visualization of prostatic nerves by polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2016, 7, 3170–3183. [Google Scholar] [CrossRef] [Green Version]
- Osman, H.; Georges, J.; Elsahy, D.; Hattab, E.; Yocom, S.; Cohen-Gadol, A.A. In Vivo Microscopy in Neurosurgical Oncology. World Neurosurg. 2018, 115, 110–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Brown, W.; Maher, J.R.; Levinson, H.; Wax, A. Functional optical coherence tomography: Principles and progress. Phys. Med. Biol. 2015, 60, R211–R237. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography—A review [Invited]. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, B. Polarization Sensitive Optical Coherence Tomography: A Review of Technology and Applications. Appl. Sci. 2017, 7, 474. [Google Scholar] [CrossRef]
- Wang, H.; Akkin, T.; Magnain, C.; Wang, R.; Dubb, J.; Kostis, W.J.; Yaseen, M.A.; Cramer, A.; Sakadžić, S.; Boas, D. Polarization sensitive optical coherence microscopy for brain imaging. Opt. Lett. 2016, 41, 2213. [Google Scholar] [CrossRef]
- Boas, D.A.; Wang, H.; Magnain, C.; Fischl, B. Polarization-sensitive optical coherence tomography of the human brain connectome. SPIE Newsroom 2017, 10, 006834. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Akkin, T. Serial optical coherence scanner for large-scale brain imaging at microscopic resolution. NeuroImage 2014, 84, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, J.; Reuter, M.; Vinke, L.N.; Yendiki, A.; Boas, D.A.; Fischl, B.; Akkin, T. Cross-validation of serial optical coherence scanning and diffusion tensor imaging: A study on neural fiber maps in human medulla oblongata. NeuroImage 2014, 100, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Gubarkova, E.V.; Dudenkova, V.V.; Feldchtein, F.I.; Timofeeva, L.B.; Kiseleva, E.B.; Kuznetsov, S.S.; Shakhov, B.E.; Moiseev, A.A.; Gelikonov, V.M.; Gelikonov, G.V.; et al. Multi-modal optical imaging characterization of atherosclerotic plaques. J. Biophotonics 2016, 9, 1009–1020. [Google Scholar] [CrossRef]
- Gladkova, N.; Kiseleva, E.; Robakidze, N.; Balalaeva, I.; Karabut, M.; Gubarkova, E.; Feldchtein, F. Evaluation of oral mucosa collagen condition with cross-polarization optical coherence tomography. J. Biophotonics 2013, 6, 321–329. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, Z. Advances in Doppler optical coherence tomography and angiography. Transl. Biophotonics 2019, 1, e201900005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lankenau, E.; Klinger, D.; Winter, C.; Malik, A.; Müller, H.H.; Oelckers, S.; Pau, H.-W.; Just, T.; Hüttmann, G. Combining Optical Coherence Tomography (OCT) with an Operating Microscope. In Advances in Medical Engineering; Springer: Berlin/Heidelberg, Germany, 2007; Volume 114, pp. 343–348. [Google Scholar] [CrossRef]
- Finke, M.; Kantelhardt, S.; Schlaefer, A.; Bruder, R.; Lankenau, E.; Giese, A.; Schweikard, A. Automatic scanning of large tissue areas in neurosurgery using optical coherence tomography. Int. J. Med. Robot. Comput. Assist. Surg. 2012, 8, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Bizheva, K.; Unterhuber, A.; Hermann, B.; Povazay, B.; Sattmann, H.; Fercher, A.F.; Drexler, W.; Preusser, M.; Budka, H.; Stingl, A.; et al. Imaging ex vivo healthy and pathological human brain tissue with ultra-high-resolution optical coherence tomography. J. Biomed. Opt. 2005, 10, 011006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashin, K.S.; Kiseleva, E.B.; Moiseev, A.A.; Kuznetsov, S.S.; Timofeeva, L.B.; Pavlova, N.P.; Gelikonov, G.V.; Medyanik, I.А.; Kravets, L.Y.; Zagaynova, E.V.; et al. Quantitative nontumorous and tumorous human brain tissue assessment using microstructural co- and cross-polarized optical coherence tomography. Sci. Rep. 2019, 9, 2024. [Google Scholar] [CrossRef] [Green Version]
- Ecclestone, B.R.; Hosseinaee, Z.; Abbasi, N.; Bell, K.; Dinakaran, D.; Mackey, J.R.; Haji Reza, P. Three-dimensional virtual histology in unprocessed resected tissues with photoacoustic remote sensing (PARS) microscopy and optical coherence tomography (OCT). Sci. Rep. 2021, 11, 13723. [Google Scholar] [CrossRef]
- Belykh, E.G.; Zhao, X.; Cavallo, C.; Bohl, M.A.; Yagmurlu, K.; Aklinski, J.L.; Byvaltsev, V.A.; Sanai, N.; Spetzler, R.F.; Lawton, M.T.; et al. Laboratory Evaluation of a Robotic Operative Microscope—Visualization Platform for Neurosurgery. Cureus 2018, 10, e3072. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Xia, Y.; Zhang, X.; Sun, Y.; Tang, J.; Zhang, L.; Liao, H. Optical coherence tomography for precision brain imaging, neurosurgical guidance and minimally invasive theranostics. Biosci. Trends 2018, 12, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Leitgeb, R.A.; Werkmeister, R.M.; Blatter, C.; Schmetterer, L. Doppler optical coherence tomography. Prog. Retin. Eye Res. 2014, 41, 26–43. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; You, J.; Du, C.; Pan, Y. Cranial window implantation on mouse cortex to study microvascular change induced by cocaine. Quant. Imaging Med. Surg. 2015, 5, 97–107. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, Y.; Pradilla, G.; Tyler, B.; Kang, J.U. Real-time intraoperative full-range complex FD-OCT guided cerebral blood vessel identification and brain tumor resection in neurosurgery. In Proceedings of the Photonic Therapeutics and Diagnostics VII, San Francisco, CA, USA, 22–24 January 2011; International Society for Optics and Photonics: Bellingham, DC, USA, 2011; pp. 1–8. [Google Scholar] [CrossRef]
- Yashin, K.S.; Kiseleva, E.B.; Gubarkova, E.V.; Matveev, L.A.; Karabut, M.M.; Elagin, V.V.; Sirotkina, M.A.; Medyanik, I.A.; Kravets, L.Y.; Gladkova, N.D. Multimodal optical coherence tomography for in vivo imaging of brain tissue structure and microvascular network at glioblastoma. In Proceedings of the Clinical and translational neurophotonics, San Francisco, CA, USA, 28–29 January 2017; Volume 10050, p. 100500Z. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, W.J.; Gong, C.-S.A.; Tsai, M.T.; Boppart, S.A.; Wojtkowski, M.; Oh, W.-Y. Characteristics of brain tumor with optical coherence tomography. In Proceedings of the European Conference on Biomedical Optics, München, Germany, 23–27 June 2019; p. 61. [Google Scholar] [CrossRef]
- Yuan, W.; Kut, C.; Liang, W.; Li, X. Robust and fast characterization of OCT-based optical attenuation using a novel frequency-domain algorithm for brain cancer detection. Sci. Rep. 2017, 7, 44909. [Google Scholar] [CrossRef] [PubMed]
- Boppart, S.A.; Brezinski, M.E.; Pitris, C.; Fujimoto, J.G. Optical coherence tomography for neurosurgical imaging of human intracortical melanoma. Neurosurgery 1998, 43, 834–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Jin, Y.; Huang, L.; Guo, S.; Zhao, G.; Chen, X. Iterative fusion convolutional neural networks for classification of optical coherence tomography images. J. Vis. Commun. Image Represent. 2019, 59, 327–333. [Google Scholar] [CrossRef]
- Treder, M.; Lauermann, J.L.; Eter, N. Automated detection of exudative age-related macular degeneration in spectral domain optical coherence tomography using deep learning. Graefe‘s Arch. Clin. Exp. Ophthalmol. 2017, 256, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, A.; Snopova, L.; Kuznetsov, S.; Buyanova, N.; Elagin, V.; Sirotkina, M.; Kiseleva, E.; Matveev, L.; Zaitsev, V.; Feldchtein, F.; et al. Pixel classification method in optical coherence tomography for tumor segmentation and its complementary usage with OCT microangiography. J. Biophotonics 2018, 11, e201700072. [Google Scholar] [CrossRef]
- Singla, N.; Dubey, K.; Srivastava, V. Automated assessment of breast cancer margin in optical coherence tomography images via pretrained convolutional neural network. J. Biophotonics 2018, 12, e201800255. [Google Scholar] [CrossRef]
- Girish, G.N.; Thakur, B.; Chowdhury, S.R.; Kothari, A.R.; Rajan, J. Segmentation of Intra-Retinal Cysts From Optical Coherence Tomography Images Using a Fully Convolutional Neural Network Model. IEEE J. Biomed. Health Inform. 2019, 23, 296–304. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Z.; Wang, Z.; Wen, C.; Jiang, Z.; Yu, Z.; Liu, J.; Liu, G.; Huang, X.; Maier, A.; et al. A deep learning based pipeline for optical coherence tomography angiography. J. Biophotonics 2019, 12, e201900008. [Google Scholar] [CrossRef]
- Lee, C.S.; Tyring, A.J.; Deruyter, N.P.; Wu, Y.; Rokem, A.; Lee, A.Y. Deep-learning based, automated segmentation of macular edema in optical coherence tomography. Biomed. Opt. Express 2017, 8, 3440. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Wang, C.; Li, S.; Rabbani, H.; Chen, X.; Liu, Z. Attention to Lesion: Lesion-Aware Convolutional Neural Network for Retinal Optical Coherence Tomography Image Classification. IEEE Trans. Med. Imaging 2019, 38, 1959–1970. [Google Scholar] [CrossRef]
- Huang, L.; He, X.; Fang, L.; Rabbani, H.; Chen, X. Automatic Classification of Retinal Optical Coherence Tomography Images With Layer Guided Convolutional Neural Network. IEEE Signal Process. Lett. 2019, 26, 1026–1030. [Google Scholar] [CrossRef]
- Prabhu, D.; Bezerra, H.G.; Kolluru, C.; Gharaibeh, Y.; Mehanna, E.; Wu, H.; Wilson, D.L. Automated A-line coronary plaque classification of intravascular optical coherence tomography images using handcrafted features and large datasets. J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef]
- Liew, Y.M.; Chee, K.H.; McLaughlin, R.A.; Tan, L.K.; Yong, Y.L. Linear-regression convolutional neural network for fully automated coronary lumen segmentation in intravascular optical coherence tomography. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef] [Green Version]
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.J. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 2002, 47, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, S.C.; Lin, W.C.; Mahadevan-Jansen, A. In vitro determination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys. Med. Biol. 2006, 51, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, F.; Piguet, D.; Marquet, P.; Gross, J.D.; Tromberg, B.J.; Depeursinge, C. In vivo local determination of tissue optical properties: Applications to human brain. Appl. Opt. 1999, 38, 4939–4950. [Google Scholar] [CrossRef] [Green Version]
- Liewald, D.; Miller, R.; Logothetis, N.; Wagner, H.-J.; Schüz, A. Distribution of axon diameters in cortical white matter: An electron-microscopic study on three human brains and a macaque. Biol. Cybern. 2014, 108, 541–557. [Google Scholar] [CrossRef] [Green Version]
- Morell, P.; Quarles, R.H. Myelin Formation, Structure and Biochemistry. In Basic Neurochemistry, 6th ed.; Raven Press: New York, NY, USA, 1999. [Google Scholar]
- Kiseleva, E.B.; Yashin, K.S.; Moiseev, A.A.; Sirotkina, M.A.; Timofeeva, L.B.; Fedoseeva, V.V.; Alekseeva, A.I.; Medyanik, I.A.; Karyakin, N.N.; Kravets, L.Y.; et al. Cross-Polarization Optical Coherence Tomography in Comparative in vivo and ex vivo Studies of the Optical Properties of Normal and Tumorous Brain Tissues. Sovrem. Tehnol. Med. 2017, 9, 177. [Google Scholar] [CrossRef]
- Rodriguez, C.L.R.; Szu, J.I.; Eberle, M.M.; Wang, Y.; Hsu, M.S.; Binder, D.K.; Park, B.H. Decreased light attenuation in cerebral cortex during cerebral edema detected using optical coherence tomography. Neurophotonics 2014, 1, 025004. [Google Scholar] [CrossRef] [Green Version]
- Tuchin, V.V. Tissue Optics and Photonics: Light-Tissue Interaction. J. Biomed. Photonics Eng. 2015, 1, 98–134. [Google Scholar] [CrossRef]
- Almasian, M.; Wilk, L.S.; Bloemen, P.R.; van Leeuwen, T.G.; ter Laan, M.; Aalders, M.C.G. Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J. Biophotonics 2019, 12, e201900037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, M.S.; Farhang, S.; Tang, R.S.; Desai, N.; Fishman, P.S.; Rohwer, R.G.; Tang, C.-M.; Schmitt, J.M. Optical coherence tomography in the diagnosis and treatment of neurological disorders. J. Biomed. Opt. 2005, 10, 051603. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Wang, R.K. Swept-source optical coherence tomography powered by a 1.3-μm vertical cavity surface emitting laser enables 2.3-mm-deep brain imaging in micein vivo. J. Biomed. Opt. 2015, 20, 106004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, K.; Stein, K.-P.; Neyazi, B.; Sandalcioglu, I.E. First in vivo visualization of the human subarachnoid space and brain cortex via optical coherence tomography. Ther. Adv. Neurol. Disord. 2019, 12, 175628641984304. [Google Scholar] [CrossRef] [Green Version]
- Zoeller, G.K.; Benveniste, R.J.; Landy, H.; Morcos, J.J.; Jagid, J. Outcomes and management strategies after nondiagnostic stereotactic biopsies of brain lesions. Stereotact. Funct. Neurosurg. 2009, 87, 174–181. [Google Scholar] [CrossRef]
- Dammers, R.; Haitsma, I.K.; Schouten, J.W.; Kros, J.M.; Avezaat, C.J.; Vincent, A.J. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir. 2008, 150, 23–29. [Google Scholar] [CrossRef]
- Dammers, R.; Schouten, J.W.; Haitsma, I.K.; Vincent, A.J.; Kros, J.M.; Dirven, C.M. Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochir. 2010, 152, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Tilgner, J.; Herr, M.; Ostertag, C.; Volk, B. Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: Intraoperative versus final diagnosis—Influence of clinical factors. Neurosurgery 2005, 56, 257–265. [Google Scholar] [CrossRef]
- Woodworth, G.; McGirt, M.J.; Samdani, A.; Garonzik, I.; Olivi, A.; Weingart, J.D. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: Comparison of biopsy and open resection specimen. Neurol. Res. 2005, 27, 358–362. [Google Scholar] [CrossRef]
- Heper, A.O.; Erden, E.; Savas, A.; Ceyhan, K.; Erden, I.; Akyar, S.; Kanpolat, Y. An analysis of stereotactic biopsy of brain tumors and nonneoplastic lesions: A prospective clinicopathologic study. Surg. Neurol. 2005, 64 (Suppl. S2), S82–S88. [Google Scholar] [CrossRef]
- Gralla, J.; Nimsky, C.; Buchfelder, M.; Fahlbusch, R.; Ganslandt, O. Frameless stereotactic brain biopsy procedures using the Stealth Station: Indications, accuracy and results. Zent. Neurochir. 2003, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Shooman, D.; Belli, A.; Grundy, P.L. Image-guided frameless stereotactic biopsy without intraoperative neuropathological examination. J. Neurosurg. 2010, 113, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, R.; Sadetzki, S.; Spiegelmann, R.; Ram, Z. Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir. 2005, 147, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Witham, T.F.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. Comprehensive assessment of hemorrhage risks and outcomes after stereotactic brain biopsy. J. Neurosurg. 2001, 94, 545–551. [Google Scholar] [CrossRef]
- Dorward, N.L.; Paleologos, T.S.; Alberti, O.; Thomas, D.G. The advantages of frameless stereotactic biopsy over frame-based biopsy. Br. J. Neurosurg. 2002, 16, 110–118. [Google Scholar] [CrossRef]
- Lunsford, L.D.; Niranjan, A.; Khan, A.A.; Kondziolka, D. Establishing a benchmark for complications using frame-based stereotactic surgery. Stereotact. Funct. Neurosurg. 2008, 86, 278–287. [Google Scholar] [CrossRef]
- Kiseleva, E.B.; Shilyagin, P.A.; Romashov, V.N.; Korzhimanova, Y.V.; Sirotkina, M.A.; Yashin, K.S.; Zagaynova, E.V.; Gelikonov, G.V.; Gladkova, N.D.; Lilge, L.D.; et al. Cross-polarization OCT needle probe for combined blood vessels detection and tissue differentiation during stereotactic biopsy of brain tumors. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 23–25 June 2019; p. 49. [Google Scholar] [CrossRef]
- Lorenser, D.; Yang, X.; Kirk, R.W.; Quirk, B.C.; McLaughlin, R.A.; Sampson, D.D. Ultrathin side-viewing needle probe for optical coherence tomography. Opt. Lett. 2011, 36, 3894–3896. [Google Scholar] [CrossRef]
- Scolaro, L.; Lorenser, D.; McLaughlin, R.A.; Quirk, B.C.; Kirk, R.W.; Sampson, D.D. High-sensitivity anastigmatic imaging needle for optical coherence tomography. Opt. Lett. 2012, 37, 5247–5249. [Google Scholar] [CrossRef]
- McLaughlin, R.A.; Quirk, B.C.; Curatolo, A.; Kirk, R.W.; Scolaro, L.; Lorenser, D.; Robbins, P.D.; Wood, B.A.; Saunders, C.M.; Sampson, D.D. Imaging of Breast Cancer With Optical Coherence Tomography Needle Probes: Feasibility and Initial Results. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1184–1191. [Google Scholar] [CrossRef]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L.; Kateb, B. Review of the potential of optical technologies for cancer diagnosis in neurosurgery: A step toward intraoperative neurophotonics. Neurophotonics 2016, 4, 011010. [Google Scholar] [CrossRef] [Green Version]
- Valdes, P.A.; Roberts, D.W.; Lu, F.K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus 2016, 40, E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martirosyan, N.L.; Georges, J.; Eschbacher, J.M.; Cavalcanti, D.D.; Elhadi, A.M.; Abdelwahab, M.G.; Scheck, A.C.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg. Focus 2014, 36, E16. [Google Scholar] [CrossRef] [PubMed]
- Belykh, E.; Patel, A.A.; Miller, E.J.; Bozkurt, B.; Yağmurlu, K.; Woolf, E.C.; Scheck, A.C.; Eschbacher, J.M.; Nakaji, P.; Preul, M.C. Probe-based three-dimensional confocal laser endomicroscopy of brain tumors: Technical note. Cancer Manag. Res. 2018, 10, 3109–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restelli, F.; Pollo, B.; Vetrano, I.G.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.C.; Petrecca, K.; Leblond, F. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 2015, 7, 274ra219. [Google Scholar] [CrossRef]
- Banerjee, H.; Verma, M. Intraoperative brain cancer detection with Raman spectroscopy in humans. Ann. Transl. Med. 2016, 4, 68. [Google Scholar] [CrossRef]
- Desroches, J.; Jermyn, M.; Pinto, M.; Picot, F.; Tremblay, M.-A.; Obaid, S.; Marple, E.; Urmey, K.; Trudel, D.; Soulez, G.; et al. A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci. Rep. 2018, 8, 1792. [Google Scholar] [CrossRef]
- Sun, Y.; Hatami, N.; Yee, M.; Phipps, J.; Elson, D.S.; Gorin, F.; Schrot, R.J.; Marcu, L. Fluorescence lifetime imaging microscopy for brain tumor image-guided surgery. J. Biomed. Opt. 2010, 15, 056022. [Google Scholar] [CrossRef] [Green Version]
- Butte, P.V.; Mamelak, A.N.; Nuno, M.; Bannykh, S.I.; Black, K.L.; Marcu, L. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. Neuroimage 2011, 54 (Suppl. S1), S125–S135. [Google Scholar] [CrossRef] [Green Version]
- Farkas, D.L.; Nicolau, D.V.; Leif, R.C.; Vasefi, F.; Kittle, D.S.; Nie, Z.; Falcone, C.; Patil, C.G.; Chu, R.M.; Mamelak, A.N.; et al. Intraoperative optical biopsy for brain tumors using spectro-lifetime properties of intrinsic fluorophores. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues IX, San Francisco, CA, USA, 15–17 February 2016; Volume 9711, p. 97111T. [Google Scholar] [CrossRef]
- Lukina, M.; Yashin, K.; Kiseleva, E.E.; Alekseeva, A.; Dudenkova, V.; Zagaynova, E.V.; Bederina, E.; Medyanic, I.; Becker, W.; Mishra, D.; et al. Label-Free Macroscopic Fluorescence Lifetime Imaging of Brain Tumors. Front. Oncol. 2021, 11, 1781. [Google Scholar] [CrossRef]
- Yashin, K.S.; Gubarkova, E.V.; Kiseleva, E.B.; Kuznetsov, S.S.; Karabut, M.M.; Timofeeva, L.B.; Snopova, L.B.; Moiseev, A.A.; Medyanik, I.A.; Kravets, L.Y.; et al. Ex vivo Visualization of Human Gliomas with Cross-Polarization Optical Coherence Tomography: Pilot Study. Sovrem. Tehnol. Med. 2016, 8, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Orillac, C.; Stummer, W.; Orringer, D.A. Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery 2021, 89, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Markwardt, N.A.; Stepp, H.; Franz, G.; Sroka, R.; Goetz, M.; Zelenkov, P.; Rühm, A. Remission spectrometry for blood vessel detection during stereotactic biopsy of brain tumors. J. Biophotonics 2016, 10, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Gobel, W.; Brucker, D.; Kienast, Y.; Johansson, A.; Kniebuhler, G.; Ruhm, A.; Eigenbrod, S.; Fischer, S.; Goetz, M.; Kreth, F.W.; et al. Optical needle endoscope for safe and precise stereotactically guided biopsy sampling in neurosurgery. Opt. Express 2012, 20, 26117–26126. [Google Scholar] [CrossRef] [PubMed]
- Wieser, W.; Biedermann, B.R.; Klein, T.; Eigenwillig, C.M.; Huber, R. Multi-Megahertz OCT: High quality 3D imaging at 20 million A-scans and 45 GVoxels per second. Opt. Express 2010, 18, 14685. [Google Scholar] [CrossRef]
- Bui, B.V.; Kolb, J.P.; Draxinger, W.; Klee, J.; Pfeiffer, T.; Eibl, M.; Klein, T.; Wieser, W.; Huber, R. Live video rate volumetric OCT imaging of the retina with multi-MHz A-scan rates. PLoS ONE 2019, 14, e0213144. [Google Scholar] [CrossRef] [Green Version]
- Münter, M.; vom Endt, M.; Pieper, M.; Casper, M.; Ahrens, M.; Kohlfaerber, T.; Rahmanzadeh, R.; König, P.; Hüttmann, G.; Schulz-Hildebrandt, H. Dynamic contrast in scanning microscopic OCT. Opt. Lett. 2020, 45, 4766. [Google Scholar] [CrossRef]
- Münter, M.; Pieper, M.; Kohlfaerber, T.; Bodenstorfer, E.; Ahrens, M.; Winter, C.; Huber, R.; König, P.; Hüttmann, G.; Schulz-Hildebrandt, H. Microscopic optical coherence tomography (mOCT) at 600 kHz for 4D volumetric imaging and dynamic contrast. Biomed. Opt. Express 2021, 12, 6024. [Google Scholar] [CrossRef]
- Strenge, P.; Lange, B.; Grill, C.; Draxinger, W.; Bonsanto, M.M.; Hagel, C.; Huber, R.; Brinkmann, R.; Izatt, J.A.; Fujimoto, J.G. Segmented OCT data set for depth resolved brain tumor detection validated by histological analysis. In Proceedings of the Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXIV, San Francisco, CA, USA, 3–5 February 2020; p. 96. [Google Scholar] [CrossRef]
- Boppart, S.A.; Herrmann, J.; Pitris, C.; Stamper, D.L.; Brezinski, M.E.; Fujimoto, J.G. High-resolution optical coherence tomography-guided laser ablation of surgical tissue. J. Surg. Res. 1999, 82, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Fan, Y.; Zhang, X.; Liao, H. An Intelligent Theranostics Method Using Optical Coherence Tomography Guided Automatic Laser Ablation for Neurosurgery. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3224–3227. [Google Scholar] [CrossRef]
- Katta, N.; McElroy, A.B.; Estrada, A.D.; Milner, T.E. Optical coherence tomography image-guided smart laser knife for surgery. Lasers Surg. Med. 2018, 50, 202–212. [Google Scholar] [CrossRef]
- Katta, N.; Estrada, A.D.; McElroy, A.B.; Gruslova, A.; Oglesby, M.; Cabe, A.G.; Feldman, M.D.; Fleming, R.Y.D.; Brenner, A.J.; Milner, T.E. Laser brain cancer surgery in a xenograft model guided by optical coherence tomography. Theranostics 2019, 9, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, D. Enhanced Tissue Ablation Efficiency with a Mid-Infrared Nonlinear Frequency Conversion Laser System and Tissue Interaction Monitoring Using Optical Coherence Tomography. Sensors 2016, 16, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Fan, Y.; Hu, C.; Mao, F.; Zhang, X.; Liao, H. Intelligent optical diagnosis and treatment system for automated image-guided laser ablation of tumors. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 2147–2157. [Google Scholar] [CrossRef]


| Study | Type of Study | Study Population | Tissue Type | The Type of Assessment | Sensitivity/Specificity |
|---|---|---|---|---|---|
| Böhringer et al., 2009 [20] | In vivo | 9 patients (Grade 2–4) | Cortex White matter Tumor | qualitative, quantitative | no data, correlation between the scoring of the optical tissue analysis and the result of the histology (χ2 test; r = 0.99). |
| Kut et al., 2015 [24] | In vivo (mice) Ex vivo (human) | in vivo—5 mice ex vivo—32 glioma patients (Grade 2–4) | Cortex White matter Tumor | quantitative color-coded maps | 100/80% for LGG 92/100% for HGG |
| Yashin et al., 2019 [46] | Ex vivo (human) | 30 glioma patients (Grade 2–4) | Cortex White matter Tumor | quantitative color-coded maps | 95.6–90.1/81.3–87.5% for all tumors 100/100% for tumor without necrosis 91.5–81/81–87.5% for tumor with necrosis |
| Yashin et al., 2019 [22] | Ex vivo (human) In vivo (human) | ex vivo—30 glioma patients (Grade 2–4) in vivo—17 glioma patients (Grade 2–4) | Cortex White matter Tumor | qualitative | 82–85/92–94% for LGG/HGG |
| Almasian et al., 2019 [76] | In vivo (human) | 5 patients (Grade 2–4) | Cortex White matter Tumor | quantitative | 100/80% for LGG 92/100% for HGG, |
| Juarez-Chambi et al., 2019 [23] | Ex vivo (human) | 9 patients (Grade 2–4) | Cortex White matter Tumor | quantitative color-coded maps | 90.16/80.95% for LGG 95.45/82.14% for HGG 90.55/82.73% for LGG/HGG |
| iMRI | ioUS | 5-ALA | Raman | Confocal Microscopy | OCT | |
|---|---|---|---|---|---|---|
| Contrast physics | Nuclear magnetic resonance | Sound | Backscattering 5-ALA fluorescence | Raman scattering | Backscattering fluorescence | Backscattering |
| Resolution | 3–20 mm3 | 0.3 mm3 | 0.0001 mm2 | 0.00000025 mm2 | <0.001 mm2 | 0.004 mm3 |
| Penetration | No limit | ~80 mm | ~300–800 µm | ~1 mm | 300–800 µm | ≤2 mm |
| Field of scanning | Whole brain | 12,500 mm3 | 75–2000 mm2 | 0.1225 mm3 | ~0.1 mm2 | 8–16 mm3 |
| Real-time imaging and continuous guidance | No | Yes | Yes | Yes | Yes | Yes |
| Supports label-free | Yes | Yes | No | Yes | Limited | Yes |
| Numerical data | No | No | Yes | Yes | Yes | Yes |
| Surgical microscope integration | No | No | Yes | No | No | Yes |
| Sensitivity | 75% | 88–95% for HGG | 82.6% for HGG | 94% for HGG91% for LGG | 85% for HGG90% for LGG | ~90–100% for LGG~92–95% for HGG |
| Specificity | 96–100% | 62–98% for HGG | 97.4% for HGG | 91% for HGG91% for LGG | 81% for HGG93% for LGG | ~80–100% for LGG~90–100% for HGG |
| GTR achieving | 38–100% | 73.4% | ~76% | No data | No data | No data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yashin, K.; Bonsanto, M.M.; Achkasova, K.; Zolotova, A.; Wael, A.-M.; Kiseleva, E.; Moiseev, A.; Medyanik, I.; Kravets, L.; Huber, R.; et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics 2022, 12, 335. https://doi.org/10.3390/diagnostics12020335
Yashin K, Bonsanto MM, Achkasova K, Zolotova A, Wael A-M, Kiseleva E, Moiseev A, Medyanik I, Kravets L, Huber R, et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics. 2022; 12(2):335. https://doi.org/10.3390/diagnostics12020335
Chicago/Turabian StyleYashin, Konstantin, Matteo Mario Bonsanto, Ksenia Achkasova, Anna Zolotova, Al-Madhaji Wael, Elena Kiseleva, Alexander Moiseev, Igor Medyanik, Leonid Kravets, Robert Huber, and et al. 2022. "OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives" Diagnostics 12, no. 2: 335. https://doi.org/10.3390/diagnostics12020335
APA StyleYashin, K., Bonsanto, M. M., Achkasova, K., Zolotova, A., Wael, A.-M., Kiseleva, E., Moiseev, A., Medyanik, I., Kravets, L., Huber, R., Brinkmann, R., & Gladkova, N. (2022). OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics, 12(2), 335. https://doi.org/10.3390/diagnostics12020335







