Corneal Confocal Microscopy Findings in Neuro Lyme Disease: A Case Report
Abstract
:1. Introduction
2. Case Presentation
2.1. Ocular Examination
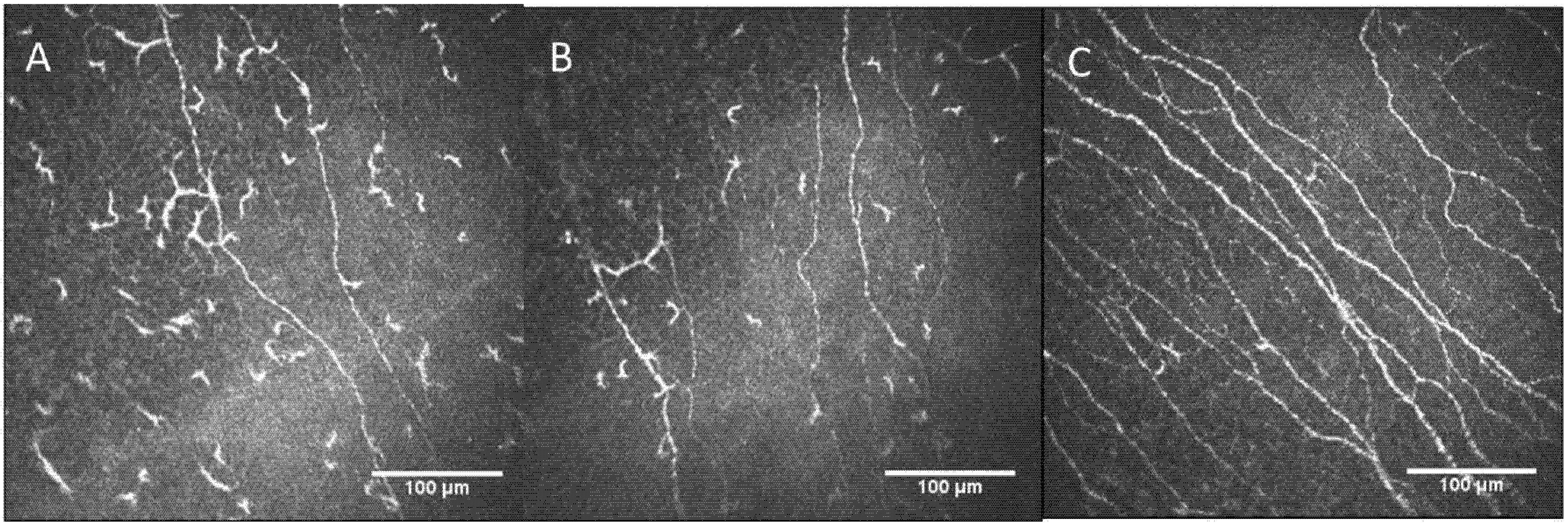
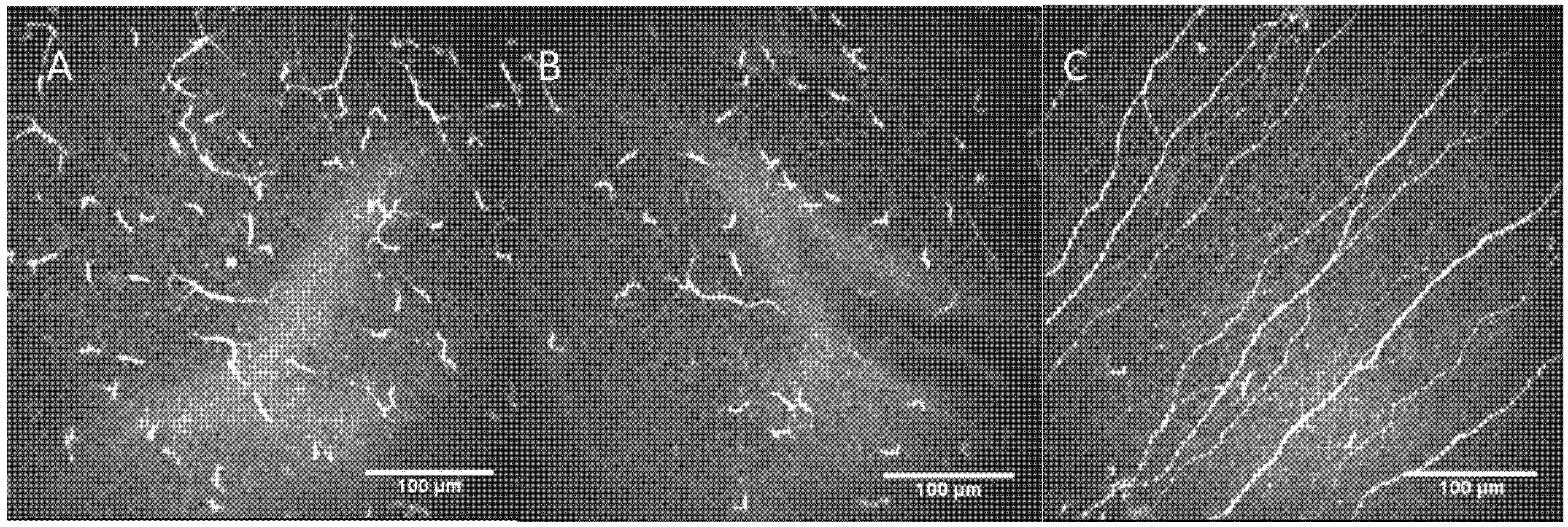
2.2. Confocal Microscopy Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Cutler, S.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Euro Surveill. 2019, 24, 1800170. [Google Scholar] [CrossRef] [Green Version]
- Telford, S.R., 3rd; Goethert, H.K.; Molloy, P.J.; Berardi, V.P.; Chowdri, H.R.; Gugliotta, J.L.; Lepore, T.J. Borrelia miyamotoi Disease: Neither Lyme Disease Nor Relapsing Fever. Clin. Lab. Med. 2015, 35, 867–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Borrelia miyamotoi-An Emerging Human Tick-Borne Pathogen in Europe. Microorganisms 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Burgmann, H.; Stanek, G.; Winkler, S.; Schötta, A.-M.; Obermüller, M.; Markowicz, M.; Lagler, H. Human Borrelia miyamotoi Infection, Austria. Emerg. Infect. Dis. 2020, 26, 2201–2204. [Google Scholar] [CrossRef]
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagemakers, A.; Staarink, P.J.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015, 31, 260–269. [Google Scholar] [CrossRef]
- Stanek, G.; Fingerle, V.; Hunfeld, K.-P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Merola, A.; Rosso, M.; Romagnolo, A.; Comi, C.; Fasano, A.; Zibetti, M.; Lopez-Castellanos, J.R.; Cocito, D.; Lopiano, L.; Espay, A.J. Peripheral neuropathy as marker of severe Parkinson’s disease phenotype. Mov. Disord 2017, 32, 1256–1258. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Leta, V.; Giannoccaro, M.P.; Scaglione, C.; Martinelli, P.; Capellari, S.; Avoni, P.; Baruzzi, A.; Liguori, R. Skin nerve α-synuclein deposits: A biomarker for idiopathic Parkinson disease. Neurology 2014, 82, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Fares, U.; Suleman, H.; Lowe, J.; Dua, H.S. Architecture and distribution of human corneal nerves. Br. J. Ophthalmol. 2010, 94, 784–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, D.V.; McGhee, C.N. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3409–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472. [Google Scholar]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Dabbah, M.A.; Chen, X.; Graham, J.; Ponirakis, G.; Boulton, A.J.M.; et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2071–2078. [Google Scholar] [CrossRef]
- Wang, E.F.; Misra, S.L.; Patel, D.V. In Vivo Confocal Microscopy of the Human Cornea in the Assessment of Peripheral Neuropathy and Systemic Diseases. Biomed. Res. Int. 2015, 2015, 951081. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Marshall, A.; Pitceathly, R.; Gow, D.; Roberts, M.E.; Malik, R.A. Corneal confocal microscopy: A novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp. Neurol. 2010, 223, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, M.; Marshall, A.; Thompson, L.; Kenny, M.; Waldek, S.; Efron, N.; Malik, R. Corneal confocal microscopy: A novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 2009, 40, 976–984. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, M.; Cañadas, P.; Gros-Otero, J.; Rodriguez-Perez, I.; Cañones-Zafra, R.; Kozobolis, V.; Teus, M.A. Long-term corneal subbasal nerve plexus regeneration after laser in situ keratomileusis. J. Cataract Refract. Surg. 2019, 45, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monco, J.C.; Benach, J.L. Lyme Neuroborreliosis: Clinical Outcomes, Controversy, Pathogenesis, and Polymicrobial Infections. Ann. Neurol. 2019, 85, 21–31. [Google Scholar] [CrossRef] [PubMed]
- MacIver, M.B.; Tanelian, D.L. Free nerve ending terminal morphology is fiber type specific for A delta and C fibers innervating rabbit corneal epithelium. J. Neurophysiol. 1993, 69, 1779–1783. [Google Scholar] [CrossRef]
- Gallar, J.; Pozo, M.A.; Tuckett, R.P.; Belmonte, C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat’s cornea. J. Physiol. 1993, 468, 609–622. [Google Scholar] [CrossRef]
- Acosta, M.C.; Tan, M.E.; Belmonte, C.; Gallar, J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2063–2067. [Google Scholar]
- Tanelian, D.L.; Beuerman, R.W. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Exp. Neurol. 1984, 84, 165–178. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Ponirakis, G.; Asghar, O.; Alam, U.; Marshall, A.; Fadavi, H.; Ferdousi, M.; et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: Comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015, 38, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Lukashenko, M.V.; Gavrilova, N.Y.; Bregovskaya, A.V.; Soprun, L.A.; Churilov, L.P.; Petropoulos, I.N.; Malik, R.A.; Shoenfeld, Y. Corneal Confocal Microscopy in the Diagnosis of Small Fiber Neuropathy: Faster, Easier, and More Efficient Than Skin Biopsy? Pathophysiology 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef]
- Tavakoli, M.; Marshall, A.; Banka, S.; Msc, I.N.P.; Fadavi, H.; Kingston, H.; Malik, R. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve 2012, 46, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szalai, E.; Szucs, G.; Szamosi, S.; Aszalos, Z.; Afra, I.; Kemeny-Beke, A. An in vivo confocal microscopy study of corneal changes in patients with systemic sclerosis. Sci. Rep. 2021, 11, 11111. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Culver, D.A.; Ferdousi, M.; Tannemaat, M.; Van Velzen, M.; Dahan, A.; Malik, R.A. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci. Rep. 2018, 8, 4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, B.A.; Santhiago, M.R.; Jorge, P.A.; Kara-José, N.; Moraes, H.V.; Kara-Junior, N. Corneal involvement in systemic inflammatory diseases. Eye Contact Lens 2015, 41, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Nubile, M.; Lanzini, M.; Carpineto, P.; Ciancaglini, M.; Pannellini, T.; DI Nicola, M.; Dua, H.S. Epithelial dendritic cell distribution in normal and inflamed human cornea: In vivo confocal microscopy study. Am. J. Ophthalmol. 2006, 142, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Darabad, D.R.R.; Cruzat, A.; Hajrasouliha, A.R.; Witki, D.; Wong, N.; Dana, R.; Hamrah, P. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7179–7185. [Google Scholar] [CrossRef]
- Alam, U.; Jeziorska, M.; Petropoulos, I.N.; Asghar, O.; Fadavi, H.; Ponirakis, G.; Marshall, A.; Tavakoli, M.; Boulton, A.J.M.; Efron, N.; et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE 2017, 12, e0180175. [Google Scholar] [CrossRef] [Green Version]
| Blood Tests | |
|---|---|
| Darkfield microscopy | Spirochetes forms-like compatible with Borrelia were detected. |
| Phage Borrelia qPCR | Borrelia Miyamotoi positive |
| Elisa e inmunoblot | Negatives |
| Elispot | Positive in B31/basal 20, Outer Surface Proteins (OSP)-mix/basal 13 |
| Basal Blood Pressure | |
| Blood pressure (systolic/diastolic) | 92/63 (mmHg) |
| Heart rate | 71 beats per minute |
| Biochemical Hormonal Specific Study | |
| Hormone values in prone position (20 min) | |
| Noradrenaline: 62 pg/mL Antidiuretic hormone (vasopressin): <1.9 pg/mL | |
| Hormone values in standing position (3 min) | |
| Noradrenaline: 346 pg/mL Antidiuretic hormone (vasopressin): <1.9 pg/mL | |
| Quantitative Sensory Test (QST) | |
| Vibratory sensitivity (A-beta fibers) | Normal |
| Cold sensitivity: (A-delta fibers) | Normal |
| Hot sensitivity (C Fibers) | Normal |
| Sudomotor Function. Electroconductance (ESC) | |
| The mean ESC in inferior limbs | 73 µS with a 0% of mean asymmetry |
| The mean ESC in superior limbs | 72 µS with a 1% mean asymmetry |
| Confocal Microscopy Parameters | Controls | Patient RE | Patient LE |
|---|---|---|---|
| Main nerves’ density (n/image ± SD) | 10.5 ± 2.86 | 3 | 5 |
| Total nerve length (mm/mm2 ± SD) | 14.50 ± 3.6 | 6.64 | 8.27 |
| Nerve branch density (n/image ± SD) | 47.9 ± 26.2 | 0 | 2 |
| Dendritic cells density (n/image ± SD) | 31.3 ± 21.2 | 58 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas, P.; García-Gonzalez, M.; Cañones-Zafra, R.; Teus, M.A. Corneal Confocal Microscopy Findings in Neuro Lyme Disease: A Case Report. Diagnostics 2022, 12, 343. https://doi.org/10.3390/diagnostics12020343
Cañadas P, García-Gonzalez M, Cañones-Zafra R, Teus MA. Corneal Confocal Microscopy Findings in Neuro Lyme Disease: A Case Report. Diagnostics. 2022; 12(2):343. https://doi.org/10.3390/diagnostics12020343
Chicago/Turabian StyleCañadas, Pilar, Montserrat García-Gonzalez, Rafael Cañones-Zafra, and Miguel A. Teus. 2022. "Corneal Confocal Microscopy Findings in Neuro Lyme Disease: A Case Report" Diagnostics 12, no. 2: 343. https://doi.org/10.3390/diagnostics12020343
APA StyleCañadas, P., García-Gonzalez, M., Cañones-Zafra, R., & Teus, M. A. (2022). Corneal Confocal Microscopy Findings in Neuro Lyme Disease: A Case Report. Diagnostics, 12(2), 343. https://doi.org/10.3390/diagnostics12020343






