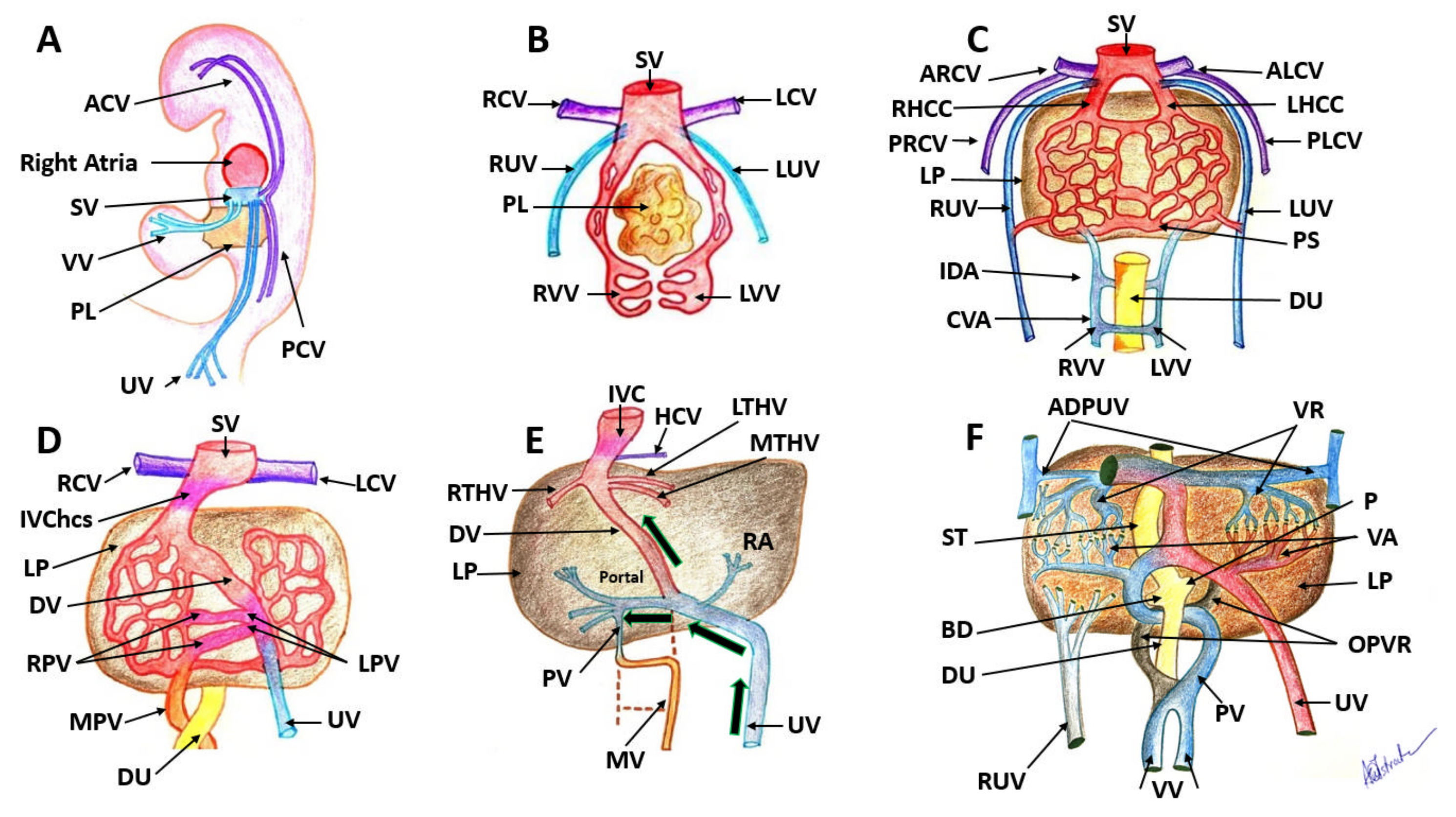
Figure 1.
Embryological development of the human venous system. (A): The embryo demonstrates the development of paired sets of ‘vitelline’ and ‘umbilical’ veins in its fifth week, which initially drain the yolk sac and allantois. (B): At 4 weeks there are three symmetric paired veins: the umbilical veins, vitelline veins and cardinal veins. All three systems converge into the sinus venosus. (C): Liver cords develop into the septum transversum and interrupt the cranial portion of the umbilical and vitelline veins. (D): this is an asymmetric stage with the intrahepatic anastomosis between umbilico–portal and –ductus venosus systems. (E): Changes in the VVs and changes in the UVs continue. Only the most caudal and the most cranial segments of this portion of the VVs will persist. (F): Small venous branches, named venæ advehentes, convey the blood from the subhepatic anastomosis to the sinusoidal plexus; small venous vessels named venæ revehentes, drain the blood of the sinusoidal plexus into the subdiaphragmatic anastomosis. SV: sinus venosus, VV: vitelline veins, PL: primordial liver, UV: umbilical veins, PCV: posterior cardinal veins, ACV: anterior cardinal veins, RUV: right umbilical vein, LUV: left umbilical vein, RVV: right vitelline vein, LVV: left vitelline vein, ARCV: anterior right cardinal vein, ALCV: anterior left cardinal vein, DU: duodenum, PS: portal sinus, IDA: intermediate dorsal anastomosis, CVA: caudal ventral anastomosis, PRCV: posterior right cardinal vein; PLCV: posterior left cardinal vein; RHCC: right hepatic common cardinal vein; IVChcs: inferior vena cava hepatocardiac segment; RPV: right portal vein; LPV: right portal vein; UV: umbilical vein; MPV: main portal vein; LP: liver parenchyma; IVC: inferior vena cava; PV: portal vein; MV: mesenteric vein; RA: ramus angularis; RTHV: right terminal hepatic vein; MTHV: median terminal hepatic vein; LTHV: median terminal hepatic vein; HCV: hepatic cardialis venula; OPVR: obliterated portion of venous rings; BD: bile duct; P: pancreas; VA: venae advehentes; VR: venae revehentes; ADPUV: anterior detached portions of umbilical vein. Images from the collection of Dr Anca-Maria Istrate-Ofiţeru.
Figure 1.
Embryological development of the human venous system. (A): The embryo demonstrates the development of paired sets of ‘vitelline’ and ‘umbilical’ veins in its fifth week, which initially drain the yolk sac and allantois. (B): At 4 weeks there are three symmetric paired veins: the umbilical veins, vitelline veins and cardinal veins. All three systems converge into the sinus venosus. (C): Liver cords develop into the septum transversum and interrupt the cranial portion of the umbilical and vitelline veins. (D): this is an asymmetric stage with the intrahepatic anastomosis between umbilico–portal and –ductus venosus systems. (E): Changes in the VVs and changes in the UVs continue. Only the most caudal and the most cranial segments of this portion of the VVs will persist. (F): Small venous branches, named venæ advehentes, convey the blood from the subhepatic anastomosis to the sinusoidal plexus; small venous vessels named venæ revehentes, drain the blood of the sinusoidal plexus into the subdiaphragmatic anastomosis. SV: sinus venosus, VV: vitelline veins, PL: primordial liver, UV: umbilical veins, PCV: posterior cardinal veins, ACV: anterior cardinal veins, RUV: right umbilical vein, LUV: left umbilical vein, RVV: right vitelline vein, LVV: left vitelline vein, ARCV: anterior right cardinal vein, ALCV: anterior left cardinal vein, DU: duodenum, PS: portal sinus, IDA: intermediate dorsal anastomosis, CVA: caudal ventral anastomosis, PRCV: posterior right cardinal vein; PLCV: posterior left cardinal vein; RHCC: right hepatic common cardinal vein; IVChcs: inferior vena cava hepatocardiac segment; RPV: right portal vein; LPV: right portal vein; UV: umbilical vein; MPV: main portal vein; LP: liver parenchyma; IVC: inferior vena cava; PV: portal vein; MV: mesenteric vein; RA: ramus angularis; RTHV: right terminal hepatic vein; MTHV: median terminal hepatic vein; LTHV: median terminal hepatic vein; HCV: hepatic cardialis venula; OPVR: obliterated portion of venous rings; BD: bile duct; P: pancreas; VA: venae advehentes; VR: venae revehentes; ADPUV: anterior detached portions of umbilical vein. Images from the collection of Dr Anca-Maria Istrate-Ofiţeru.
![Diagnostics 12 00361 g001]()
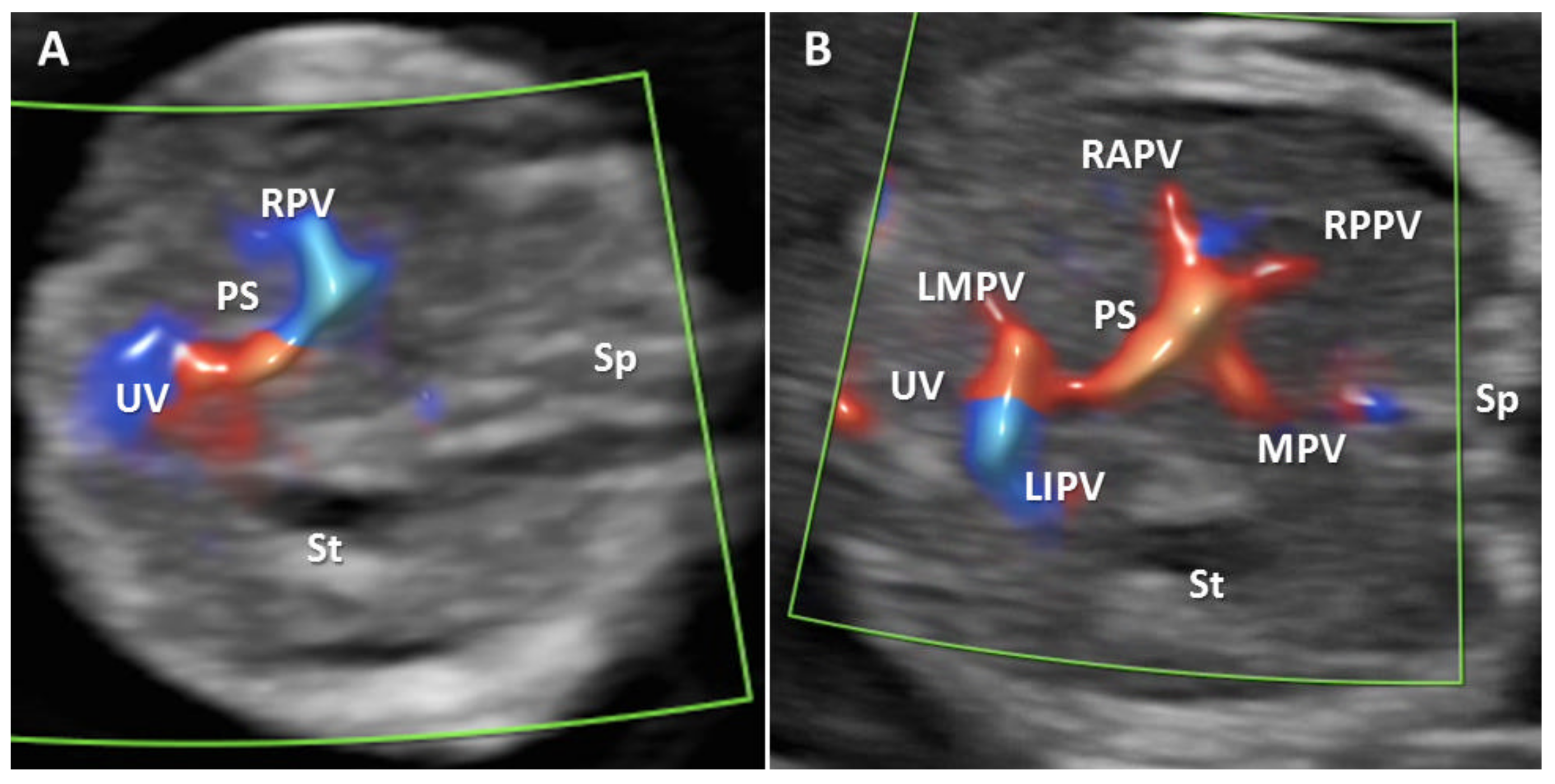
Figure 2.
Transverse plane of the FT fetal abdomen, with high-definition directional power Doppler applied. (A): Transabdominal approach demonstrating the normal L-shaped UV confluence. (B): Transabdominal approach demonstrating all PVS features. MPV main portal vein, PS portal sinus, UV umbilical vein, RAPV anterior branch of right portal vein, RPPV posterior branch of right portal vein, LIPV left portal vein inferior branch; LMPV left portal vein medial branch, St stomach, Ao aorta, Sp spine.
Figure 2.
Transverse plane of the FT fetal abdomen, with high-definition directional power Doppler applied. (A): Transabdominal approach demonstrating the normal L-shaped UV confluence. (B): Transabdominal approach demonstrating all PVS features. MPV main portal vein, PS portal sinus, UV umbilical vein, RAPV anterior branch of right portal vein, RPPV posterior branch of right portal vein, LIPV left portal vein inferior branch; LMPV left portal vein medial branch, St stomach, Ao aorta, Sp spine.
Figure 3.
Agenesis of ductus venosus (ADV) in a first trimester case with umbilical vein drainage into inferior vena cava (IVC) and increased nuchal translucency. (A): Transverse plane of the FT fetal abdomen, with high-definition directional power Doppler applied. An ”H”-shaped variant of the intrahepatic portal veins connection is identified; (B): high-definition directional power Doppler in the sagittal plane of the fetal abdomen (same case) showing ADV with umbilical vein drainage into the inferior vena cava; (C): mid-sagittal view of the fetal face with the measurement of the thickened NT. MPV main portal vein, St stomach, LPV left portal vein, UV umbilical vein, RPVa anterior branch of right portal vein, RPVp posterior branch of right portal vein, Ao aorta, IVC inferior vena cava, ADV ductus venosus agenesis, P palate, NB nasal bone, NT nuchal translucency.
Figure 3.
Agenesis of ductus venosus (ADV) in a first trimester case with umbilical vein drainage into inferior vena cava (IVC) and increased nuchal translucency. (A): Transverse plane of the FT fetal abdomen, with high-definition directional power Doppler applied. An ”H”-shaped variant of the intrahepatic portal veins connection is identified; (B): high-definition directional power Doppler in the sagittal plane of the fetal abdomen (same case) showing ADV with umbilical vein drainage into the inferior vena cava; (C): mid-sagittal view of the fetal face with the measurement of the thickened NT. MPV main portal vein, St stomach, LPV left portal vein, UV umbilical vein, RPVa anterior branch of right portal vein, RPVp posterior branch of right portal vein, Ao aorta, IVC inferior vena cava, ADV ductus venosus agenesis, P palate, NB nasal bone, NT nuchal translucency.
Figure 4.
Absence of the portal system in a first trimester case associated with hygroma and aorto-umbilical fistula. (A): Transverse plane of the upper abdomen with color Doppler applied, showing umbilical cord insertion, stomach, the prominent hepatic artery and no afferent liver venous perfusion; (B): midsagittal plane reconstructed from a three-dimensional volume acquisition were the crown-rump length is measured and fetal cystic hygroma can be observed (white arrow); (C): transverse sonographic view of the neck showing the septated nuchal cystic mass (white arrow); (D): 4D STIC showing in the longitudinal view of the fetal abdomen an abnormal connection (white arrow) between umbilical vein and aorta. (E): same aspects as (D), using two dimensional color Doppler assessment. UV umbilical vein, HA hepatic artery, Ao aorta, St stomach, Sp spine, CHy cystic hygroma, AoUf aorto-umbilical fistula.
Figure 4.
Absence of the portal system in a first trimester case associated with hygroma and aorto-umbilical fistula. (A): Transverse plane of the upper abdomen with color Doppler applied, showing umbilical cord insertion, stomach, the prominent hepatic artery and no afferent liver venous perfusion; (B): midsagittal plane reconstructed from a three-dimensional volume acquisition were the crown-rump length is measured and fetal cystic hygroma can be observed (white arrow); (C): transverse sonographic view of the neck showing the septated nuchal cystic mass (white arrow); (D): 4D STIC showing in the longitudinal view of the fetal abdomen an abnormal connection (white arrow) between umbilical vein and aorta. (E): same aspects as (D), using two dimensional color Doppler assessment. UV umbilical vein, HA hepatic artery, Ao aorta, St stomach, Sp spine, CHy cystic hygroma, AoUf aorto-umbilical fistula.
Figure 5.
Transverse planes at the level of PVS in the second trimester of pregnancy. (A):Transverse plane of the upper abdomen with color Doppler evaluation, showing hepatic course of the umbilical vein (UV), the L-shaped portal sinus (PS), the junction of the PS with the main portal vein (MPV), the left portal vein, the right portal vein and its branches. (B): Normal macroscopically appearance of the liver demonstrating the PS, MPV, UV, LSPV, RAPV, RPPV. St-stomach. Sp- spleen. PS: portal sinus, MPV: main portal vein, UV: umbilical vein, LSPV: left portal vein and the superior branch, RAPV: right anterior portal branch, RPPV: right posterior portal branch. (C): Transverse section in the hepatic parenchyma which allowed the identification of the PS, UV, LSPV, RAPV, RPPV. Classic hematoxylin-eosin staining, ×100.
Figure 5.
Transverse planes at the level of PVS in the second trimester of pregnancy. (A):Transverse plane of the upper abdomen with color Doppler evaluation, showing hepatic course of the umbilical vein (UV), the L-shaped portal sinus (PS), the junction of the PS with the main portal vein (MPV), the left portal vein, the right portal vein and its branches. (B): Normal macroscopically appearance of the liver demonstrating the PS, MPV, UV, LSPV, RAPV, RPPV. St-stomach. Sp- spleen. PS: portal sinus, MPV: main portal vein, UV: umbilical vein, LSPV: left portal vein and the superior branch, RAPV: right anterior portal branch, RPPV: right posterior portal branch. (C): Transverse section in the hepatic parenchyma which allowed the identification of the PS, UV, LSPV, RAPV, RPPV. Classic hematoxylin-eosin staining, ×100.
Figure 6.
Histological appearance of the FT liver with normal PVS. (A): Histopathological aspect of FT fetal liver. It is observed how UV enters the hepatic structure (yellow arrow) and reaches its visceral surface near the intestinal loops; (B): LSPV is identified in the structure of the liver parenchyma, toward its posterior face, in the vicinity of the intestinal loops and the stomach; (C): the L-shaped umbilical vein confluence and the PS (star) is observed in the structure of the liver parenchyma; (D): the two main branches of the RPV are identified as RAPV and RPPV. Classic hematoxylin-eosin staining, ×100. UV: umbilical vein, HP: hepatic parenchyma, INT: intestines, MZT: mesentery, LSPV: left superior portal vein, PS: portal sinus, RPV: right portal vein, MPV: main portal vein, P: pancreas tissue, St: stomach, Ao: aorta, RAPV: right anterior portal vein, RPPV: right posterior portal vein.
Figure 6.
Histological appearance of the FT liver with normal PVS. (A): Histopathological aspect of FT fetal liver. It is observed how UV enters the hepatic structure (yellow arrow) and reaches its visceral surface near the intestinal loops; (B): LSPV is identified in the structure of the liver parenchyma, toward its posterior face, in the vicinity of the intestinal loops and the stomach; (C): the L-shaped umbilical vein confluence and the PS (star) is observed in the structure of the liver parenchyma; (D): the two main branches of the RPV are identified as RAPV and RPPV. Classic hematoxylin-eosin staining, ×100. UV: umbilical vein, HP: hepatic parenchyma, INT: intestines, MZT: mesentery, LSPV: left superior portal vein, PS: portal sinus, RPV: right portal vein, MPV: main portal vein, P: pancreas tissue, St: stomach, Ao: aorta, RAPV: right anterior portal vein, RPPV: right posterior portal vein.
Figure 7.
Histopathological and ultrasound aspect of normal FT liver. (A): Normal histological appearance of the FT liver, demonstrating the UV, LSPV, PS, RAPV, RPPV. Classic hematoxylin-eosin staining, ×100; (B): image processed in negative format which highlights more strongly the elements described in image (A); (C): transverse view of the fetal abdomen on grey scale showing the UV, MPV, PS, RAPV, RPPV, St, Ao. UV: umbilical vein, LSPV: left superior portal vein, PS: portal sinus, RAPV: right anterior portal vein, RPPV: right posterior portal vein, St: stomach, Ao: aorta, Sp: Spleen.
Figure 7.
Histopathological and ultrasound aspect of normal FT liver. (A): Normal histological appearance of the FT liver, demonstrating the UV, LSPV, PS, RAPV, RPPV. Classic hematoxylin-eosin staining, ×100; (B): image processed in negative format which highlights more strongly the elements described in image (A); (C): transverse view of the fetal abdomen on grey scale showing the UV, MPV, PS, RAPV, RPPV, St, Ao. UV: umbilical vein, LSPV: left superior portal vein, PS: portal sinus, RAPV: right anterior portal vein, RPPV: right posterior portal vein, St: stomach, Ao: aorta, Sp: Spleen.
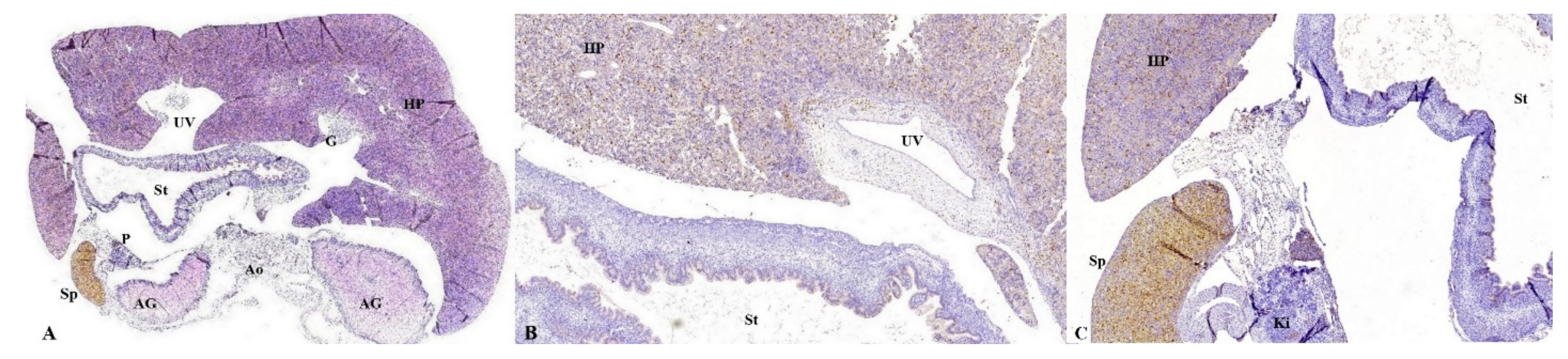
Figure 8.
Anatomic and histopathological aspects of FT organs. (A): Anatomopathological specimen showing fetal liver which occupies most of the abdominal cavity. (B,C): Transverse section at the level of the abdomen. We can identify: the hepatic parenchyma located anteriorly, with the disorganized hepatocyte cords, weakly immunolabeled with CK AE1-AE3, the stomach (St) at which the gastric epithelium was intensely immunolabeled, the pancreas (P) with immunoreactive acinar cells, the two adrenal glands (AG) and one kidney (Ki). Moreover, part of gallbladder (G) are immunolabeled. Immunohistochemical staining with anti-CK antibody AE1-AE3; (B) the hepatic parenchyma (HP) is observed, with the disorganized hepatocyte cords, weakly immunolabeled with CK AE1-AE3, the stomach (St) with the gastric epithelium intensely immunolabeled, the pancreas (P) with immunoreactive acinar cells. Immunohistochemical staining with anti-CK antibody AE1-AE3. CK: Cytokeratin, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, St: stomach, AG: adrenal glands, Ao: Aorta, Ki: kidney, In: intestine, UC: umbilical cord, RHL: right hepatic lobe, LHL: left hepatic lobe, LLL: left lower lobe of the lung.
Figure 8.
Anatomic and histopathological aspects of FT organs. (A): Anatomopathological specimen showing fetal liver which occupies most of the abdominal cavity. (B,C): Transverse section at the level of the abdomen. We can identify: the hepatic parenchyma located anteriorly, with the disorganized hepatocyte cords, weakly immunolabeled with CK AE1-AE3, the stomach (St) at which the gastric epithelium was intensely immunolabeled, the pancreas (P) with immunoreactive acinar cells, the two adrenal glands (AG) and one kidney (Ki). Moreover, part of gallbladder (G) are immunolabeled. Immunohistochemical staining with anti-CK antibody AE1-AE3; (B) the hepatic parenchyma (HP) is observed, with the disorganized hepatocyte cords, weakly immunolabeled with CK AE1-AE3, the stomach (St) with the gastric epithelium intensely immunolabeled, the pancreas (P) with immunoreactive acinar cells. Immunohistochemical staining with anti-CK antibody AE1-AE3. CK: Cytokeratin, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, St: stomach, AG: adrenal glands, Ao: Aorta, Ki: kidney, In: intestine, UC: umbilical cord, RHL: right hepatic lobe, LHL: left hepatic lobe, LLL: left lower lobe of the lung.
![Diagnostics 12 00361 g008]()
Figure 9.
Transverse section at the level of the abdomen. (A): The hepatic parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach (St) with macrophages in the gastric wall, the posterior adrenal glands (AG) and the spleen (Sp), intensely immunolabeled with anti-CD68, which demonstrates the increased number of the macrophages present in the tissue structure. Immunohistochemical staining with anti-CD68 antibody; (B): the hepatic parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach (St) with macrophages in the gastric wall; (C): the hepatic parenchyma (HP) anteriorly, disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach with macrophages in the gastric wall, the posterior kidney (Ki) located posteriorly and the spleen (Sp) on the left, intensely immunolabeled with anti-CD68, which demonstrates increased number of macrophages present in the tissue structure. Immunohistochemical staining with anti-CD68 antibody. CD: Cluster of differentiation, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, P: pancreas, St: stomach, AG: adrenal glands, Ao: Aorta, Sp: spleen, Ki: kidney.
Figure 9.
Transverse section at the level of the abdomen. (A): The hepatic parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach (St) with macrophages in the gastric wall, the posterior adrenal glands (AG) and the spleen (Sp), intensely immunolabeled with anti-CD68, which demonstrates the increased number of the macrophages present in the tissue structure. Immunohistochemical staining with anti-CD68 antibody; (B): the hepatic parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach (St) with macrophages in the gastric wall; (C): the hepatic parenchyma (HP) anteriorly, disorganized hepatocyte cords, with the presence of Kuffer cells among the hepatocyte cords, the stomach with macrophages in the gastric wall, the posterior kidney (Ki) located posteriorly and the spleen (Sp) on the left, intensely immunolabeled with anti-CD68, which demonstrates increased number of macrophages present in the tissue structure. Immunohistochemical staining with anti-CD68 antibody. CD: Cluster of differentiation, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, P: pancreas, St: stomach, AG: adrenal glands, Ao: Aorta, Sp: spleen, Ki: kidney.
![Diagnostics 12 00361 g009]()
Figure 10.
Transverse section at the level of the abdomen. (A): The liver parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, the muscular tunics of the blood vessels immunolabeled with brown in both the liver parenchyma and the great hepatic vessels, the muscular and the middle tunic of the stomach (St), muscular tunic of the aorta (Ao); (B): the hepatic parenchyma, disorganized hepatocyte cords, muscle tunics of the blood vessels immunolabeled with brown in both the hepatic parenchyma (HP) and the great hepatic vessels are observed. Moreover, the muscular and the middle tunic of the stomach (St), muscular tunic of the aorta (Ao) are identified. Immunohistochemical staining with anti-αSMA antibody. αSMA: alpha actin smooth muscle, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, St: stomach, AG: adrenal glands, Ao: Aorta, Sp: spleen.
Figure 10.
Transverse section at the level of the abdomen. (A): The liver parenchyma (HP) located anteriorly, the disorganized hepatocyte cords, the muscular tunics of the blood vessels immunolabeled with brown in both the liver parenchyma and the great hepatic vessels, the muscular and the middle tunic of the stomach (St), muscular tunic of the aorta (Ao); (B): the hepatic parenchyma, disorganized hepatocyte cords, muscle tunics of the blood vessels immunolabeled with brown in both the hepatic parenchyma (HP) and the great hepatic vessels are observed. Moreover, the muscular and the middle tunic of the stomach (St), muscular tunic of the aorta (Ao) are identified. Immunohistochemical staining with anti-αSMA antibody. αSMA: alpha actin smooth muscle, HP: hepatic parenchyma, UV: umbilical vein, G: gallbladder, St: stomach, AG: adrenal glands, Ao: Aorta, Sp: spleen.
Table 1.
Immunohistochemical panel of antibodies used in the study.
Table 1.
Immunohistochemical panel of antibodies used in the study.
| Antibody | Manufacturer | Clone | Antigenic Exposure | Secondary Antibody | Dilution | Labeling |
|---|
| Anti-CK AE1-AE3 | Dako | AE1/AE3 | Citrate, pH 6 | Monoclonal Mouse Anti-Human Cytokeratin | 1:50 | Epithelial tissues |
| Anti-CD68 | Dako | KP1 | Citrate, pH 6 | Monoclonal Mouse Anti-Human
CD68 | 1:100 | Macrophages |
| Anti-αSMA | Dako | 1A4 | Citrate, pH 6 | Monoclonal Mouse Anti-Human Smooth Muscle Actin | 1:100 | α actin smooth muscle |
Table 2.
The comparative satisfactory evaluation rate of the PVS items in the two groups, reasons for not-satisfactory visualization and the results of alternative approaches.
Table 2.
The comparative satisfactory evaluation rate of the PVS items in the two groups, reasons for not-satisfactory visualization and the results of alternative approaches.
| | Identification of All PVS Features by TAUS | Associated Unfavorable Conditions | Visualization of the L-Shaped UV Confluence, TA US | Satisfactory Visualization of PVS Features after Reschedule/TV
Evaluation | Associate Unfavorable Conditions at Reschedule/TV Evaluation | Visualization of the L-Shaped UV Confluence at Reschedule/TV
Evaluation | Final Visualization Rate of PVS Features | Final Visualization Rate of L-Shaped UV Confluence |
|---|
| Group I | 27 (of 100 cases), 27% | Fibroids 3 cases, 4.11% | 91 (of 100 cases) 91% (one atypical, one abnormal-TPVSA) | 61 (of 73 cases), 83.56% | persistent unfavorable fetal position- vertical lie, 5 cases 41.66%) | 7 of 9 cases (77.77%) | 88% | 98% |
| BMI > 24 19 cases, 26.02% |
| unfavorable fetal position 9 cases, 12.32% |
retroverted uterus
9 cases, 12.32% | persistent unfavorable fetal position- far from the probe 6 cases (50%) |
Abdominal scar
8 cases, 10.96% | Istmic fibromyoma 1 case (8.33%) |
| Fibroids and abdominal scar, 4 cases, 5.48% |
| Unfavorable fetal position and increased BMI, 9 cases, 12.32% |
| Increased BMI and abdominal scar, 12 cases, 16.43% |
| Group II | 14 (of 100 cases), 14% | Fibroids 6 cases, 6.97% | 79 (of 100 cases), 79% | 58 (of 86 cases), 67.44% | low-lying fibroids
2 cases, 7.14% | 16 of 21 cases (76.19%) | 72% | 95% |
BMI > 24
22 cases, 25.58% |
unfavorable
fetal position
17 cases, 19.76% | persistent
unfavorable fetal position -vertical lie, 12 cases, 42.85% |
retroverted uterus
16 cases, 18.60% | persistent
unfavorable
fetal position-
fetus far from the probe
14 cases, 50% |
Abdominal scar
4 cases, 4.65% |
| Fibroids and abdominal scar, 3 cases, 3.49% |
| Unfavorable fetal position and increased BMI, 18 cases, 20.93% |