Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Acquisition
2.3. CT Image Reconstruction
2.4. PET Reconstruction
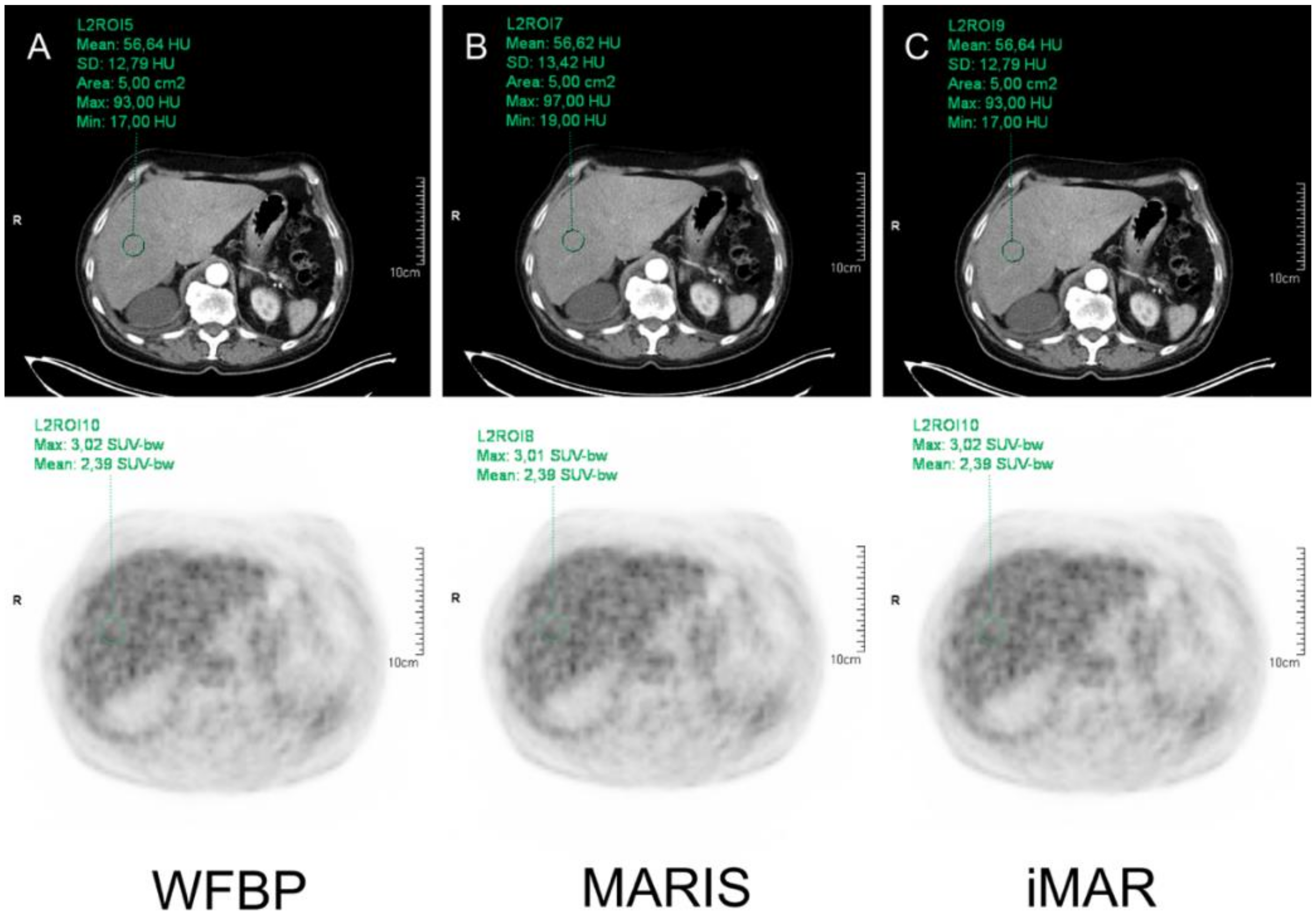
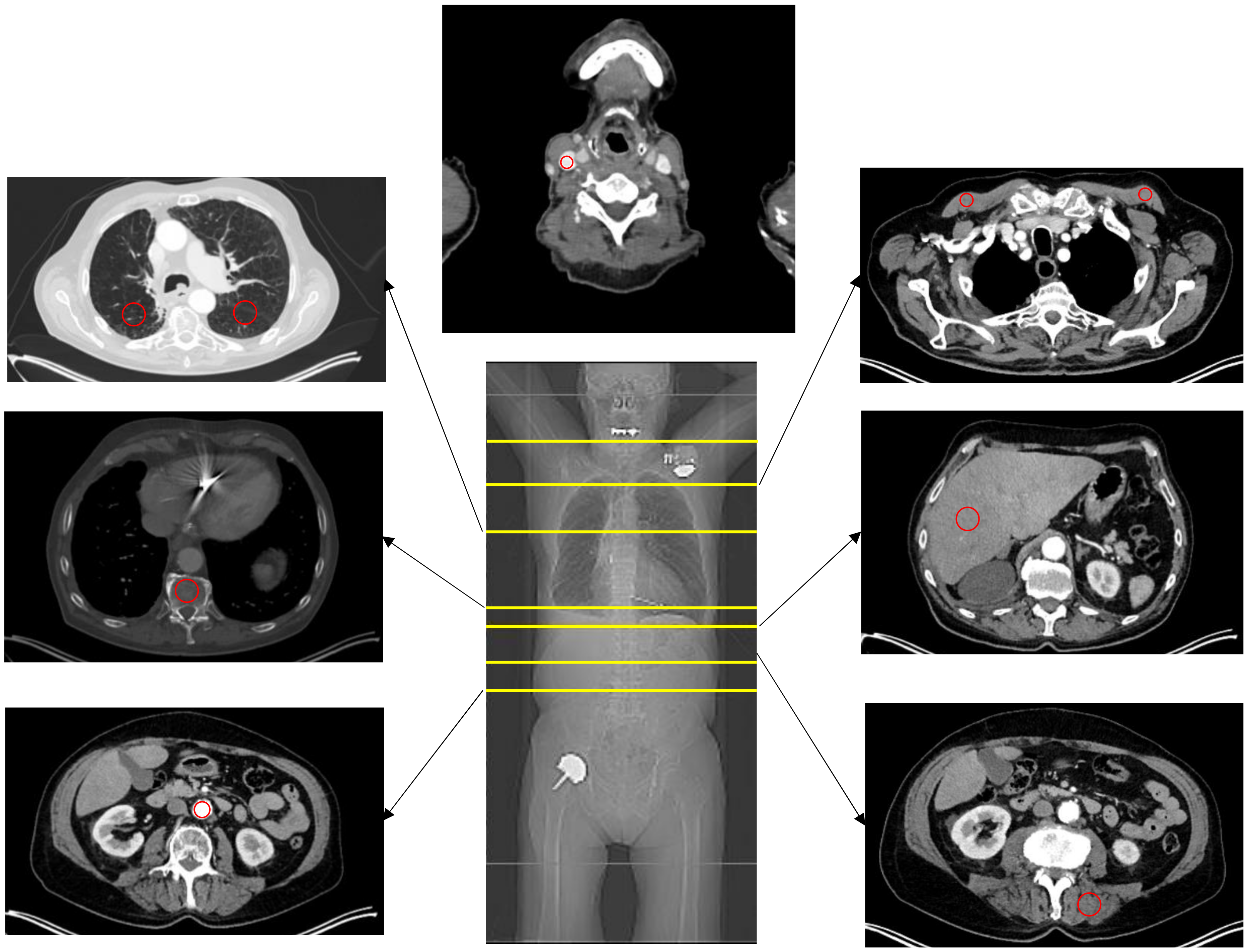
2.5. Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Population
3.2. HU Measurements
3.3. SUV Measurements
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goerres, G.W.; Ziegler, S.I.; Burger, C.; Berthold, T.; Von Schulthess, G.K.; Buck, A. Artifacts at PET and PET/CT caused by metallic hip prosthetic material. Radiology 2003, 226, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; la Fougere, C.; Nikolaou, K.; Pfannenberg, C.; Gatidis, S. Value of CT iterative metal artifact reduction in PET/CT-clinical evaluation in 100 patients. Br. J. Radiol. 2019, 92, 20180756. [Google Scholar] [CrossRef] [PubMed]
- van der Vos, C.S.; Arens, A.I.J.; Hamill, J.J.; Hofmann, C.; Panin, V.Y.; Meeuwis, A.P.W.; Visser, E.P.; de Geus-Oei, L.F. Metal Artifact Reduction of CT Scans to Improve PET/CT. J. Nucl. Med. 2017, 58, 1867–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdoli, M.; Dierckx, R.A.; Zaidi, H. Metal artifact reduction strategies for improved attenuation correction in hybrid PET/CT imaging. Med. Phys. 2012, 39, 3343–3360. [Google Scholar] [CrossRef]
- Nahmias, C.; Lemmens, C.; Faul, D.; Carlson, E.; Long, M.; Blodgett, T.; Nuyts, J.; Townsend, D. Does reducing CT artifacts from dental implants influence the PET interpretation in PET/CT studies of oral cancer and head and neck cancer? J. Nucl. Med. 2008, 49, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Wellenberg, R.H.H.; Hakvoort, E.T.; Slump, C.H.; Boomsma, M.F.; Maas, M.; Streekstra, G.J. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur. J. Radiol. 2018, 107, 60–69. [Google Scholar] [CrossRef]
- Catalano, C.; Francone, M.; Ascarelli, A.; Mangia, M.; Iacucci, I.; Passariello, R. Optimizing radiation dose and image quality. Eur. Radiol. 2007, 17 (Suppl. 6), F26–F32. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Subhas, N.; Primak, A.N.; Obuchowski, N.A.; Gupta, A.; Polster, J.M.; Krauss, A.; Iannotti, J.P. Iterative metal artifact reduction: Evaluation and optimization of technique. Skelet. Radiol. 2014, 43, 1729–1735. [Google Scholar] [CrossRef]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Martin, O.; Aissa, J.; Boos, J.; Wingendorf, K.; Latz, D.; Buchbender, C.; Gaspers, S.; Antke, C.; Sedlmair, M.; Antoch, G.; et al. Impact of different metal artifact reduction techniques on attenuation correction in 18F-FDG PET/CT examinations. Br. J. Radiol. 2020, 93, 20190069. [Google Scholar] [CrossRef]
- Raatikainen, M.J.; Arnar, D.O.; Zeppenfeld, K.; Merino, J.L.; Levya, F.; Hindriks, G.; Kuck, K.H. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace 2015, 17 (Suppl. 1), i1–i75. [Google Scholar] [CrossRef] [Green Version]
- Anzahl der Implantationen Künstlicher Hüftgelenke in Ausgewählten OECD-Ländern in den Jahren 2013 bis 2017 (je 100,000 Einwohner). Available online: https://de.statista.com/statistik/daten/studie/182669/umfrage/hueftgelenksoperationen-in-ausgewaehlten-oecd-laendern/ (accessed on 4 October 2021).
- Gallamini, A.; Fiore, F.; Sorasio, R.; Meignan, M. Interim positron emission tomography scan in Hodgkin lymphoma: Definitions, interpretation rules, and clinical validation. Leuk. Lymphoma 2009, 50, 1761–1764. [Google Scholar] [CrossRef]
- Marcus, C.; Ciarallo, A.; Tahari, A.K.; Mena, E.; Koch, W.; Wahl, R.L.; Kiess, A.P.; Kang, H.; Subramaniam, R.M. Head and neck PET/CT: Therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J. Nucl. Med. 2014, 55, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. 1), 122S–150S. [Google Scholar] [CrossRef] [Green Version]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Mueller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, A.L.; Lymphoma, G.; Eastern Cooperative Oncology, G.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Hagen, M.; Kretschmer, M.; Wurschmidt, F.; Gauer, T.; Giro, C.; Karsten, E.; Lorenzen, J. Clinical relevance of metal artefact reduction in computed tomography (iMAR) in the pelvic and head and neck region: Multi-institutional contouring study of gross tumour volumes and organs at risk on clinical cases. J. Med. Imaging Radiat. Oncol. 2019, 63, 842–851. [Google Scholar] [CrossRef] [Green Version]
- Martin, O.; Boos, J.; Aissa, J.; Vay, C.; Heusch, P.; Gaspers, S.; Antke, C.; Sedlmair, M.; Antoch, G.; Schaarschmidt, B.M. Impact of different iterative metal artifact reduction (iMAR) algorithms on PET/CT attenuation correction after port implementation. Eur. J. Radiol. 2020, 129, 109065. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Israel, O.; Frenkel, A.; Bar-Shalom, R.; Azhari, H. The reduction of artifacts due to metal hip implants in CT-attenuation corrected PET images from hybrid PET/CT scanners. Med. Biol. Eng. Comput. 2007, 45, 553–562. [Google Scholar] [CrossRef]
- Wayer, D.R.; Kim, N.Y.; Otto, B.J.; Grayev, A.M.; Kuner, A.D. Unintended Consequences: Review of New Artifacts Introduced by Iterative Reconstruction CT Metal Artifact Reduction in Spine Imaging. AJNR Am. J. Neuroradiol. 2019, 40, 1973–1975. [Google Scholar] [CrossRef]
- Schabel, C.; Gatidis, S.; Bongers, M.; Huttig, F.; Bier, G.; Kupferschlaeger, J.; Bamberg, F.; la Fougere, C.; Nikolaou, K.; Pfannenberg, C. Improving CT-Based PET Attenuation Correction in the Vicinity of Metal Implants by an Iterative Metal Artifact Reduction Algorithm of CT Data and Its Comparison to Dual-Energy-Based Strategies: A Phantom Study. Invest. Radiol. 2017, 52, 61–65. [Google Scholar] [CrossRef]
- Aissa, J.; Boos, J.; Sawicki, L.M.; Heinzler, N.; Krzymyk, K.; Sedlmair, M.; Kropil, P.; Antoch, G.; Thomas, C. Iterative metal artefact reduction (MAR) in postsurgical chest CT: Comparison of three iMAR-algorithms. Br. J. Radiol. 2017, 90, 20160778. [Google Scholar] [CrossRef]
- Rehfeld, N.S.; Heismann, B.J.; Kupferschlager, J.; Aschoff, P.; Christ, G.; Pfannenberg, A.C.; Pichler, B.J. Single and dual energy attenuation correction in PET/CT in the presence of iodine based contrast agents. Med. Phys. 2008, 35, 1959–1969. [Google Scholar] [CrossRef]
- Sai, K.K.S.; Zachar, Z.; Bingham, P.M.; Mintz, A. Metabolic PET Imaging in Oncology. AJR Am. J. Roentgenol. 2017, 209, 270–276. [Google Scholar] [CrossRef]
| HU | SUVmax | SUVmean | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WFBP | MARIS | iMAR | WFBP | MARIS | iMAR | WFBP | MARIS | iMAR | ||
| Liver | 82.72 ± 26.95 | 82.80 ± 26.94 | 82.69 ± 26.92 | 3.05 ± 0.61 | 3.04 ± 0.61 | 3.05 ± 0.61 | 2.41 ± 0.46 | 2.40 ± 0.46 | 2.41 ± 0.46 | |
| p-value | WFBP vs. MARIS | 0.295 | 0.095 | 0.353 | ||||||
| WFBP vs. iMAR | 0.055 | 0.568 | 0.159 | |||||||
| MARIS vs. iMAR | 0.157 | 0.084 | 0.278 | |||||||
| Right lung | −685.79 ± 103.02 | −684.41 ± 104.38 | −685.94 ± 103.12 | 0.93 ± 0.37 | 0.92 ± 0.37 | 0.93 ± 0.37 | 0.69 ± 0.27 | 0.69 ± 0.27 | 0.69 ± 0.27 | |
| p-value | WFBP vs. MARIS | 0.229 | 0.603 | 0.536 | ||||||
| WFBP vs. iMAR | 0.276 | 1.0 | 0.568 | |||||||
| MARIS vs. iMAR | 0.179 | 0.602 | 0.480 | |||||||
| Left lung | −679.63 ± 95.31 | −679.63 ± 95.31 | −679.63 ± 95.31 | 0.91 ± 0.38 | 0.91 ± 0.38 | 0.91 ± 0.38 | 0.67 ± 0.26 | 0.67 ± 0.26 | 0.67 ± 0.26 | |
| p-value | WFBP vs. MARIS | 1.0 | 0.049 | 0.418 | ||||||
| WFBP vs. iMAR | 0.630 | 0.109 | 0.045 | |||||||
| MARIS vs. iMAR | 0.629 | 0.251 | 0.159 | |||||||
| Vertebral body | 157.06 ± 67.58 | 157.38 ± 64.33 | 157.08 ± 67.53 | 2.56 ± 1.09 | 2.56 ± 1.09 | 2.54 ± 1.11 | 1.97 ± 0.73 | 1.96 ± 0.72 | 1.96 ± 0.72 | |
| p-value | WFBP vs. MARIS | 0.835 | 0.871 | 0.412 | ||||||
| WFBP vs. iMAR | 0.725 | 0.392 | 0.515 | |||||||
| MARIS vs. iMAR | 0.837 | 0.383 | 0.490 | |||||||
| Descending aorta | 119.91 ± 56.20 | 119.75 ± 56.11 | 119.68 ± 56.13 | 2.28 ± 0.49 | 2.27 ± 0.52 | 2.28 ± 0.49 | 1.86 ± 0.37 | 1.86 ± 0.38 | 1.86 ± 0.37 | |
| p-value | WFBP vs. MARIS | 0.038 | 0.241 | 0.080 | ||||||
| WFBP vs. iMAR | 0.333 | 0.083 | 0.159 | |||||||
| MARIS vs. iMAR | 0.801 | 0.229 | 0.109 | |||||||
| Autochthonous back muscles | 43.79 ± 17.66 | 44.05 ± 17.58 | 44.93 ± 15.06 | 0.91 ± 0.28 | 0.89 ± 0.20 | 0.91 ± 0.25 | 0.72 ± 0.20 | 0.70 ± 0.13 | 0.71 ± 0.19 | |
| p-value | WFBP vs. MARIS | 0.164 | 0.316 | 0.342 | ||||||
| WFBP vs. iMAR | 0.281 | 0.839 | 0.937 | |||||||
| MARIS vs. iMAR | 0.402 | 0.306 | 0.322 | |||||||
| Pectoral muscle (right) | 51.31 ± 13.97 | 51.14 ± 13.87 | 51.01 ± 13.06 | 0.79 ± 0.28 | 0.78 ± 0.28 | 0.79 ± 0.28 | 0.66 ± 0.26 | 0.66 ± 0.26 | 0.66 ± 0.26 | |
| p-value | WFBP vs. MARIS | 0.055 | 0.097 | 0.117 | ||||||
| WFBP vs. iMAR | 0.481 | 0.277 | 0.410 | |||||||
| MARIS vs. iMAR | 0.767 | 0.047 | 0.070 | |||||||
| Pectoral muscle (left) | 53.57 ± 14.42 | 53.38 ± 14.66 | 52.05 ± 10.16 | 0.78 ± 0.24 | 0.78 ± 0.23 | 0.78 ± 0.24 | 0.65 ± 0.20 | 0.65 ± 0.20 | 0.65 ± 0.20 | |
| p-value | WFBP vs. MARIS | 0.184 | 0.054 | 0.223 | ||||||
| WFBP vs. iMAR | 0.313 | 0.859 | 0.260 | |||||||
| MARIS vs. iMAR | 0.393 | 0.029 | 0.028 | |||||||
| Jugular vein | 152.85 ± 41.89 | 152.93 ± 41.83 | 153.34 ± 45.30 | 1.85 ± 0.39 | 1.84 ± 0.39 | 1.85 ± 0.39 | 1.63 ± 0.37 | 1.64 ± 0.39 | 1.64 ± 0.38 | |
| p-value | WFBP vs. MARIS | 0.435 | 0.512 | 0.169 | ||||||
| WFBP vs. iMAR | 0.343 | 0.194 | 0.332 | |||||||
| MARIS vs. iMAR | 0.362 | 0.251 | 0.328 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawitz, J.; Martin, O.; Boos, J.; Sawicki, L.M.; Wingendorf, K.; Sedlmair, M.; Mamlins, E.; Antke, C.; Antoch, G.; Schaarschmidt, B.M. Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT. Diagnostics 2022, 12, 375. https://doi.org/10.3390/diagnostics12020375
Morawitz J, Martin O, Boos J, Sawicki LM, Wingendorf K, Sedlmair M, Mamlins E, Antke C, Antoch G, Schaarschmidt BM. Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT. Diagnostics. 2022; 12(2):375. https://doi.org/10.3390/diagnostics12020375
Chicago/Turabian StyleMorawitz, Janna, Ole Martin, Johannes Boos, Lino M. Sawicki, Katrin Wingendorf, Martin Sedlmair, Eduards Mamlins, Christina Antke, Gerald Antoch, and Benedikt M. Schaarschmidt. 2022. "Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT" Diagnostics 12, no. 2: 375. https://doi.org/10.3390/diagnostics12020375
APA StyleMorawitz, J., Martin, O., Boos, J., Sawicki, L. M., Wingendorf, K., Sedlmair, M., Mamlins, E., Antke, C., Antoch, G., & Schaarschmidt, B. M. (2022). Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT. Diagnostics, 12(2), 375. https://doi.org/10.3390/diagnostics12020375






