Acute Retinal Necrosis: Signs, Treatment, Complications and Outcome
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandorfer, A.; Thurau, S. Acute retinal necrosis. Ophthalmology 2009, 106, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N. Standard Diagnostic Criteria for the Acute Retinal Necrosis Syndrome. Am. J. Ophthalmol 1994, 117, 663–666. [Google Scholar] [CrossRef]
- Bonfioli, A.A.; Eller, A.W. Acute retinal necrosis. Semin. Ophthalmol. 2005, 20, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Rautenberg, P.; Grancicova, L.; Hillenkamp, J.; Nölle, B.; Roider, J.; Fickenscher, H. Acute retinal necrosis from the virologist’s perspective. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2009, 106, 1065–1073. [Google Scholar]
- Cochrane, T.F.; Silvestri, G.; McDowell, C.; Foot, B.E.; McAvoy, C. Acute retinal necrosis in the United Kingdom: Results of a prospective surveillance study. Eye 2012, 26, 370–378. [Google Scholar] [CrossRef]
- Freeman, W.R.; Lerner, C.W.; Mines, J.A.; Lash, R.S.; Nadel, A.J.; Starr, M.B.; Tapper, M.L. A Prospective Study of the Ophthalmologic Findings in the Acquired Immune Deficiency Syndrome. Am. J. Ophthalmol. 1984, 97, 133–142. [Google Scholar] [CrossRef]
- Freeman, W.R.; O’Connor, G.R. Acquired Immune Deficiency Syndrome Retinopathy, Pneumocystis, and Cotton-Wool Spots. Am. J. Ophthalmol. 1984, 98, 235–237. [Google Scholar] [CrossRef]
- Pleyer, U.; Metzner, S.; Hofmann, J. Diagnostics and differential diagnosis of acute retinal necrosis. Ophthalmol. Z. Dtsch. Ophthalmol. Ges. 2009, 106, 1074–1082. [Google Scholar]
- Hillenkamp, J. Diagnosis and therapy of acute retinal necrosis. Ophthalmol. Z. Dtsch. Ophthal-Mologischen Ges. 2009, 106, 1057. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Jiang, R.; Wang, Z.; Xu, G.; Wu, X.; Zhou, M. Bilateral Acute Retinal Necrosis: A Case Series. Retina 2020, 40, 145–153. [Google Scholar] [CrossRef]
- Williams, A.M.; Nguyen, V.Q.; Botsford, B.W.; Eller, A.W. Bilateral acute retinal necrosis caused by two separate viral etiologies. Am. J. Ophthalmol. Case Rep. 2020, 18, 100636. [Google Scholar] [CrossRef] [PubMed]
- Young, N.J.; Bird, A.C. Bilateral acute retinal necrosis. Br. J. Ophthalmol. 1978, 62, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Palay, D.A.; Sternberg, P., Jr.; Davis, J.; Lewis, H.; Holland, G.N.; Mieler, W.F.; Jabs, D.A.; Drews, C. Decrease in the Risk of Bilateral Acute Retinal Necrosis by Acyclovir Therapy. Am. J. Ophthalmol. 1991, 112, 250–255. [Google Scholar] [CrossRef]
- Lau, C.H.; Missotten, T.; Salzmann, J.; Lightman, S.L. Acute Retinal Necrosis: Features, Management, and Outcomes. Ophthalmology 2007, 114, 756–762.e1. [Google Scholar] [CrossRef]
- Butler, N.J.; Moradi, A.; Salek, S.S.; Burkholder, B.M.; Leung, T.G.; Dunn, J.P.; Thorne, J.E. Acute Retinal Necrosis: Presenting Characteristics and Clinical Outcomes in a Cohort of Polymerase Chain Reaction–Positive Patients. Am. J. Ophthalmol. 2017, 179, 179–189. [Google Scholar] [CrossRef]
- Muthiah, M.N.; Michaelides, M.; Child, C.S.; Mitchell, S.M. Acute retinal necrosis: A national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br. J. Ophthalmol. 2007, 91, 1452–1455. [Google Scholar] [CrossRef] [Green Version]
- Hillenkamp, J.; Nölle, B.; Bruns, C.; Rautenberg, P.; Fickenscher, H.; Roider, J. Acute Retinal Necrosis: Clinical Features, Early Vitrectomy, and Outcomes. Ophthalmology 2009, 116, 1971–1975.e2. [Google Scholar] [CrossRef]
- Yeh, S.; Suhler, E.B.; Smith, J.R.; Bruce, B.; Fahle, G.; Bailey, S.T.; Hwang, T.S.; Stout, J.T.; Lauer, A.K.; Wilson, D.J.; et al. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 399–407. [Google Scholar] [CrossRef]
- Lachin, J.M. Worst-Rank Score Methods—A Nonparametric Approach to Informatively Missing Data. J. Am. Med. Assoc. 2020, 324, 1670–1671. [Google Scholar] [CrossRef]
- Urayama, A. Unilateral acute uveitis with retinal periarteritis and detachment. Rinsho Ganka (Jpn. J. Clin. Ophthalmol.) 1971, 25, 607–619. [Google Scholar]
- Culbertson, W.W.; Blumenkranz, M.S.; Haines, H.; Gass, J.D.M.; Mitchell, K.B.; Norton, E.W. The acute retinal necrosis syndrome: Part 2: Histopathology and etiology. Ophthalmology 1982, 89, 1317–1325. [Google Scholar] [CrossRef]
- Falcone, P.M.; Brockhurst, R.J. Delayed onset of bilateral acute retinal necrosis syndrome: A 34-year interval. Ann. Ophthalmol. 1993, 25, 373–374. [Google Scholar] [PubMed]
- Forster, D.J.; Dugel, P.U.; Frangieh, G.T.; Liggett, P.E.; Rao, N.A. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 1990, 110, 341–348. [Google Scholar] [CrossRef]
- Matsuo, T.; Morimoto, K.; Matsuo, N. Factors associated with poor visual outcome in acute retinal necrosis. Br. J. Ophthalmol. 1991, 75, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, T.W.; Miller, D.; Schiffman, J.C.; Davis, J.L. Polymerase Chain Reaction Analysis of Aqueous and Vitreous Specimens in the Diagnosis of Posterior Segment Infectious Uveitis. Am. J. Ophthalmol. 2009, 147, 140–147.e2. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; Velhagen, K.; Pleyer, U. Acute retinal necrosis syndrome: Analysis, therapy and long-term follow up of 14 eyes. Klin. Mon. Augenheilkd. 2000, 217, 345–350. [Google Scholar] [CrossRef]
- Gargiulo, F.; De Francesco, M.A.; Nascimbeni, G.; Turano, R.; Perandin, F.; Gandolfo, E.; Manca, N. Polymerase chain reaction as a rapid diagnostic tool for therapy of acute retinal necrosis syndrome. J. Med. Virol. 2003, 69, 397–400. [Google Scholar] [CrossRef]
- Aizman, A.; Johnson, M.W.; Elner, S.G. Treatment of Acute Retinal Necrosis Syndrome with Oral Antiviral Medications. Ophthalmology 2007, 114, 307–312. [Google Scholar] [CrossRef]
- Emerson, G.G.; Smith, J.R.; Wilson, D.J.; Rosenbaum, J.T.; Flaxel, C.J. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology 2006, 113, 2259–2261. [Google Scholar] [CrossRef]
- Tibbetts, M.D.; Shah, C.P.; Young, L.; Duker, J.S.; Maguire, J.I.; Morley, M.G. Treatment of Acute Retinal Necrosis. Ophthalmology 2010, 117, 818–824. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Spencer, D.B.; Mochizuki, M. Therapy for acute retinal necrosis. In Seminars in Ophthalmology; Taylor & Francis: Tokyo, Japan, 2008. [Google Scholar]
- Kopplin, L.J.; Thomas, A.S.; Cramer, S.; Kim, Y.H.; Yeh, S.; Lauer, A.K.; Flaxel, C.J. Long-Term Surgical Outcomes of Retinal Detachment Associated With Acute Retinal Necrosis. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.P.; Pappuru, R.R.; Pathengay, A.; Tyagi, M.; Narayanan, R.; Jalali, S. Vitrectomy with Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment following Acute Retinal Necrosis: Clinical Outcomes and Prognostic Factors. In Seminars in Ophthalmology; Taylor & Francis: Tokyo, Japan, 2019. [Google Scholar] [CrossRef]
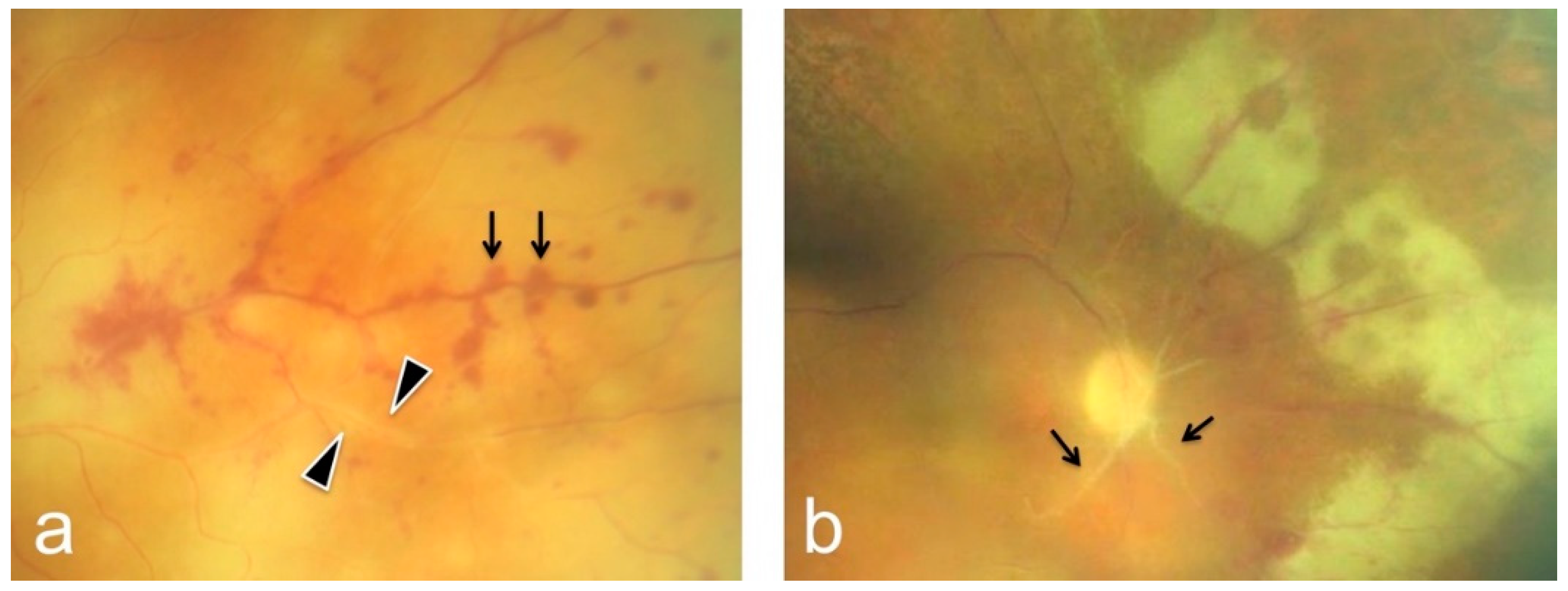
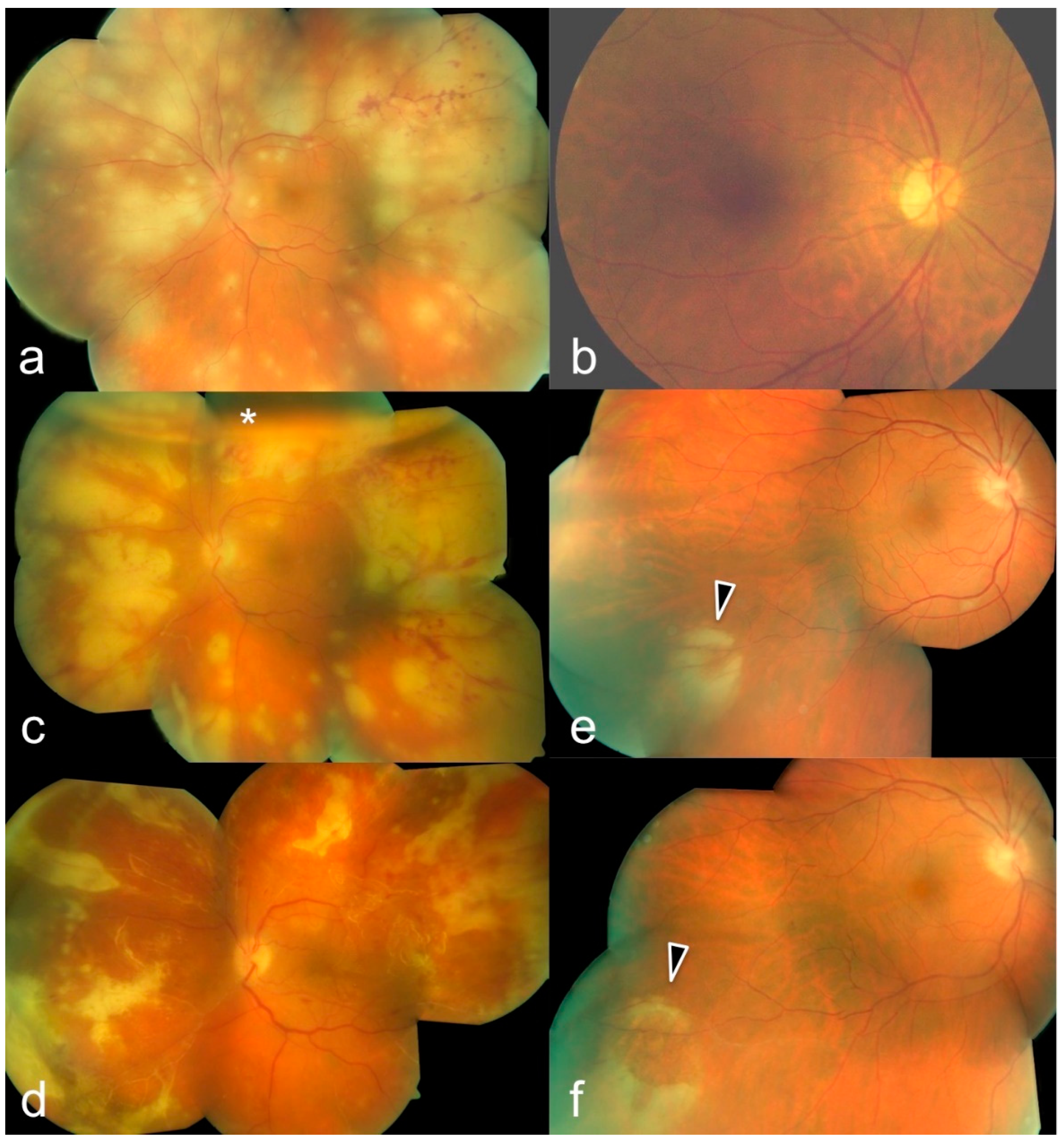
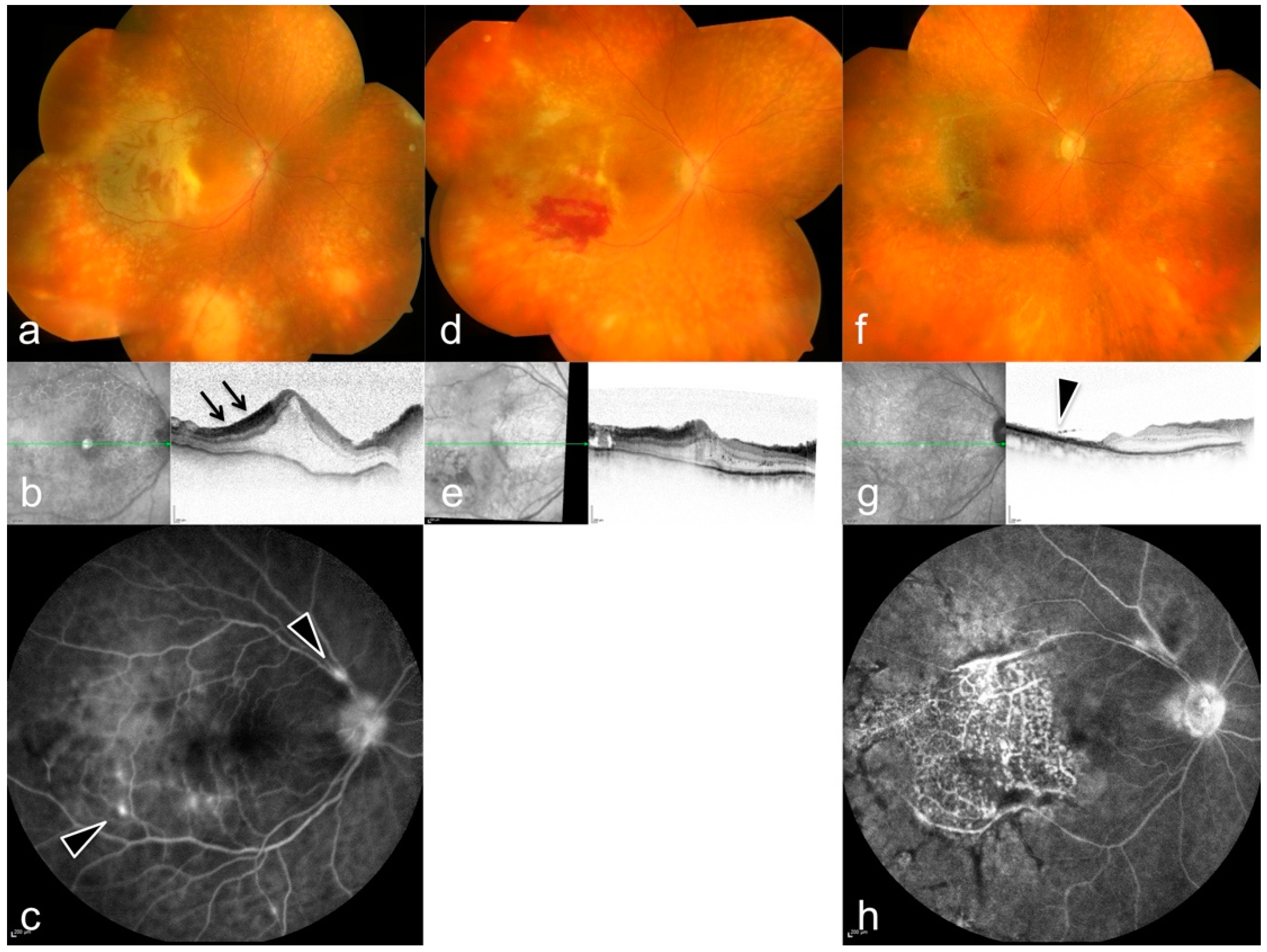
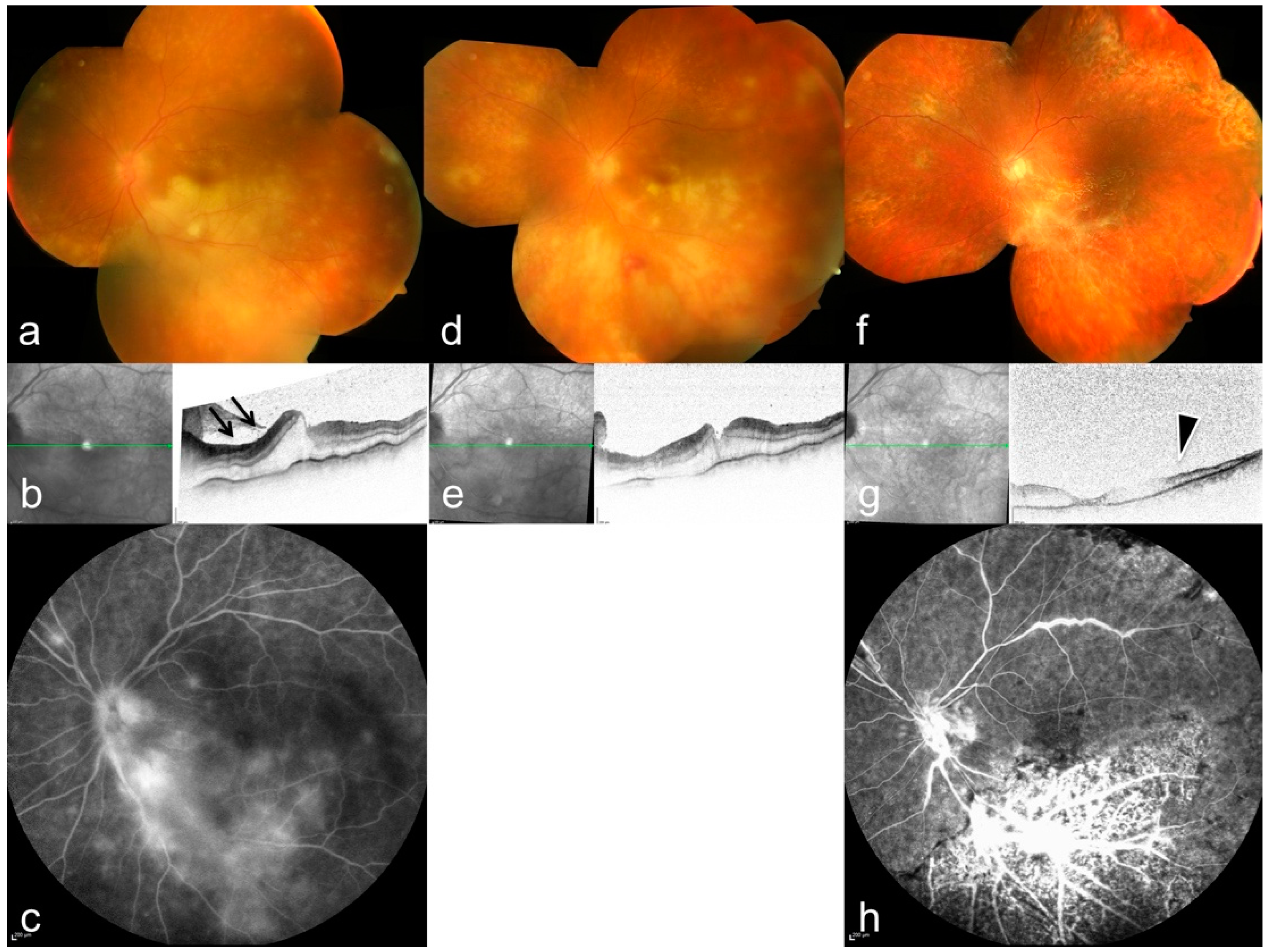
| # | Sex | Age | Eye | BCVA at Onset | Local Tx | Systemic Tx | Pathogen | Complications | Timepoint of 2nd Involvement | BCVA after 3 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 53 | OD | 0.6 | vitrectomy | Aciclovir | HSV | none | 3 months later | 0.2 |
| OS | 0.2 | vitrectomy | IOL luxation | - | 0.1 | |||||
| 2 | M | 54 | OD | 0.5 | IVT Ganciclovir | Aciclovir | EBV, VZV | none | 7 weeks later | 0.8 |
| OS | 0.1 | vitrectomy + IVT | cataract surgery | - | 0.2 | |||||
| 3 | M | 65 | OS | 0.063 | IVT Ganciclovir | Aciclovir | HSV | multiple retinal detachments | - | hand motion |
| 4 | M | 59 | OD | 1.0 | IVT Ganciclovir | Aciclovir | VZV | none | 1 month later | 1.0 |
| OS | 0.6 | vitrectomy + IVT Ganciclovir | retinal detachment | - | 0.2 | |||||
| 5 | M | 66 | OD | 0.3 | IVT Ganciclovir | Aciclovir | VZV | retinal detachment | - | 0.2 |
| 6 | F | 86 | OD | 0.4 | vitrectomy + IVT Ganciclovir | Aciclovir | - | Vitreous hemorrage, vitr. Silicone oil tamponade, endophthalmitis, secondary glaucoma, retinal branch occlusion | same time | 0.4 |
| OS | 0.1 | vitrectomy + IVT Ganciclovir | Vitreous hemorrage, vitr. Silicone oil tamponade | - | 0.5 | |||||
| 7 | F | 76 | OD | 0.5 | IVT Ganciclovir | Aciclovir | CMV | none | same time | light perception |
| OS | 0.1 | vitrectomy + IVT Ganciclovir | Recurrence 3 months later | - | light perception | |||||
| 8 | F | 77 | OD | 0.4 | IVT Aciclovir | Aciclovir | VZV | none | same time | 0.5 |
| OS | hand motion | vitrectomy + IVT Ganciclovir | retinal detachment | - | counting fingers | |||||
| 9 | F | 60 | OS | hand motion | vitrectomy + IVT Ganciclovir | Aciclovir | VZV | retinal detachment | - | light perception |
| 10 | F | 32 | OD | 0.6 | IVT Ganciclovir | Aciclovir | VZV | retinal detachment | - | counting fingers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, C.S.; Blobner, K.; Storr, J.; Baur, I.D.; Khoramnia, R. Acute Retinal Necrosis: Signs, Treatment, Complications and Outcome. Diagnostics 2022, 12, 386. https://doi.org/10.3390/diagnostics12020386
Mayer CS, Blobner K, Storr J, Baur ID, Khoramnia R. Acute Retinal Necrosis: Signs, Treatment, Complications and Outcome. Diagnostics. 2022; 12(2):386. https://doi.org/10.3390/diagnostics12020386
Chicago/Turabian StyleMayer, Christian S., Katharina Blobner, Julia Storr, Isabella D. Baur, and Ramin Khoramnia. 2022. "Acute Retinal Necrosis: Signs, Treatment, Complications and Outcome" Diagnostics 12, no. 2: 386. https://doi.org/10.3390/diagnostics12020386
APA StyleMayer, C. S., Blobner, K., Storr, J., Baur, I. D., & Khoramnia, R. (2022). Acute Retinal Necrosis: Signs, Treatment, Complications and Outcome. Diagnostics, 12(2), 386. https://doi.org/10.3390/diagnostics12020386







