Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment
Abstract
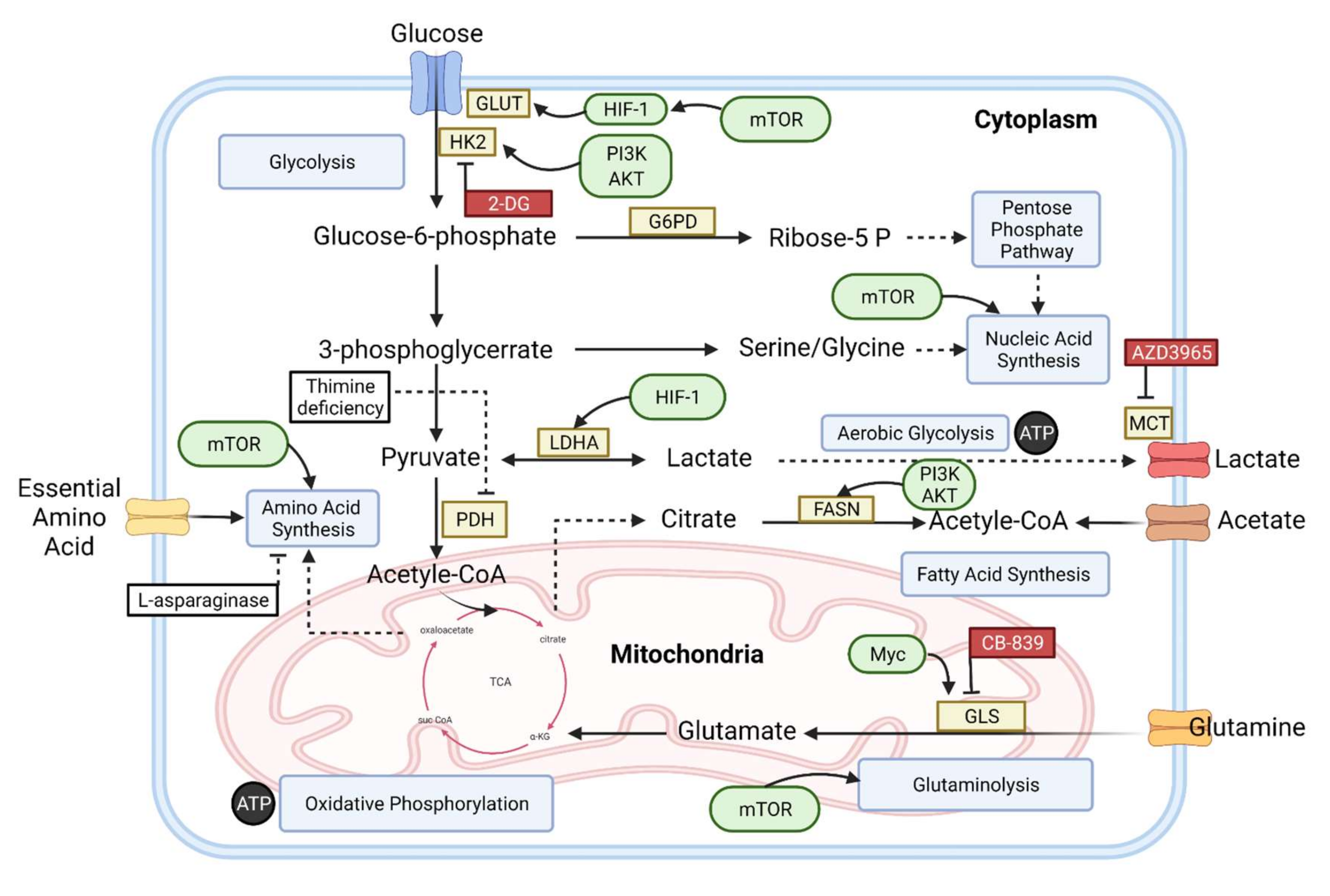
:1. Introduction
2. Basic Metabolism in Normal Cells
3. Metabolic Alteration in B-Cell Lymphoma
4. An Overview of Metabolic Biomarkers in B-Cell Lymphomas
4.1. Glucose Uptake
4.2. CHK α
4.3. LDH and β2-MG
4.4. NEK2
4.5. Glutamine
4.6. HK2
4.7. Notch2
4.8. Metabolic Profile
4.8.1. Hypoxanthine and Elaidic Acid
4.8.2. B Vitamins
4.9. Genetic Alterations
4.9.1. PI3K/AKT/mTOR
4.9.2. PTEN
4.9.3. MCT1
4.10. TMTV
5. The Novel and Future Therapeutic Aspect for B-Cell Lymphoma
5.1. Energetic Pathways Targeting
5.2. Amino Acids Targeting
5.3. A New Therapeutic Approache from a Metabolic Perspective for B-Cell Lymphoma
5.3.1. Metabolic Interventions with Immunotherapy
5.3.2. Metabolic Evaluation of the Immunomodulatory Therapy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin Lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Afonso, J.; Pinto, T.; Simões-Sousa, S.; Schmitt, F.; Longatto-Filho, A.; Pinheiro, C.; Marques, H.; Baltazar, F. Clinical Significance of Metabolism-Related Biomarkers in Non-Hodgkin Lymphoma—MCT1 as Potential Target in Diffuse Large B Cell Lymphoma. Cell. Oncol. 2019, 42, 303–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin Lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood J. Am. Soc. Hematol. 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, E.G.; Ciborowski, M. Applications of Metabolomics in Cancer Studies. Metab. Fundam. Clin. Appl. 2017, 965, 209–234. [Google Scholar]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; B’ez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor Cell Metabolism: An Integral View. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Tennant, D.A.; Durán, R.V.; Gottlieb, E. Targeting Metabolic Transformation for Cancer Therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Oncogene-Driven Metabolic Alterations in Cancer. Biomol. Ther. 2018, 26, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Kerr, E.M.; Martins, C.P. Metabolic Rewiring in Mutant Kras Lung Cancer. FEBS J. 2018, 285, 28–41. [Google Scholar] [CrossRef]
- Rizzieri, D.; Paul, B.; Kang, Y. Metabolic Alterations and the Potential for Targeting Metabolic Pathways in the Treatment of Multiple Myeloma. J. Cancer Metastasis Treat. 2019, 2019, 26. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Shin, Y.; Kim, T.H.; Kim, D.H.; Lee, A. Plasma Metabolites as Possible Biomarkers for Diagnosis of Breast Cancer. PLoS ONE 2019, 14, e0225129. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- De Berardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Lane, A.N.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Zhang, H.; et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in b Cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.A.; Bell, N.; Blair, H.; Sikka, A.; Thomas, H.; Phillips, N.; Nakjang, S.; Miwa, S.; Crossland, R.; Rand, V.; et al. Inhibition of Monocarboxyate Transporter 1 by AZD3965 as a Novel Therapeutic Approach for Diffuse Large B-Cell Lymphoma and Burkitt Lymphoma. Haematologica 2017, 102, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, M.; Vermeersch, K. Applications of Metabolomics in Cancer Research. J. Carcinog. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Maciel, T.T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting Glutamine Uptake Represents an Attractive New Strategy for Treating Acute Myeloid Leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Jurado, B.; Sosa-Quintero, L.S.; Carrasco-Martinez, I.L.; Norato-Delgado, A.; Garcia-Luna, E.; Guzmán-Silahua, S.; Riebeling-Navarro, C.; Nava-Zavala, A.H. New Biomarkers in Non-Hodgkin Lymphoma and Acute Leukemias. Adv. Clin. Chem. 2020, 96, 19–53. [Google Scholar] [CrossRef]
- Bansode, S. Cancer Biology-Causes & Biomarkers of Cancer. Curr. Res. Oncol. 2019, 2019, 1. [Google Scholar]
- Ricci, J.E.; Chiche, J. Metabolic Reprogramming of Non-Hodgkin’s B-Cell Lymphomas and Potential Therapeutic Strategies. Front. Oncol. 2018, 8, 556. [Google Scholar] [CrossRef]
- Zheng, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccaluga, P.P.; Weber, A.; Ambrosio, M.R.; Ahmed, Y.; Leoncini, L. Epstein-Barr Virus-Induced Metabolic Rearrangements in Human B-Cell Lymphomas. Front. Microbiol. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s Contributions to Current Concepts of Cancer Metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Jiang, B. Aerobic Glycolysis and High Level of Lactate in Cancer Metabolism and Microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer Metabolic Reprogramming: Importance, Main Features, and Potentials for Precise Targeted Anti-Cancer Therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lu, M.; Jia, D.; Ma, J.; Ben-Jacob, E.; Levine, H.; Kaipparettu, B.A.; Onuchic, J.N. Modeling the Genetic Regulation of Cancer Metabolism: Interplay between Glycolysis and Oxidative Phosphorylation. Cancer Res. 2017, 77, 1564–1574. [Google Scholar] [CrossRef] [Green Version]
- Kinnaird, A.; Zhao, S.; Wellen, K.E.; Michelakis, E.D. Metabolic Control of Epigenetics in Cancer. Nat. Rev. Cancer 2016, 16, 694–707. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 2013, 342, 1254. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, M.; Baur, R.; Stoll, A.; Mackensen, A.; Mougiakakos, D. Linking Immunoevasion and Metabolic Reprogramming in B-Cell–Derived Lymphomas. Front. Oncol. 2020, 10, 594782. [Google Scholar] [CrossRef]
- Yadav, C.; Ahmad, A.; D’Souza, B.; Agarwal, A.; Nandini, M.; Ashok Prabhu, K.; D’Souza, V. Serum Lactate Dehydrogenase in Non-Hodgkin’s Lymphoma: A Prognostic Indicator. Indian J. Clin. Biochem. 2016, 31, 240–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claudino, W.M.; Dias, A.; Tse, W.; Sharma, V.R. Type B Lactic Acidosis: A Rare but Life Threatening Hematologic Emergency. A Case Illustration and Brief Review. Am. J. Blood Res. 2015, 5, 25–29. [Google Scholar] [PubMed]
- De Groot, R.; Sprenger, R.A.; Imholz, A.L.T.; Gerding, M.N. Type B Lactic Acidosis in Solid Malignancies. Neth. J. Med. 2011, 69, 120–123. [Google Scholar] [PubMed]
- Ruiz, J.P.; Singh, A.K.; Hart, P. Type B Lactic Acidosis Secondary to Malignancy: Case Report, Review of Published Cases, Insights into Pathogenesis, and Prospects for Therapy. Sci. World J. 2011, 11, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and Therapeutic Approach to Elevated Lactate Levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sia, P.; Plumb, T.J.; Fillaus, J.A. Type B Lactic Acidosis Associated with Multiple Myeloma. Am. J. Kidney Dis. 2013, 62, 633–637. [Google Scholar] [CrossRef]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical Applications of Metabolomics in Oncology: A Review. Clin. Cancer Res. 2009, 15, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Mamas, M.; Dunn, W.B.; Neyses, L.; Goodacre, R. The Role of Metabolites and Metabolomics in Clinically Applicable Biomarkers of Disease. Arch. Toxicol. 2011, 85, 5–17. [Google Scholar] [CrossRef]
- Barberini, L.; Noto, A.; Fattuoni, C.; Satta, G.; Zucca, M.; Cabras, M.G.; Mura, E.; Cocco, P. The Metabolomic Profile of Lymphoma Subtypes: A Pilot Study. Molecules 2019, 24, 2367. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.P.; Jacobs, S.R.; Freemerman, A.J.; Makowski, L.; Rathmell, J.C.; Dittmer, D.P.; Damania, B. Dysregulation of Fatty Acid Synthesis and Glycolysis in Non-Hodgkin Lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, 11818–11823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Dortz, L.; de Guibert, S.; Bayat, S.; Devillers, A.; Houot, R.; Rolland, Y.; Cuggia, M.; le Jeune, F.; Bahri, H.; Barge, M.L.; et al. Diagnostic and Prognostic Impact of 18F-FDG PET/CT in Follicular Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2307–2314. [Google Scholar] [CrossRef]
- Paes, F.M.; Kalkanis, D.G.; Sideras, P.A.; Serafini, A.N. FDG PET/CT of Extranodal Involvement in Non-Hodgkin Lymphoma and Hodgkin Disease. Radiographics 2010, 30, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.; Noy, A.; Gönen, M.; Weng, L.; Green, D.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W.D. Intensity of 18fluorodeoxyglucose Uptake in Positron Emission Tomography Distinguishes between Indolent and Aggressive Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2005, 23, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Oki, Y.; Onoda, H.; Taji, H.; Yamamoto, K.; Tamaki, T.; Morishima, Y. High Maximum Standard Uptake Value (SUVmax) on PET Scan Is Associated with Shorter Survival in Patients with Diffuse Large B Cell Lymphoma. Int. J. Hematol. 2011, 93, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Marzec, M.; Liu, X.; Wehrli, S.; Kantekure, K.; Ragunath, P.N.; Nelson, D.S.; Delikatny, E.J.; Glickson, J.D.; Wasik, M.A. Decreased Lactate Concentration and Glycolytic Enzyme Expression Reflect Inhibition of MTOR Signal Transduction Pathway in B-Cell Lymphoma. NMR Biomed. 2013, 26, 106–114. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Popov, A.V.; Delikatny, E.J. Choline Kinase Alpha—Putting the ChoK-Hold on Tumor Metabolism. Prog. Lipid Res. 2016, 63, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, T.H.; Filho, R.S.; Castro, A.C.G.; Paulino, E.; Mamede, M. Targeting Personalized Medicine in a Non-Hodgkin Lymphoma Patient with 18F-FDG and 18F-Choline PET/CT. Rev. Assoc. Med. Bras. 2017, 63, 109–111. [Google Scholar] [CrossRef] [Green Version]
- Gokhale, S.; Xie, P. Chok-Full of Potential: Choline Kinase in b Cell and t Cell Malignancies. Pharmaceutics 2021, 13, 911. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Gui, W.; Wang, T.; Wang, J.; Wang, L.; He, J.; Yang, B.; Zhao, Z.; Zhang, H.; Zhang, Q. An Improved Prognostic Parameter for Non-Hodgkin’s Lymphoma Based on the Combination of Three Serum Tumor Markers. Int. J. Biol. Markers 2008, 23, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, X.; Peng, C.; Wei, J.; Song, Z.; Cong, Z. Prognostic Values of Serum LDH and Β2-MG in Patients with Non-Hodgkin’s Lymphoma. Chin.-Ger. J. Clin. Oncol. 2009, 8, 353–355. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, L.; Gong, Y.; Zhao, J.; Zhang, J.; Mao, Z.; Wang, Z.; Zhang, W.; Zhou, R. NEK2 Promotes Cell Proliferation and Glycolysis by Regulating PKM2 Abundance via Phosphorylation in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2021, 11, 677763. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhou, W.; Huang, J.; Yang, Y.; Wendlandt, E.; Xu, H.; He, X.; Tricot, G.; Zhan, F. Nek2 Is a Novel Regulator of B Cell Development and Immunological Response. Biomed Res. Int. 2014, 2014, 621082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Dang, C.V. Studying Myc’s Role in Metabolism Regulation. Methods Mol. Biol. 2013, 1012, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Lozy, F.; Karantza, V. Autophagy and Cancer Cell Metabolism. Semin. Cell Dev. Biol. 2012, 23, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, L.; Jiang, W.; Lu, W.; Yang, J.; Yang, W. B Cell Lymphoma with Different Metabolic Characteristics Show Distinct Sensitivities to Metabolic Inhibitors. J. Cancer 2018, 9, 1582–1591. [Google Scholar] [CrossRef]
- Bhalla, K.; Jaber, S.; Nahid, N.M.; Underwood, K.; Beheshti, A.; Landon, A.; Bhandary, B.; Bastain, P.; Evens, A.M.; Haley, J.; et al. Role of Hypoxia in Diffuse Large B-Cell Lymphoma: Metabolic Repression and Selective Translation of HK2 Facilitates Development of DLBCL. Sci. Rep. 2018, 8, 744. [Google Scholar] [CrossRef] [Green Version]
- Ambrosio, M.R.; Piccaluga, P.P.; Ponzoni, M.; Rocca, B.J.; Malagnino, V.; Onorati, M.; De Falco, G.; Calbi, V.; Ogwang, M.; Naresh, K.N.; et al. The Alteration of Lipid Metabolism in Burkitt Lymphoma Identifies a Novel Marker: Adipophilin. PLoS ONE 2012, 7, e44315. [Google Scholar] [CrossRef]
- Arruga, F.; Vaisitti, T.; Deaglio, S. The NOTCH Pathway and Its Mutations in Mature B Cell Malignancies. Front. Oncol. 2018, 8, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Kumano, K.; Nakazaki, K.; Sanada, M.; Matsumoto, A.; Yamamoto, G.; Nannya, Y.; Suzuki, R.; Ota, S.; Ota, Y.; et al. Gain-of-Function Mutations and Copy Number Increases of Notch2 in Diffuse Large B-Cell Lymphoma. Cancer Sci. 2009, 100, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miranda, N.F.C.C.; Georgiou, K.; Chen, L.; Wu, C.; Gao, Z.; Zaravinos, A.; Lisboa, S.; Enblad, G.; Teixeira, M.R.; Zeng, Y.; et al. Exome Sequencing Reveals Novel Mutation Targets in Diffuse Large B-Cell Lymphomas Derived from Chinese Patients. Blood 2014, 124, 2544–2553. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Innao, V.; Gerace, D.; Bianco, O.; Musolino, C. The Metabolomic Signature of Hematologic Malignancies. Leuk. Res. 2016, 49, 22–35. [Google Scholar] [CrossRef]
- Stillwell, W. Introduction to Biological Membranes. In An Introduction to Biological Membranes Composition Structure and Function; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–15, 532. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and Epigenetics: An Interplay of Dietary Methyl Donors, One-Carbon Metabolism and DNA Methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Chen, P.; Cai, M.; Shi, Q.; Xu, P.; Wang, L.; He, Y.; Wang, H.; Zhao, W. Prognostic Impact of B-Vitamins Involved in One-Carbon Metabolism in Patients with Diffuse Large B-Cell Lymphoma. Hematol. Oncol. 2020, 38, 456–466. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef] [Green Version]
- Korac, P.; Dotlic, S.; Matulic, M.; Petranovic, M.Z.; Dominis, M. Role of MYC in B Cell Lymphomagenesis. Genes 2017, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of MTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty Acid Synthase as a Potential Therapeutic Target in Cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, S.H.; Roy, D.; Wang, L.; Staudt, M.R.; Fakhari, F.D.; Patel, D.D.; Henry, D.; Harrington, W.J.; Damania, B.A.; Dittmer, D.P. Rapamycin Is Efficacious against Primary Effusion Lymphoma (PEL) Cell Lines in Vivo by Inhibiting Autocrine Signaling. Blood 2007, 109, 2165–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro, P.; Kishan, A.U.; Norberg, E.; Stanley, I.A.; Chapuy, B.; Ficarro, S.B.; Polak, K.; Tondera, D.; Gounarides, J.; Yin, H.; et al. Metabolic Signatures Uncover Distinct Targets in Molecular Subsets of Diffuse Large B Cell Lymphoma. Cancer Cell 2012, 22, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Havranek, O.; Xu, J.; Köhrer, S.; Wang, Z.; Becker, L.; Comer, J.M.; Henderson, J.; Ma, W.; Man Chun Ma, J.; Westin, J.R. Tonic B-Cell Receptor Signaling in Diffuse Large B-Cell Lymphoma. Blood J. Am. Soc. Hematol. 2017, 130, 995–1006. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.M.; Chandel, N.S.; Hay, N. AMPK Regulates NADPH Homeostasis to Promote Tumour Cell Survival during Energy Stress. Nature 2012, 485, 661–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.M.; Shaffer, A.L.; Phelan, J.D.; Staudt, L.M. B-Cell Receptor Signaling in Diffuse Large B-Cell Lymphoma. Semin. Hematol. 2015, 52, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.T.; Moreno, M.V.; Lodi, A.; Ronen, S.M.; Ruggero, D. Protein and Nucleotide Biosynthesis Are Coupled by a Single Rate-Limiting Enzyme, PRPS2, to Drive Cancer. Cell 2014, 157, 1088–1103. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Metabolism: A Therapeutic Perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the Test of Time: Targeting Thymidylate Biosynthesis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298. [Google Scholar] [CrossRef]
- Dang, C.V. Rethinking the Warburg Effect with Myc Micromanaging Glutamine Metabolism. Cancer Res. 2010, 70, 859–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folmes, C.D.L.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, S.; Hussain, A.R.; Siraj, A.K.; Manogaran, P.S.; Al-Jomah, N.A.; Moorji, A.; Atizado, V.; Al-Dayel, F.; Belgaumi, A.; El-Solh, H.; et al. Role of Phosphatidylinositol 3′-Kinase/AKT Pathway in Diffuse Large B-Cell Lymphoma Survival. Blood 2006, 108, 4178–4186. [Google Scholar] [CrossRef] [Green Version]
- Wanner, K.; Hipp, S.; Oelsner, M.; Ringshausen, I.; Bogner, C.; Peschel, C.; Decker, T. Mammalian Target of Rapamycin Inhibition Induces Cell Cycle Arrest in Diffuse Large B Cell Lymphoma (DLBCL) Cells and Sensitises DLBCL Cells to Rituximab. Br. J. Haematol. 2006, 134, 475–484. [Google Scholar] [CrossRef]
- Bhende, P.M.; Park, S.I.; Lim, M.S.; Dittmer, D.P.; Damania, B. The Dual PI3K/MTOR Inhibitor, NVP-BEZ235, Is Efficacious against Follicular Lymphoma. Leukemia 2010, 24, 1781–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.H.; Yap, T.A.; Yan, L.; Cunningham, D. Targeting the PI3K-AKT-MTOR Singnaling Network in Cancer. Chin. J. Cancer 2013, 32, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandolfi, P.P. Breast Cancer—Loss of PTEN Predicts Resistance to Treatment. N. Engl. J. Med. 2004, 351, 2337–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Yu, J.S.; Malmström, P.O.; Mansukhani, M.; Enoksson, J.; et al. PIK3CA Mutations Correlate with Hormone Receptors, Node Metastasis, and ERBB2, and Are Mutually Exclusive with PTEN Loss in Human Breast Carcinoma. Cancer Res. 2005, 65, 2554–2559. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Ostrander, J.H.; Faivre, E.J.; Olsen, A.; Fitzsimmons, D.; Lange, C.A. Regulated Association of Protein Kinase B/Akt with Breast Tumor Kinase. J. Biol. Chem. 2005, 280, 1982–1991. [Google Scholar] [CrossRef] [Green Version]
- Paplomata, E.; O’regan, R. The PI3K/AKT/MTOR Pathway in Breast Cancer: Targets, Trials and Biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [Green Version]
- West, K.A.; Castillo, S.S.; Dennis, P.A. Activation of the PI3K/Akt Pathway and Chemotherapeutic Resistance. Drug Resist. Updates 2002, 5, 234–248. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and Apoptosis: Size Matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollizzi, K.N.; Powell, J.D. Regulation of T Cells by MTOR: The Known Knowns and the Known Unknowns. Trends Immunol. 2015, 36, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cao, X.; Sun, R.; Tang, C.; Tzankov, A.; Zhang, J.; Manyam, G.C.; Xiao, M.; Miao, Y.; Jabbar, K.; et al. Clinical Significance of PTEN Deletion, Mutation, and Loss of PTEN Expression in De Novo Diffuse Large B-Cell Lymphoma. Neoplasia 2018, 20, 574–593. [Google Scholar] [CrossRef]
- Pfeifer, M.; Grau, M.; Lenze, D.; Wenzel, S.S.; Wolf, A.; Wollert-Wulf, B.; Dietze, K.; Nogai, H.; Storek, B.; Madle, H.; et al. PTEN Loss Defines a PI3K/AKT Pathway-Dependent Germinal Center Subtype of Diffuse Large B-Cell Lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 12420–12425. [Google Scholar] [CrossRef] [Green Version]
- Parks, S.K.; Chiche, J.; Pouysségur, J. Disrupting Proton Dynamics and Energy Metabolism for Cancer Therapy. Nat. Rev. Cancer 2013, 13, 611–623. [Google Scholar] [CrossRef]
- Basu, S.; Zaidi, H.; Salavati, A.; Hess, S.; Carlsen, P.F.H.; Alavi, A. FDG PET/CT Methodology for Evaluation of Treatment Response in Lymphoma: From “Graded Visual Analysis” and “Semiquantitative SUVmax” to Global Disease Burden Assessment. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2158–2160. [Google Scholar] [CrossRef] [Green Version]
- Sasanelli, M.; Meignan, M.; Haioun, C.; Berriolo-Riedinger, A.; Casasnovas, R.O.; Biggi, A.; Gallamini, A.; Siegel, B.A.; Cashen, A.F.; Véra, P.; et al. Pretherapy Metabolic Tumour Volume Is an Independent Predictor of Outcome in Patients with Diffuse Large B-Cell Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2017–2022. [Google Scholar] [CrossRef]
- Al Tabaa, Y.; Bailly, C.; Kanoun, S. FDG-PET/CT in Lymphoma: Where Do We Go Now? Cancers 2021, 13, 5222. [Google Scholar] [CrossRef]
- Kirsch, B.J.; Chang, S.J.; Betenbaugh, M.J.; Le, A. Non-Hodgkin Lymphoma Metabolism. Adv. Exp. Med. Biol. 2021, 1311, 103–116. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farge, T.; Saland, E.; de Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bost, F.; Decoux-Poullot, A.-G.; Tanti, J.F.; Clavel, S. Energy Disruptors: Rising Stars in Anticancer Therapy? Oncogenesis 2016, 5, e188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic MTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Sahra, I.B.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, Independent of AMPK, Induces MTOR Inhibition and Cell-Cycle Arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Dowling, R.J.O.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin Inhibits Mammalian Target of Rapamycin-Dependent Translation Initiation in Breast Cancer Cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.Y.; Xiao, D.; Wang, L.; Dong, L.H.; Yan, Z.X.; Shen, Z.X.; Chen, S.J.; Chen, Y.; Zhao, W.L. Therapeutic Metformin/AMPK Activation Blocked Lymphoma Cell Growth via Inhibition of MTOR Pathway and Induction of Autophagy. Cell Death Dis. 2012, 3, e275. [Google Scholar] [CrossRef]
- Pusapati, R.V.; Daemen, A.; Wilson, C.; Sandoval, W.; Gao, M.; Haley, B.; Baudy, A.R.; Hatzivassiliou, G.; Evangelista, M.; Settleman, J. MTORC1-Dependent Metabolic Reprogramming Underlies Escape from Glycolysis Addiction in Cancer Cells. Cancer Cell 2016, 29, 548–562. [Google Scholar] [CrossRef] [Green Version]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A Expression Uncovers a Link between Glycolysis, Mitochondrial Physiology, and Tumor Maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Le Floch, R.; Chiche, J.; Marchiq, I.; Naïken, T.; Ilk, K.; Murray, C.M.; Critchlow, S.E.; Roux, D.; Simon, M.P.; Pouysségur, J. CD147 Subunit of Lactate/H+ Symporters MCT1 and Hypoxia-Inducible MCT4 Is Critical for Energetics and Growth of Glycolytic Tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 16663–16668. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Sasayama, T.; Irino, Y.; Takata, K.; Nagashima, H.; Satoh, N.; Kyotani, K.; Mizowaki, T.; Imahori, T.; Ejima, Y.; et al. Compensatory Glutamine Metabolism Promotes Glioblastoma Resistance to MTOR Inhibitor Treatment. J. Clin. Investig. 2015, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor Activity of the Glutaminase Inhibitor CB-839 in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacque, N.; Ronchetti, A.M.; Larrue, C.; Meunier, G.; Birsen, R.; Willems, L.; Saland, E.; Decroocq, J.; Maciel, T.T.; Lambert, M.; et al. Targeting Glutaminolysis Has Antileukemic Activity in Acute Myeloid Leukemia and Synergizes with BCL-2 Inhibition. Blood 2015, 126, 1346–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Trand, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC Oncogene Overexpression Drives Renal Cell Carcinoma in a Mouse Model through Glutamine Metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 6539–6544. [Google Scholar] [CrossRef] [Green Version]
- Pishko, A.; Nasta, S.D. The Role of Novel Immunotherapies in Non-Hodgkin Lymphoma. Transl. Cancer Res. 2017, 6, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Greve, P.; Meyer-Wentrup, F.A.G.; Peperzak, V.; Boes, M. Upcoming Immunotherapeutic Combinations for B-Cell Lymphoma. Immunother. Adv. 2021, 1, ltab001. [Google Scholar] [CrossRef]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.C.; Fendt, S.M.; Ho, P.C. Navigating Metabolic Pathways to Enhance Antitumour Immunity and Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 425–441. [Google Scholar] [CrossRef]
- Luo, Y. A Novel Molecular Classification of Diffuse Large B Cell Lymphoma Based on Metabolism-Related Genes. BMC Cancer 2020. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to Differentiate Pseudoprogression from True Progression in Cancer Patients Treated with Immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar] [PubMed]
| Metabolism Influence | Genes | Ref. |
|---|---|---|
| Increase glycolysis and FAS | PI3K, mTOR | [42,72,73,74] |
| Reduces PPP activity and increases FAO | AMPK | [75,76,77,78] |
| Increase nucleotide biosynthesis | PRPS2 | [79,80,81] |
| Organize glycolysis, TCA, glutamine, and proteins | MYC | [82,83] |
| Metabolic Therapy | Immunotherapy | Metabolic Target | Tumor Type | Study Phase | Status |
|---|---|---|---|---|---|
| Trigriluzole | Nivolumab or pembrolizumab | Glutamine and glutamate pathway inhibitors | Solid malignancies or lymphoma | II | Completed |
| CPI-006 (anti-CD73 antibody) | Pembrolizumab | Adenosine pathway inhibitors | Advanced-stage cancers, non-Hodgkin’s lymphoma | I | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaifi, A.; Bahashwan, S.; Alsaadi, M.; Malhan, H.; Aqeel, A.; Al-Kahiry, W.; Almehdar, H.; Qadri, I. Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment. Diagnostics 2022, 12, 394. https://doi.org/10.3390/diagnostics12020394
Alfaifi A, Bahashwan S, Alsaadi M, Malhan H, Aqeel A, Al-Kahiry W, Almehdar H, Qadri I. Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment. Diagnostics. 2022; 12(2):394. https://doi.org/10.3390/diagnostics12020394
Chicago/Turabian StyleAlfaifi, Abdullah, Salem Bahashwan, Mohammed Alsaadi, Hafiz Malhan, Aqeel Aqeel, Waiel Al-Kahiry, Hussein Almehdar, and Ishtiaq Qadri. 2022. "Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment" Diagnostics 12, no. 2: 394. https://doi.org/10.3390/diagnostics12020394
APA StyleAlfaifi, A., Bahashwan, S., Alsaadi, M., Malhan, H., Aqeel, A., Al-Kahiry, W., Almehdar, H., & Qadri, I. (2022). Metabolic Biomarkers in B-Cell Lymphomas for Early Diagnosis and Prediction, as Well as Their Influence on Prognosis and Treatment. Diagnostics, 12(2), 394. https://doi.org/10.3390/diagnostics12020394