Cardiovascular Magnetic Resonance in Myocarditis
Abstract
:1. Introduction
2. Clinically Suspected Myocarditis
2.1. Clinical Presentation
2.2. Diagnostic Work-Up
3. CMR Imaging of Myocardial Inflammation
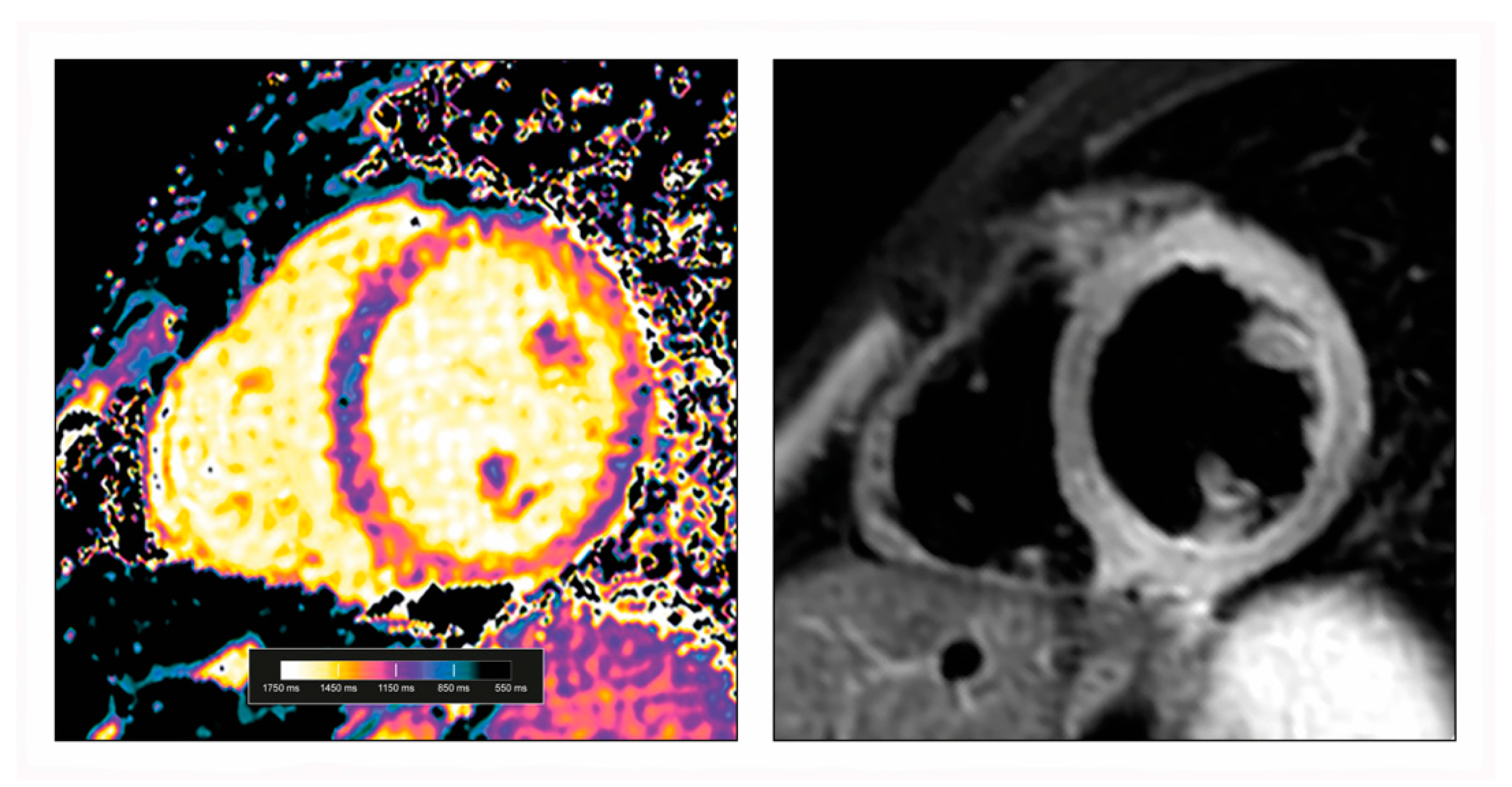
3.1. CMR Mapping Techniques
3.2. Myocardial Oedema
3.3. Myocardial Hyperaemia and Capillary Leak
3.4. Myocardial Necrosis and Fibrosis
3.5. Functional and Pericardial Alterations
4. Updated Lake Louise Criteria
5. CMR in Different Forms of Myocarditis
5.1. Viral Myocarditis
5.2. COVID-19 and Post-Vaccination Associated Myocarditis
5.3. Giant Cell Myocarditis
5.4. Cardiac Sarcoidosis
5.5. Eosinophilic Myocarditis
5.6. Myocarditis in Systemic Immune-Mediated Diseases
5.7. Immune Checkpoint Inhibitor-Induced Myocarditis
5.8. Myocarditis in Children and Adolescence
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on myocarditis. J. Am. Coll. Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, A.; Patti, G.; Manzoli, A.; Sinagra, G.; Di Lenarda, A.; Silvestri, F.; Di Sciascio, G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: A review. Heart 2001, 85, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Fabre, A.; Sheppard, M.N. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart 2006, 92, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T., Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Biesbroek, P.S.; Hirsch, A.; Zweerink, A.; van de Ven, P.M.; Beek, A.M.; Groenink, M.; Windhausen, F.; Planken, R.N.; van Rossum, A.C.; Nijveldt, R. Additional diagnostic value of CMR to the European Society of Cardiology (ESC) position statement criteria in a large clinical population of patients with suspected myocarditis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1397–1407. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR with Late Gadolinium Enhancement in Acute Myocarditis with Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef]
- Grani, C.; Eichhorn, C.; Biere, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients with Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Martino, T.; Opavsky, M.A.; Penninger, J. Viral myocarditis: Balance between viral infection and immune response. Can. J. Cardiol. 1996, 12, 935–943. [Google Scholar] [PubMed]
- Cooper, L.T., Jr. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukena, C.; Mahfoud, F.; Kindermann, I.; Kandolf, R.; Kindermann, M.; Bohm, M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur. J. Heart Fail. 2011, 13, 398–405. [Google Scholar] [CrossRef]
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Morgera, T.; Di Lenarda, A.; Dreas, L.; Pinamonti, B.; Humar, F.; Bussani, R.; Silvestri, F.; Chersevani, D.; Camerini, F. Electrocardiography of myocarditis revisited: Clinical and prognostic significance of electrocardiographic changes. Am. Heart J. 1992, 124, 455–467. [Google Scholar] [CrossRef]
- Lauer, B.; Niederau, C.; Kuhl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Ukena, C.; Kindermann, M.; Mahfoud, F.; Geisel, J.; Lepper, P.M.; Kandolf, R.; Bohm, M.; Kindermann, I. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin. Res. Cardiol. 2014, 103, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Gartner, B.; Kindermann, M.; Ukena, C.; Gadomski, K.; Klingel, K.; Kandolf, R.; Bohm, M.; Kindermann, I. Virus serology in patients with suspected myocarditis: Utility or futility? Eur. Heart J. 2011, 32, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Jeserich, M.; Konstantinides, S.; Pavlik, G.; Bode, C.; Geibel, A. Non-invasive imaging in the diagnosis of acute viral myocarditis. Clin. Res. Cardiol. 2009, 98, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logstrup, B.B.; Nielsen, J.M.; Kim, W.Y.; Poulsen, S.H. Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitman, M.; Vered, Z.; Tyomkin, V.; Macogon, B.; Moravsky, G.; Peleg, E.; Copel, L. Speckle tracking imaging in inflammatory heart diseases. Int. J. Cardiovasc. Imaging 2018, 34, 787–792. [Google Scholar] [CrossRef]
- Uppu, S.C.; Shah, A.; Weigand, J.; Nielsen, J.C.; Ko, H.H.; Parness, I.A.; Srivastava, S. Two-dimensional speckle-tracking-derived segmental peak systolic longitudinal strain identifies regional myocardial involvement in patients with myocarditis and normal global left ventricular systolic function. Pediatr. Cardiol. 2015, 36, 950–959. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Vita, T.; Okada, D.R.; Veillet-Chowdhury, M.; Bravo, P.E.; Mullins, E.; Hulten, E.; Agrawal, M.; Madan, R.; Taqueti, V.R.; Steigner, M.; et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2018, 11, e007030. [Google Scholar] [CrossRef]
- Kruse, M.J.; Kovell, L.; Kasper, E.K.; Pomper, M.G.; Moller, D.R.; Solnes, L.; Chen, E.S.; Schindler, T.H. Myocardial Blood Flow and Inflammatory Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 157–167. [Google Scholar] [CrossRef]
- Kadkhodayan, A.; Chareonthaitawee, P.; Raman, S.V.; Cooper, L.T. Imaging of Inflammation in Unexplained Cardiomyopathy. JACC Cardiovasc. Imaging 2016, 9, 603–617. [Google Scholar] [CrossRef]
- Polte, C.L.; Burck, I.; Gjertsson, P.; Lomsky, M.; Nekolla, S.G.; Nagel, E. Cardiac Positron Emission Tomography: A Clinical Perspective. Curr. Cardiovasc. Imaging Rep. 2016, 9, 9. [Google Scholar] [CrossRef]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J., Jr.; Olsen, E.G.; Schoen, F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Raisanen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ. Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, R.C.; Tazelaar, H.D.; Berry, G.J.; Cooper, L.T., Jr. The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J. Card. Fail. 2002, 8, 74–78. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Lurz, P.; Luecke, C.; Eitel, I.; Fohrenbach, F.; Frank, C.; Grothoff, M.; de Waha, S.; Rommel, K.P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients with Suspected Myocarditis: The MyoRacer-Trial. J. Am. Coll. Cardiol. 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Dabir, D.; Kuetting, D.L.; Marx, C.; Doerner, J.; Schlesinger-Irsch, U.; Andrie, R.; Sprinkart, A.M.; Schmeel, F.C.; et al. Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis. J. Am. Heart Assoc. 2016, 5, e003603. [Google Scholar] [CrossRef] [Green Version]
- Francone, M.; Chimenti, C.; Galea, N.; Scopelliti, F.; Verardo, R.; Galea, R.; Carbone, I.; Catalano, C.; Fedele, F.; Frustaci, A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc. Imaging 2014, 7, 254–263. [Google Scholar] [CrossRef]
- Nagel, E.; Kwong, R.Y.; Chandrashekhar, Y.S. CMR in Nonischemic Myocardial Inflammation: Solving the Problem of Diagnosing Myocarditis or Still Diagnostic Ambiguity? JACC Cardiovasc. Imaging 2020, 13, 163–166. [Google Scholar] [CrossRef]
- Laissy, J.P.; Hyafil, F.; Feldman, L.J.; Juliard, J.M.; Schouman-Claeys, E.; Steg, P.G.; Faraggi, M. Differentiating acute myocardial infarction from myocarditis: Diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology 2005, 237, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc. Imaging 2013, 6, 1048–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabir, D.; Child, N.; Kalra, A.; Rogers, T.; Gebker, R.; Jabbour, A.; Plein, S.; Yu, C.Y.; Otton, J.; Kidambi, A.; et al. Reference values for healthy human myocardium using a T1 mapping methodology: Results from the International T1 Multicenter cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2014, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Baessler, B.; Schaarschmidt, F.; Stehning, C.; Schnackenburg, B.; Maintz, D.; Bunck, A.C. A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur. J. Radiol. 2015, 84, 2161–2170. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [Green Version]
- Radunski, U.K.; Lund, G.K.; Stehning, C.; Schnackenburg, B.; Bohnen, S.; Adam, G.; Blankenberg, S.; Muellerleile, K. CMR in patients with severe myocarditis: Diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc. Imaging 2014, 7, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Radunski, U.K.; Lund, G.K.; Saring, D.; Bohnen, S.; Stehning, C.; Schnackenburg, B.; Avanesov, M.; Tahir, E.; Adam, G.; Blankenberg, S.; et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin. Res. Cardiol. 2017, 106, 10–17. [Google Scholar] [CrossRef]
- Nadjiri, J.; Nieberler, H.; Hendrich, E.; Greiser, A.; Will, A.; Martinoff, S.; Hadamitzky, M. Performance of native and contrast-enhanced T1 mapping to detect myocardial damage in patients with suspected myocarditis: A head-to-head comparison of different cardiovascular magnetic resonance techniques. Int. J. Cardiovasc. Imaging 2017, 33, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Von Knobelsdorff-Brenkenhoff, F.; Schuler, J.; Doganguzel, S.; Dieringer, M.A.; Rudolph, A.; Greiser, A.; Kellman, P.; Schulz-Menger, J. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e005242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetkens, J.A.; Homsi, R.; Sprinkart, A.M.; Doerner, J.; Dabir, D.; Kuetting, D.L.; Block, W.; Andrie, R.; Stehning, C.; Fimmers, R.; et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Doerner, J.; Thomas, D.K.; Dabir, D.; Gieseke, J.; Sprinkart, A.M.; Fimmers, R.; Stehning, C.; Homsi, R.; Schwab, J.O.; et al. Acute myocarditis: Multiparametric cardiac MR imaging. Radiology 2014, 273, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Lagan, J.; Schmitt, M.; Miller, C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int. J. Cardiovasc. Imaging 2018, 34, 35–54. [Google Scholar] [CrossRef]
- Kotanidis, C.P.; Bazmpani, M.A.; Haidich, A.B.; Karvounis, C.; Antoniades, C.; Karamitsos, T.D. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2018, 11, 1583–1590. [Google Scholar] [CrossRef]
- Verhaert, D.; Thavendiranathan, P.; Giri, S.; Mihai, G.; Rajagopalan, S.; Simonetti, O.P.; Raman, S.V. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc. Imaging 2011, 4, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aty, H.; Simonetti, O.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance imaging. J. Magn. Reson. Imaging 2007, 26, 452–459. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Dall’Armellina, E.; Karamitsos, T.D.; Francis, J.M.; Ntusi, N.; Holloway, C.; Choudhury, R.P.; Kardos, A.; Robson, M.D.; et al. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J. Cardiovasc. Magn. Reson. 2014, 16, 36. [Google Scholar] [CrossRef]
- Hinojar, R.; Foote, L.; Arroyo Ucar, E.; Jackson, T.; Jabbour, A.; Yu, C.Y.; McCrohon, J.; Higgins, D.M.; Carr-White, G.; Mayr, M.; et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: A proposed diagnostic algorithm using CMR. JACC Cardiovasc. Imaging 2015, 8, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Rottgen, R.; Christiani, R.; Freyhardt, P.; Gutberlet, M.; Schultheiss, H.P.; Hamm, B.; Kuhl, U. Magnetic resonance imaging findings in acute myocarditis and correlation with immunohistological parameters. Eur. Radiol. 2011, 21, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potet, J.; Rahmouni, A.; Mayer, J.; Vignaud, A.; Lim, P.; Luciani, A.; Dubois-Rande, J.L.; Kobeiter, H.; Deux, J.F. Detection of myocardial edema with low-b-value diffusion-weighted echo-planar imaging sequence in patients with acute myocarditis. Radiology 2013, 269, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, H.; Boye, P.; Zagrosek, A.; Wassmuth, R.; Kumar, A.; Messroghli, D.; Bock, P.; Dietz, R.; Friedrich, M.G.; Schulz-Menger, J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: Comparison of different approaches. J. Am. Coll. Cardiol. 2005, 45, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, G.C.; Flewitt, J.A.; Mikami, Y.; Vermes, E.; Friedrich, M.G. Assessment of acute myocarditis by cardiovascular MR: Diagnostic performance of shortened protocols. Int. J. Cardiovasc. Imaging 2013, 29, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Rieker, O.; Mohrs, O.; Oberholzer, K.; Kreitner, K.F.; Thelen, M. Cardiac MRI in suspected myocarditis. Rofo 2002, 174, 1530–1536. [Google Scholar] [CrossRef]
- Jeserich, M.; Konstantinides, S.; Olschewski, M.; Pavlik, G.; Bode, C.; Geibel, A. Diagnosis of early myocarditis after respiratory or gastrointestinal tract viral infection: Insights from cardiovascular magnetic resonance. Clin. Res. Cardiol. 2010, 99, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Schwab, J.; Rogg, H.J.; Pauschinger, M.; Fessele, K.; Bareiter, T.; Bar, I.; Loose, R. Functional and Morphological Parameters with Tissue Characterization of Cardiovascular Magnetic Imaging in Clinically Verified “Infarct-like Myocarditis”. Rofo 2016, 188, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Laissy, J.P.; Messin, B.; Varenne, O.; Iung, B.; Karila-Cohen, D.; Schouman-Claeys, E.; Steg, P.G. MRI of acute myocarditis: A comprehensive approach based on various imaging sequences. Chest 2002, 122, 1638–1648. [Google Scholar] [CrossRef] [Green Version]
- Nordlund, D.; Klug, G.; Heiberg, E.; Koul, S.; Larsen, T.H.; Hoffmann, P.; Metzler, B.; Erlinge, D.; Atar, D.; Aletras, A.H.; et al. Multi-vendor, multicentre comparison of contrast-enhanced SSFP and T2-STIR CMR for determining myocardium at risk in ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Kellman, P.; Aletras, A.H.; Mancini, C.; McVeigh, E.R.; Arai, A.E. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn. Reson. Med. 2007, 57, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Childs, H.; Aljizeeri, A.; Merchant, N.; Friedrich, M.G. Importance of Reference Muscle Selection in Quantitative Signal Intensity Analysis of T2-Weighted Images of Myocardial Edema Using a T2 Ratio Method. Biomed. Res. Int. 2015, 2015, 232649. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Schaarschmidt, F.; Dick, A.; Stehning, C.; Schnackenburg, B.; Michels, G.; Maintz, D.; Bunck, A.C. Mapping tissue inhomogeneity in acute myocarditis: A novel analytical approach to quantitative myocardial edema imaging by T2-mapping. J. Cardiovasc. Magn. Reson. 2015, 17, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, F.H.; Bertrand, P.B.; Willems, E.; Gielen, E.; Mullens, W.; Giri, S.; Tang, W.H.W.; Raman, S.V.; Verhaert, D. Global myocardial oedema in advanced decompensated heart failure. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 787–794. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Strohm, O.; Schulz-Menger, J.; Marciniak, H.; Luft, F.C.; Dietz, R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998, 97, 1802–1809. [Google Scholar] [CrossRef] [Green Version]
- Jeserich, M.; Merkely, B.; Schlosser, P.; Kimmel, S.; Pavlik, G.; Achenbach, S. Assessment of edema using STIR+ via 3D cardiovascular magnetic resonance imaging in patients with suspected myocarditis. Magn. Reson. Mater. Phys. Biol. Med. 2017, 30, 309–316. [Google Scholar] [CrossRef]
- Yilmaz, A.; Mahrholdt, H.; Athanasiadis, A.; Vogelsberg, H.; Meinhardt, G.; Voehringer, M.; Kispert, E.M.; Deluigi, C.; Baccouche, H.; Spodarev, E.; et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 2008, 94, 1456–1463. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.T.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef] [Green Version]
- Ammirati, E.; Moroni, F.; Sormani, P.; Peritore, A.; Milazzo, A.; Quattrocchi, G.; Cipriani, M.; Oliva, F.; Giannattasio, C.; Frigerio, M.; et al. Quantitative changes in late gadolinium enhancement at cardiac magnetic resonance in the early phase of acute myocarditis. Int. J. Cardiol. 2017, 231, 216–221. [Google Scholar] [CrossRef]
- Bogaert, J.; Francone, M. Cardiovascular magnetic resonance in pericardial diseases. J. Cardiovasc. Magn. Reson. 2009, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Gutberlet, M.; Spors, B.; Thoma, T.; Bertram, H.; Denecke, T.; Felix, R.; Noutsias, M.; Schultheiss, H.P.; Kuhl, U. Suspected chronic myocarditis at cardiac MR: Diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 2008, 246, 401–409. [Google Scholar] [CrossRef] [PubMed]
- De Cobelli, F.; Pieroni, M.; Esposito, A.; Chimenti, C.; Belloni, E.; Mellone, R.; Canu, T.; Perseghin, G.; Gaudio, C.; Maseri, A.; et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J. Am. Coll. Cardiol. 2006, 47, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satterfield, B.A.; Bhatt, D.L.; Gersh, B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered from COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, Z.; Luo, L.; Wu, W.; Jia, T.; Lu, L.; Liu, W.V.; Qin, Y.; Hu, F.; Ding, X.; et al. Cardiac T1 and T2 Mapping Showed Myocardial Involvement in Recovered COVID-19 Patients Initially Considered Devoid of Cardiac Damage. J. Magn. Reson. Imaging 2021, 54, 421–428. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.H.; Liu, Y.X.; Yuan, J.; Wang, F.X.; Wu, W.B.; Li, J.X.; Wang, L.F.; Gao, H.; Wang, Y.; Dong, C.F.; et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated with COVID-19 Vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Bang, V.; Ganatra, S.; Shah, S.P.; Dani, S.S.; Neilan, T.G.; Thavendiranathan, P.; Resnic, F.S.; Piemonte, T.C.; Barac, A.; Patel, R.; et al. Management of Patients with Giant Cell Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1122–1134. [Google Scholar] [CrossRef]
- Shonk, J.R.; Vogel-Claussen, J.; Halushka, M.K.; Lima, J.A.; Bluemke, D.A. Giant cell myocarditis depicted by cardiac magnetic resonance imaging. J. Comput. Assist. Tomogr. 2005, 29, 742–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarine, A.; Guillemain, R.; Bruneval, P. Different focal delayed gadolinium-enhancement patterns using cardiac magnetic resonance in a case of diffuse giant cell myocarditis. Eur. Heart J. 2009, 30, 1485. [Google Scholar] [CrossRef] [Green Version]
- Sujino, Y.; Kimura, F.; Tanno, J.; Nakano, S.; Yamaguchi, E.; Shimizu, M.; Okano, N.; Tamura, Y.; Fujita, J.; Cooper, L.T.; et al. Cardiac magnetic resonance imaging in giant cell myocarditis: Intriguing associations with clinical and pathological features. Circulation 2014, 129, e467–e469. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, X.; Li, J.; Sun, Y.; Song, J.; Wang, H.; Zhao, S. Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Fail. 2021, 8, 2320–2327. [Google Scholar] [CrossRef]
- Bogabathina, H.; Olson, P.; Rathi, V.K.; Biederman, R.W. Cardiac sarcoidosis or giant cell myocarditis? On treatment improvement of fulminant myocarditis as demonstrated by cardiovascular magnetic resonance imaging. Case Rep. Cardiol. 2012, 2012, 647041. [Google Scholar] [CrossRef] [Green Version]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kouranos, V.; Tzelepis, G.E.; Rapti, A.; Mavrogeni, S.; Aggeli, K.; Douskou, M.; Prasad, S.; Koulouris, N.; Sfikakis, P.; Wells, A.; et al. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1437–1447. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.R.; Klein, M.R.; Chandra, S.; Spencer, K.T.; Decara, J.M.; Lang, R.M.; Burke, M.C.; Garrity, E.R.; Hogarth, D.K.; Archer, S.L.; et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur. J. Heart Fail. 2011, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, G.; Laffin, L.J.; Beshai, J.F.; Maffessanti, F.; Bonham, C.A.; Patel, A.V.; Yu, Z.; Addetia, K.; Mor-Avi, V.; Moss, J.D.; et al. Prognosis of Myocardial Damage in Sarcoidosis Patients with Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2016, 9, e003738. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Deluigi, C.C.; Gloekler, S.; Wahl, A.; Zurn, C.; Kramer, U.; Nothnagel, D.; Bultel, H.; Schumm, J.; Grun, S.; et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc. Imaging 2013, 6, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef]
- Kuchynka, P.; Palecek, T.; Masek, M.; Cerny, V.; Lambert, L.; Vitkova, I.; Linhart, A. Current Diagnostic and Therapeutic Aspects of Eosinophilic Myocarditis. Biomed. Res. Int. 2016, 2016, 2829583. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dai, Z.; Wang, B.; Huang, W. A case report of eosinophilic myocarditis and a review of the relevant literature. BMC Cardiovasc. Disord. 2015, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Ntusi, N.A.B.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Matthews, P.M.; Robson, M.D.; Wordsworth, P.B.; Neubauer, S.; Karamitsos, T.D. Diffuse Myocardial Fibrosis and Inflammation in Rheumatoid Arthritis: Insights from CMR T1 Mapping. JACC Cardiovasc. Imaging 2015, 8, 526–536. [Google Scholar] [CrossRef] [Green Version]
- Ntusi, N.A.; Piechnik, S.K.; Francis, J.M.; Ferreira, V.M.; Rai, A.B.; Matthews, P.M.; Robson, M.D.; Moon, J.; Wordsworth, P.B.; Neubauer, S.; et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—A clinical study using myocardial T1-mapping and extracellular volume quantification. J. Cardiovasc. Magn. Reson. 2014, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Sano, M.; Satoh, H.; Suwa, K.; Nobuhara, M.; Saitoh, T.; Saotome, M.; Urushida, T.; Katoh, H.; Shimoyama, K.; Suzuki, D.; et al. Characteristics and clinical relevance of late gadolinium enhancement in cardiac magnetic resonance in patients with systemic sclerosis. Heart Vessels 2015, 30, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puntmann, V.O.; D’Cruz, D.; Smith, Z.; Pastor, A.; Choong, P.; Voigt, T.; Carr-White, G.; Sangle, S.; Schaeffter, T.; Nagel, E. Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ. Cardiovasc. Imaging 2013, 6, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Banka, P.; Robinson, J.D.; Uppu, S.C.; Harris, M.A.; Hasbani, K.; Lai, W.W.; Richmond, M.E.; Fratz, S.; Jain, S.; Johnson, T.R.; et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J. Cardiovasc. Magn. Reson. 2015, 17, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornicelli, M.D.; Rigsby, C.K.; Rychlik, K.; Pahl, E.; Robinson, J.D. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 40. [Google Scholar] [CrossRef] [Green Version]
- Mavrogeni, S.; Bratis, K.; Georgakopoulos, D.; Karanasios, E.; Kolovou, G.; Pavlides, G.; Papadopoulos, G. Evaluation of myocarditis in a pediatric population using cardiovascular magnetic resonance and endomyocardial biopsy. Int. J. Cardiol. 2012, 160, 192–195. [Google Scholar] [CrossRef]
- Martins, D.S.; Ait-Ali, L.; Khraiche, D.; Festa, P.; Barison, A.; Martini, N.; Benadjaoud, Y.; Anjos, R.; Boddaert, N.; Bonnet, D.; et al. Evolution of acute myocarditis in a pediatric population: An MRI based study. Int. J. Cardiol. 2021, 329, 226–233. [Google Scholar] [CrossRef]
- Dubey, S.; Agarwal, A.; Nguyen, S.; Adebo, D. Persistence of Late Gadolinium Enhancement on Follow-Up CMR Imaging in Children with Acute Myocarditis. Pediatr. Cardiol. 2020, 41, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Luecke, C.; Lurz, J.; Klingel, K.; von Roeder, M.; de Waha, S.; Besler, C.; Maintz, D.; Gutberlet, M.; Thiele, H.; et al. Cardiac MRI Texture Analysis of T1 and T2 Maps in Patients with Infarctlike Acute Myocarditis. Radiology 2018, 289, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baessler, B.; Luecke, C.; Lurz, J.; Klingel, K.; Das, A.; von Roeder, M.; de Waha-Thiele, S.; Besler, C.; Rommel, K.P.; Maintz, D.; et al. Cardiac MRI and Texture Analysis of Myocardial T1 and T2 Maps in Myocarditis with Acute versus Chronic Symptoms of Heart Failure. Radiology 2019, 292, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef]
- Polte, C.L.; Bollano, E.; Oldfors, A.; Dudas, A.; Lagerstrand, K.M.; Himmelman, J.; Bobbio, E.; Karason, K.; van Essen, M.; Bergh, N. Somatostatin Receptor Positron Emission Tomography/Computed Tomography in Giant Cell Myocarditis: A Promising Approach to Molecular Myocardial Inflammation Imaging. Circ. Cardiovasc. Imaging 2021, 15, e013551. [Google Scholar] [CrossRef]
- Polte, C.L.; Bergh, N.; Oldfors, A.; Hanna, B.; Bollano, E. Cardiac involvement in immune-mediated necrotizing myopathy: Insights from CMR and somatostatin receptor PET/CT. Eur. Heart J. Cardiovasc. Imaging 2021, jeab262. [Google Scholar] [CrossRef]
- Lapa, C.; Reiter, T.; Li, X.; Werner, R.A.; Samnick, S.; Jahns, R.; Buck, A.K.; Ertl, G.; Bauer, W.R. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int. J. Cardiol. 2015, 194, 44–49. [Google Scholar] [CrossRef]

| Clinical presentation * |
|
| Diagnostic criteria |
|
|
|
| Oedema | Hyperaemia/Capillary Leak | Necrosis | Fibrosis (Focal/Diffuse) | |
|---|---|---|---|---|
| Acute (active) | T2↑ (T2 SIR/T2 map) T1↑ (native T1/ECV) | T1↑ (native T1/ECV) EGE - or + | LGE - or + | - |
| Chronic | T2 - or↑ (T2 SIR/T2 map) T1 - or↑ (native T1/ECV) | T1 - or↑ (native T1/ECV) EGE - or + | LGE - or + | LGE - or + T1 - or↑ (native T1/ECV) |
| Healed | - | - | - | LGE - or + T1 - or↑ (native T1/ECV) |
| Main criteria (“2 out of 2”) |
|
| Supportive criteria |
|
| Viral Myocarditis | Cardiac Sarcoidosis | Giant Cell Myocarditis | Eosinophilic Myocarditis | |
|---|---|---|---|---|
| Demographics | Mostly young adults, both genders | Mostly middle-aged, both genders | Mostly middle-aged, both genders | Mostly adults <40 years, both genders |
| Most common clinical presentation | Acute coronary syndrome-like with eventual infectious prodrome | Ventricular arrhythmia, heart block, worsening heart failure- Often associated with extra-cardiac sarcoidosis | Ventricular arrhythmia, heart block, worsening heart failure | Acute coronary syndrome-like with fever and dyspnoea |
| Clinical course | Entire spectrum from asymptomatic to fulminant course | Entire spectrum from asymptomatic to fulminant course | Usually fulminant course | Usually acute |
| Characteristic LGE pattern | Subepicardial and/or mid-wall LGE, predominantly basal to mid-lateral and inferolateral wall segments | Varying, usually complex * LGE involving both ventricles including right ventricular insertion points | Often extensive, complex * LGE involving both ventricles including right ventricular insertion points | Diffuse subendocardial LGE with high signal intensity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. https://doi.org/10.3390/diagnostics12020399
Polte CL, Bobbio E, Bollano E, Bergh N, Polte C, Himmelman J, Lagerstrand KM, Gao SA. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics. 2022; 12(2):399. https://doi.org/10.3390/diagnostics12020399
Chicago/Turabian StylePolte, Christian L., Emanuele Bobbio, Entela Bollano, Niklas Bergh, Christina Polte, Jakob Himmelman, Kerstin M. Lagerstrand, and Sinsia A. Gao. 2022. "Cardiovascular Magnetic Resonance in Myocarditis" Diagnostics 12, no. 2: 399. https://doi.org/10.3390/diagnostics12020399
APA StylePolte, C. L., Bobbio, E., Bollano, E., Bergh, N., Polte, C., Himmelman, J., Lagerstrand, K. M., & Gao, S. A. (2022). Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics, 12(2), 399. https://doi.org/10.3390/diagnostics12020399






