Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Medical History of the Subjects
2.3. Measurements and Blood Sampling
2.4. NKA Measurement
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
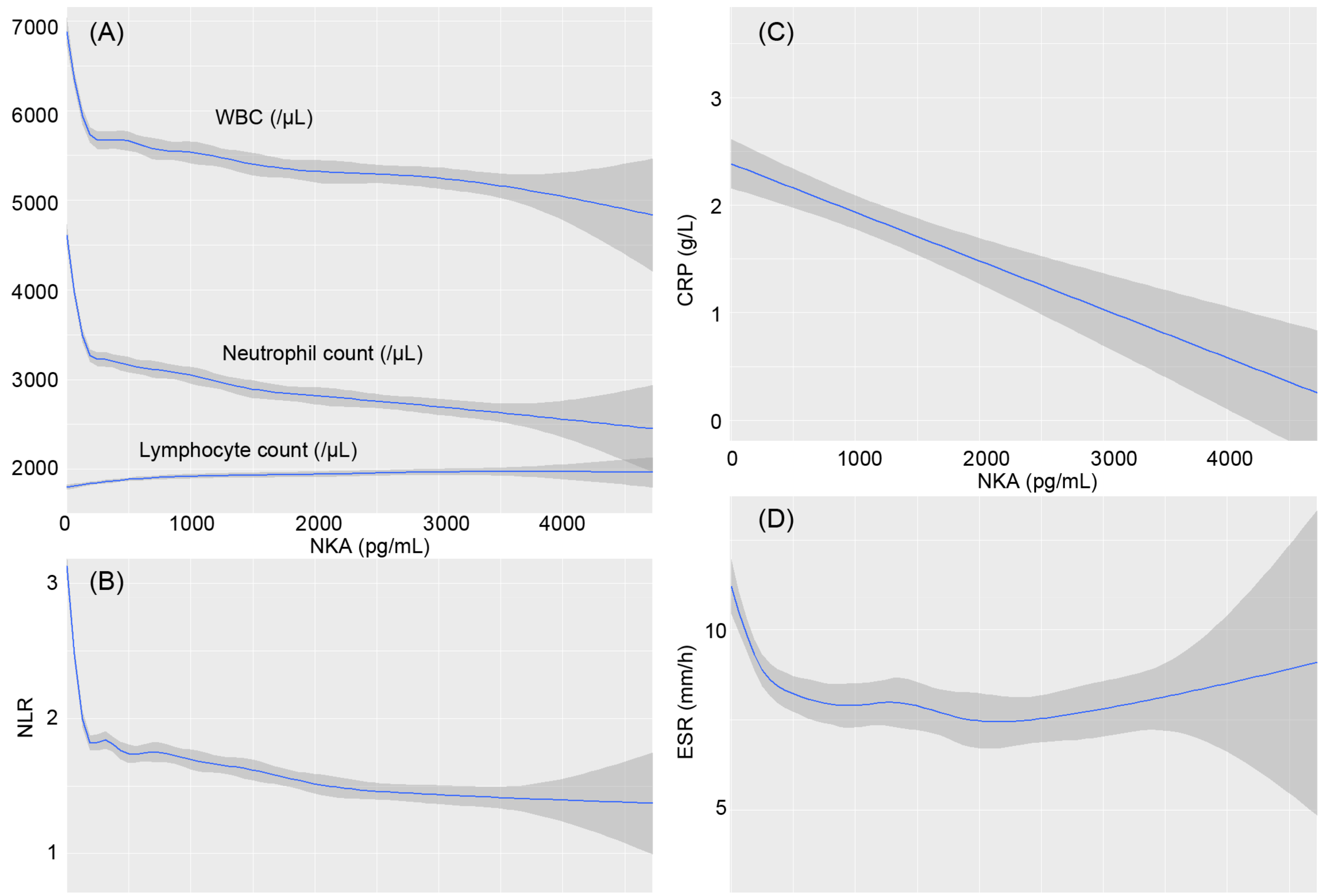
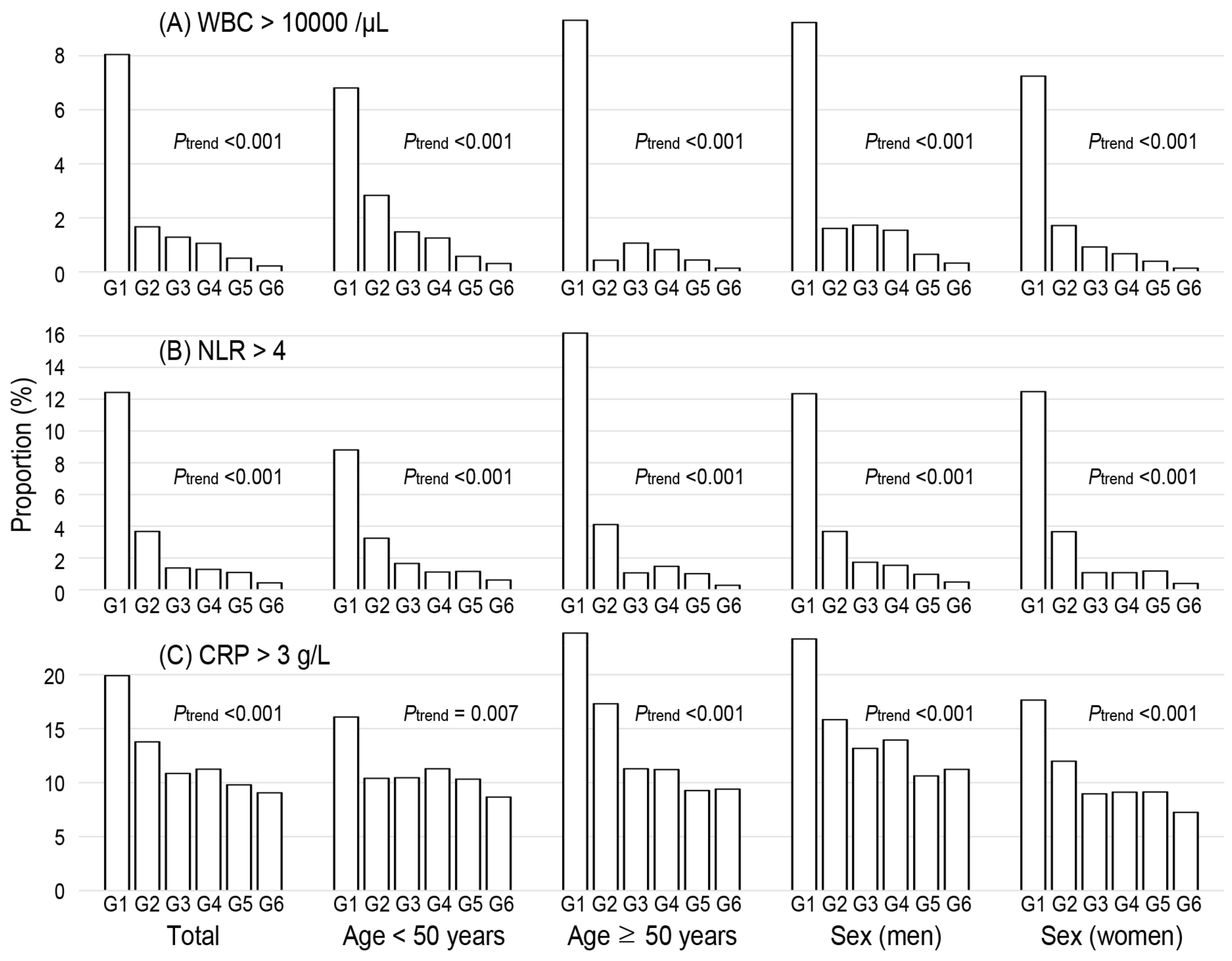
3.2. Association of NKA with CBC Parameters and Inflammatory Indices
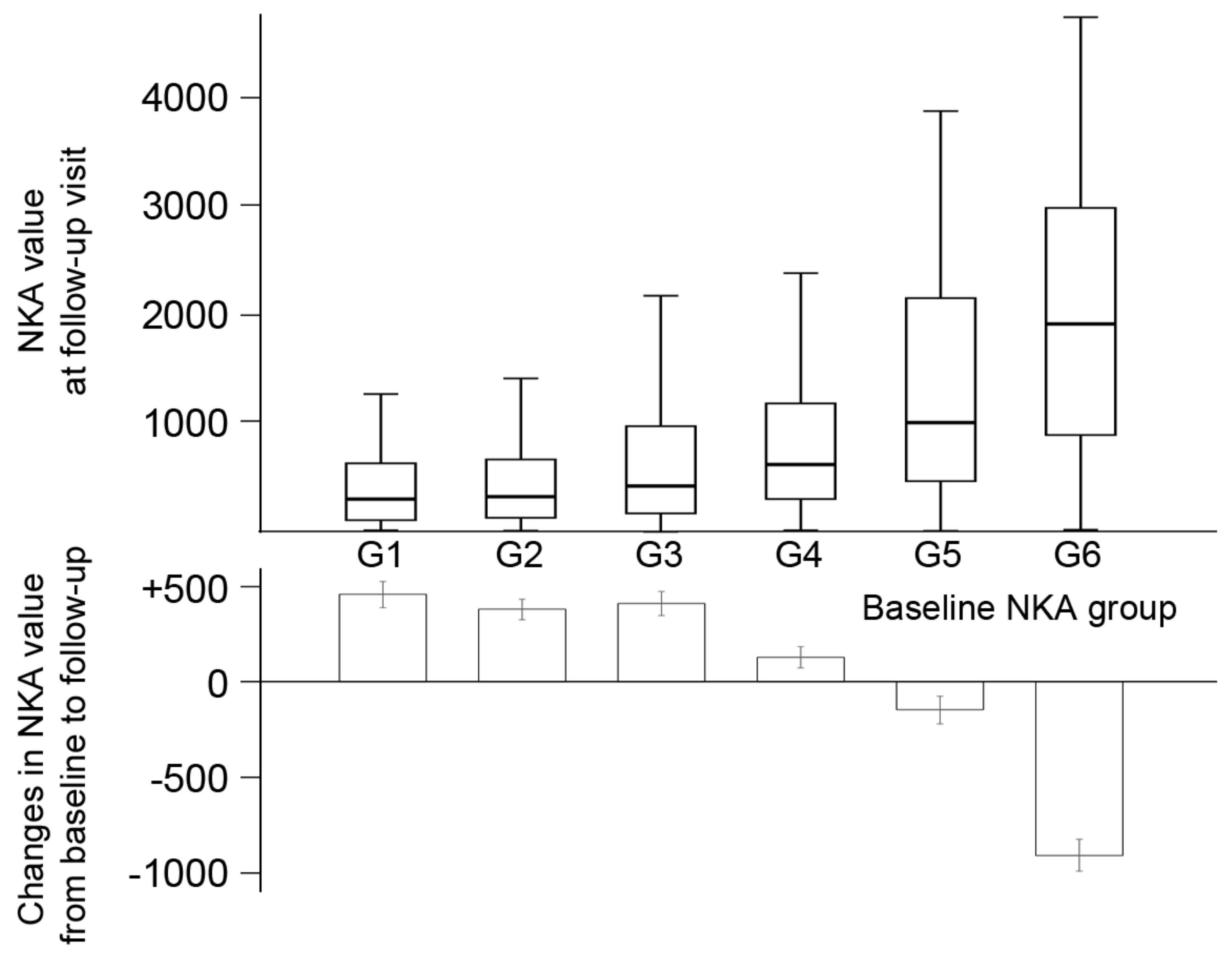
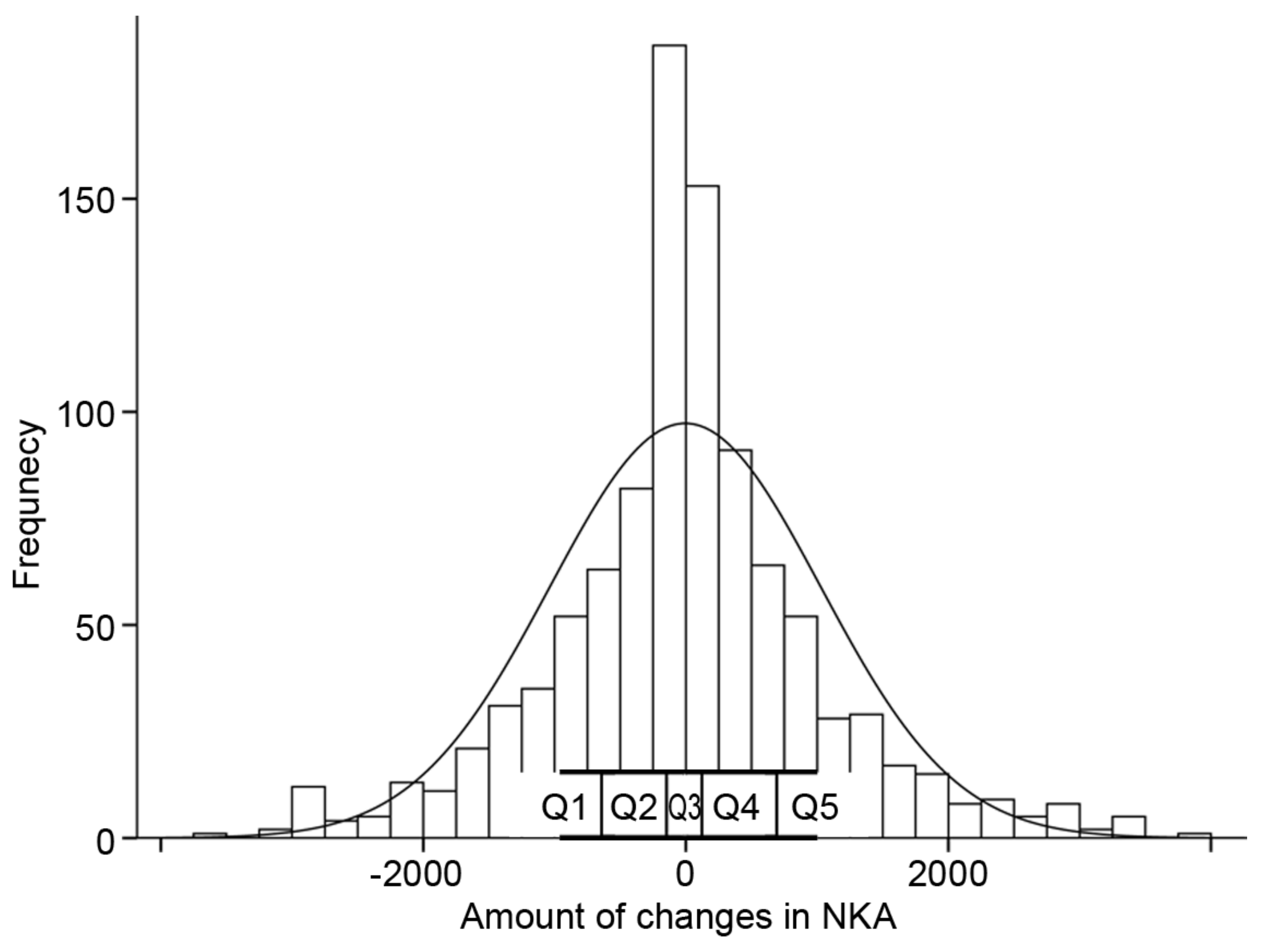
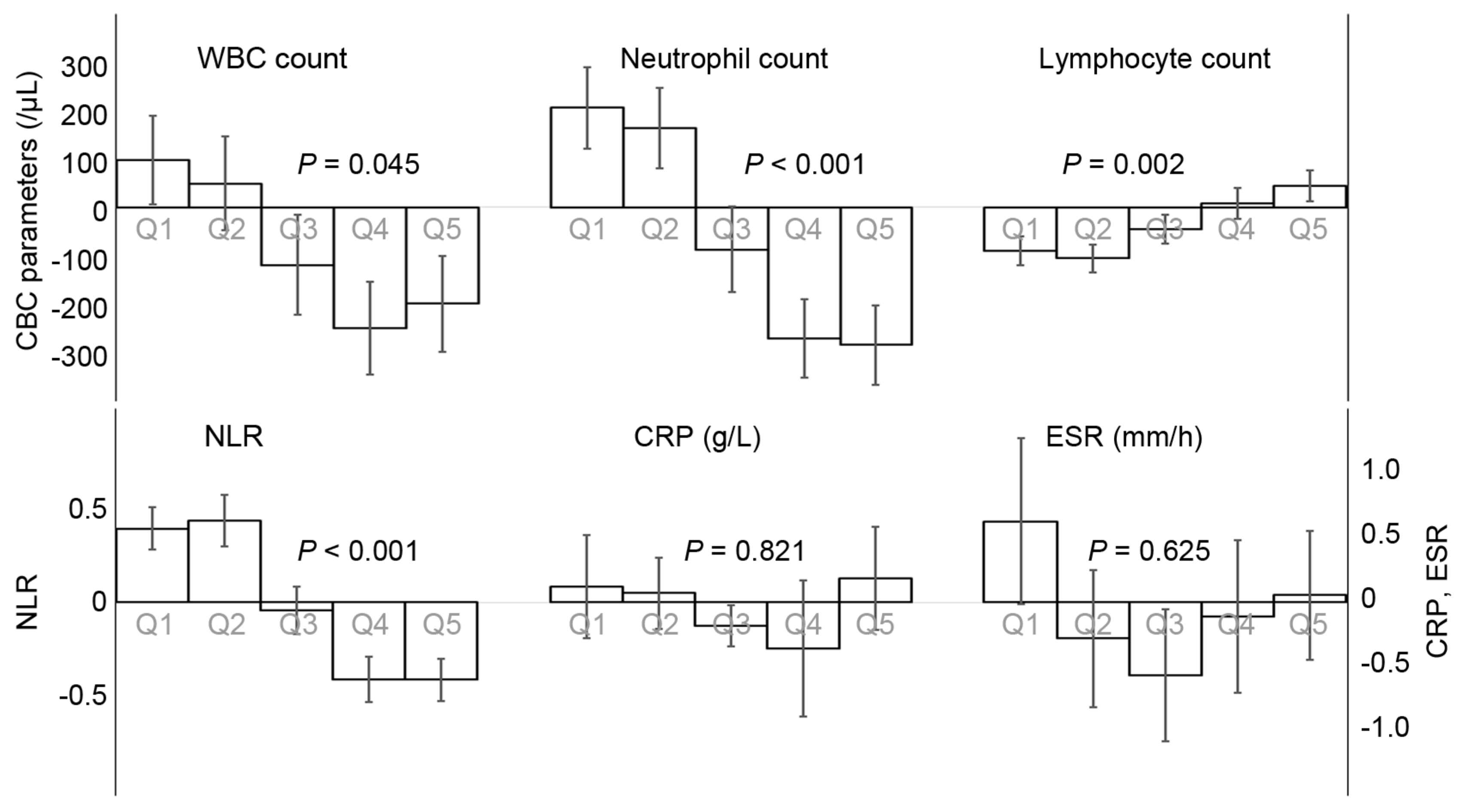
3.3. The Time-Dependent Changes of NKA and Concomitant Change of CBC Parameters and Inflammatory Indices
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bryceson, Y.T.; Chiang, S.; Darmanin, S.; Fauriat, C.; Schlums, H.; Theorell, J.; Wood, S.M. Molecular mechanisms of natural killer cell activation. J. Innate Immun. 2011, 3, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.; March, M.; Ljunggren, H.-G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauriat, C.; Long, E.O.; Ljunggren, H.-G.; Bryceson, Y. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosello-Trampont, A.; Surette, F.A.; Ewald, S.E.; Hahn, Y.S. Immunoregulatory Role of NK Cells in Tissue Inflammation and Regeneration. Front. Immunol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitti, B.; Bryceson, Y.T. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 2018, 42, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.; Lewis, J.E.; Melillo, A.B.; Leonard, S.; Ali, K.H.; Asthana, D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand. J. Immunol. 2012, 75, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Cha, J.; Kim, I.-K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.-Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Jobin, G.; Rodriguez-Suarez, R.; Betito, K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology 2017, 153, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J.; Rodriguez-Suarez, R.; Betito, K. Association between natural killer cell activity and prostate cancer: A pilot study. Can. J. Urol. 2017, 24, 8708–8713. [Google Scholar]
- Choi, S.I.; Lee, S.H.; Park, J.-Y.; Kim, K.-A.; Lee, E.J.; Lee, S.Y.; In, K.H. Clinical utility of a novel natural killer cell activity assay for diagnosing non-small cell lung cancer: A prospective pilot study. Onco Targets Ther. 2019, 12, 1661–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Chun, S.; Hwang, S.; Kim, J.; Cho, Y.; Lee, J.; Kwack, K.; Choi, S.-W. Vitamin D and Exercise Are Major Determinants of Natural Killer Cell Activity, Which Is Age- and Gender-Specific. Front. Immunol. 2021, 12, 594356. [Google Scholar] [CrossRef]
- Harrison, M. Erythrocyte sedimentation rate and C-reactive protein. Aust. Prescr. 2015, 38, 93–94. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K. Neutrophils suppress intraluminal NK cell–mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, B.N.; Donadieu, J.; Cognet, C.; Bernat, C.; Ordoñez-Rueda, D.; Barlogis, V.; Mahlaoui, N.; Fenis, A.; Narni-Mancinelli, E.; Beaupain, B.; et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012, 209, 565–580. [Google Scholar] [CrossRef]
- Barker, T.; Fulde, G.; Moulton, B.; Nadauld, L.D.; Rhodes, T. An elevated neutrophil-to-lymphocyte ratio associates with weight loss and cachexia in cancer. Sci. Rep. 2020, 10, 7535. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhatip, A.; Kamel, M.G.; Hamza, M.K.; Farag, E.M.; Yassin, H.M.; Elayashy, M.; Naguib, A.A.; Wagih, M.; Abd-Elhay, F.A.-E.; Algameel, H.Z.; et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021, 21, 505–514. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Jiang, X.; Zhu, H.; Lu, Z.; Xu, L. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: A meta-analysis of observational studies. Atherosclerosis 2014, 234, 206–213. [Google Scholar] [CrossRef]
- Kim, B.-R.; Chun, S.; Cho, D.; Kim, K.-H. Association of neutrophil-to-lymphocyte ratio and natural killer cell activity revealed by measurement of interferon-gamma levels in a healthy population. J. Clin. Lab. Anal. 2019, 33, e22640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nederby, L.; Jakobsen, A.; Hokland, M.; Hansen, T.F. Quantification of NK cell activity using whole blood: Methodological aspects of a new test. J. Immunol. Methods 2018, 458, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef] [PubMed]
- Vano, Y.-A.; Oudard, S.; By, M.-A.; Têtu, P.; Thibault, C.; Aboudagga, H.; Scotté, F.; ElAidi, R. Optimal cut-off for neutrophil-to-lymphocyte ratio: Fact or fantasy? A prospective cohort study in metastatic cancer patients. PLoS ONE 2018, 13, e0195042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Pera, A.; Lopez-Fernandez, I.; Alonso, C.; Tarazona, R.; Solana, R. Proinflammatory status influences NK cells subsets in the elderly. Immunol. Lett. 2014, 162, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Mocikat, R.; Braumüller, H.; Gumy, A.; Egeter, O.; Ziegler, H.; Reusch, U.; Bubeck, A.; Louis, J.; Mailhammer, R.; Riethmüller, G.; et al. Natural killer cells activated by MHC class I low targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 2003, 19, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Gaudino, S.J.; Kumar, P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, S.; Romero-Weaver, A.; Scarzello, A.; Gamero, A. Interferon: Cellular executioner or white knight? Curr. Med. Chem. 2007, 14, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.; Faridi, R.M.; Sligl, W.; Shabani-Rad, M.-T.; Dharmani-Khan, P.; Parker, A.; Kalra, A.; Tripathi, M.B.; Storek, J.; Tervaert, J.W.C.; et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020, 4, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, C.; Khan, L.; Osman, M.S.; Tervaert, J.W.C. Natural Killer Cell Dysfunction and Its Role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, L.; Backteman, K.; Ernerudh, J. Loss of natural killer cell activity in patients with coronary artery disease. Atherosclerosis 2005, 183, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Rose, N.R.; Čiháková, D. Natural killer cells in inflammatory heart disease. Clin. Immunol. 2017, 175, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Feldman, M.; Aziz, B.; Kang, G.N.; Opondo, M.A.; Belz, R.K.; Sellers, C. C-reactive protein and erythrocyte sedimentation rate discordance: Frequency and causes in adults. Transl. Res. 2013, 161, 37–43. [Google Scholar] [CrossRef]
- Oda, E.; Kawai, R. Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern. Med. 2010, 49, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, J.M.; Trompet, S.; Blauw, G.J.; Westendorp, R.G.J.; de Craen, A.J.M. White blood cell count and C-reactive protein are independent predictors of mortality in the oldest old. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 764–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Subjects at Baseline | |||
|---|---|---|---|
| Total (N = 7031) | Men (N = 3098) | Women (N = 3933) | |
| Age (years) | 49.1 ± 12.5 | 50.7 ± 12.3 | 47.9 ± 12.5 |
| Body mass index (kg/m2) | 23.49 ± 3.75 | 25.3 ± 3.3 | 21.9 ± 3.4 |
| NKA (pg/mL) | 674 (248–1656) | 683 (254–1677) | 664 (240–1648) |
| WBC (/µL) | 5390 (4500–6490) | 5600 (4700–6713) | 5230 (4360–6290) |
| Neutrophil count (/µL) | 2928 (2301–3715) | 3019 (2409–3802) | 2851 (2228–3621) |
| Lymphocyte count (/µL) | 1831 (1499–2218) | 1886 (1532–2282) | 1789 (1468–2162) |
| NLR | 1.59 (1.24–2.07) | 1.59 (1.25–2.07) | 1.59 (1.23–2.08) |
| CRP (g/L) | 0.7 (0.4–1.5) | 0.9 (0.5–1.8) | 0.6 (0.3–1.3) |
| ESR (mm/h) | 6.0 (3.0–11.0) | 4.0 (2.0–9.0) | 7.0 (3.0–13.0) |
| Subjects with repeated measurement (N = 1005) | |||
| Baseline | Follow-up | p | |
| Age (years) | 51.6 ± 12.0 | ||
| Sex (men) | 478 (47.6%) | ||
| Hypertension | 177 (17.6%) | ||
| Diabetes | 58 (5.8%) | ||
| Dyslipidemia | 237 (23.6%) | ||
| Alcohol consumer | 399 (39.7%) | ||
| Smoking | |||
| Non-smoker | 673 (67.0%) | ||
| Current smoker | 123 (12.2%) | ||
| Ex-smoker | 209 (20.8%) | ||
| NKA (pg/mL) | 681 (252–1692) | 667 (249–1576) | 0.616 |
| WBC (/µL) | 5400 (4450–6415) | 5290 (4445–6325) | 0.013 |
| Neutrophil count (/µL) | 2905 (2244–3695) | 2870 (2249–3676) | 0.065 |
| Lymphocyte count (/µL) | 1831 (1519–2230) | 1822 (1509–2160) | 0.007 |
| NLR | 1.59 (1.22–2.04) | 1.60 (1.23–2.03) | 0.681 |
| CRP (g/L) | 0.6 (0.3–1.3) | 0.7 (0.4–1.4) | 0.037 |
| ESR (mm/h) | 6.0 (3.0–11.0) | 6.0 (3.0–11.0) | 0.917 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-K.; Haam, J.-H.; Cho, S.-H.; Kim, Y.-S. Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity. Diagnostics 2022, 12, 448. https://doi.org/10.3390/diagnostics12020448
Lee Y-K, Haam J-H, Cho S-H, Kim Y-S. Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity. Diagnostics. 2022; 12(2):448. https://doi.org/10.3390/diagnostics12020448
Chicago/Turabian StyleLee, Yun-Kyong, Ji-Hee Haam, Sung-Hoon Cho, and Young-Sang Kim. 2022. "Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity" Diagnostics 12, no. 2: 448. https://doi.org/10.3390/diagnostics12020448
APA StyleLee, Y.-K., Haam, J.-H., Cho, S.-H., & Kim, Y.-S. (2022). Cross-Sectional and Time-Dependent Analyses on Inflammatory Markers following Natural Killer Cell Activity. Diagnostics, 12(2), 448. https://doi.org/10.3390/diagnostics12020448






