Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients—A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
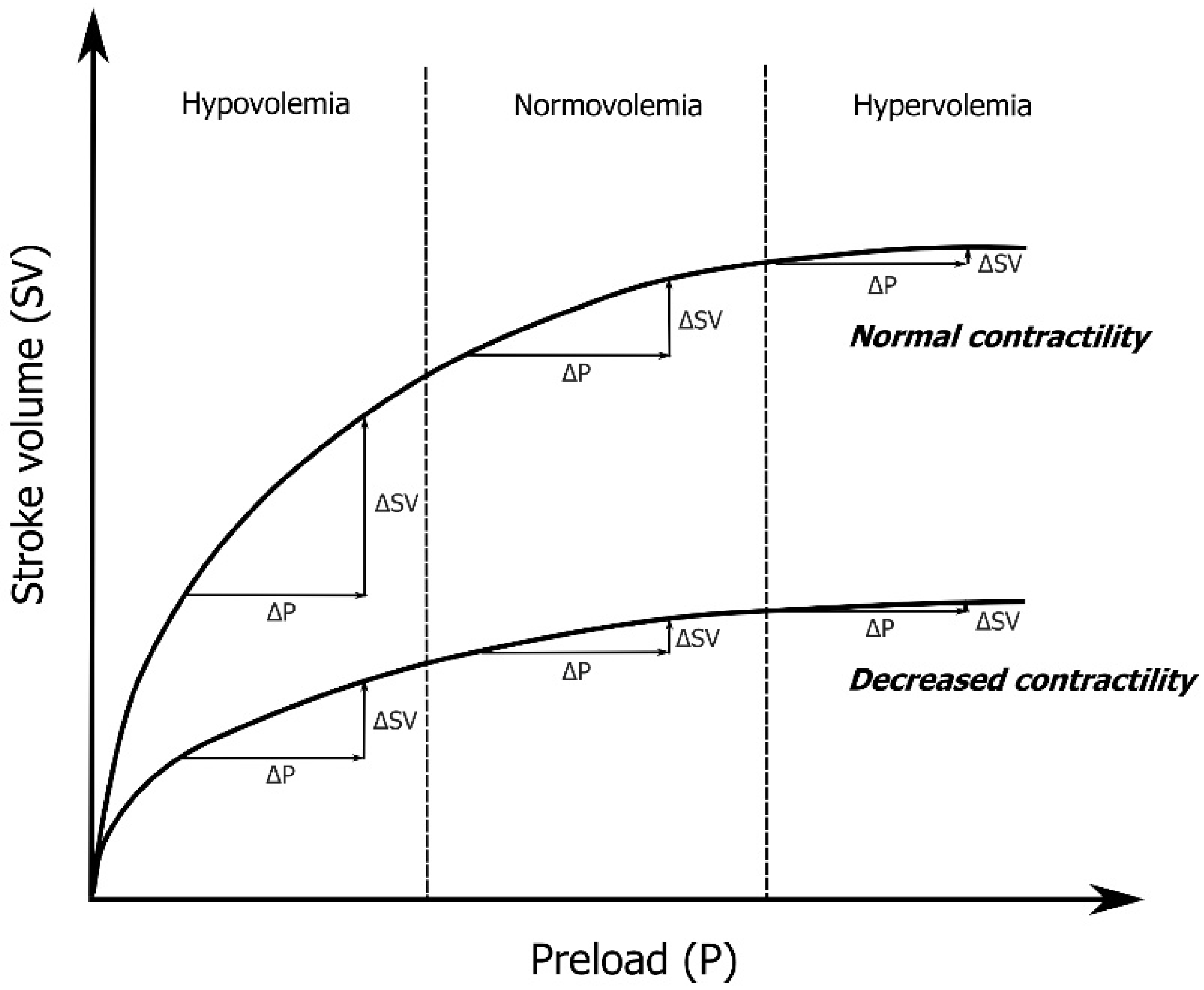
3. End-Expiratory and End-Inspiratory Occlusion
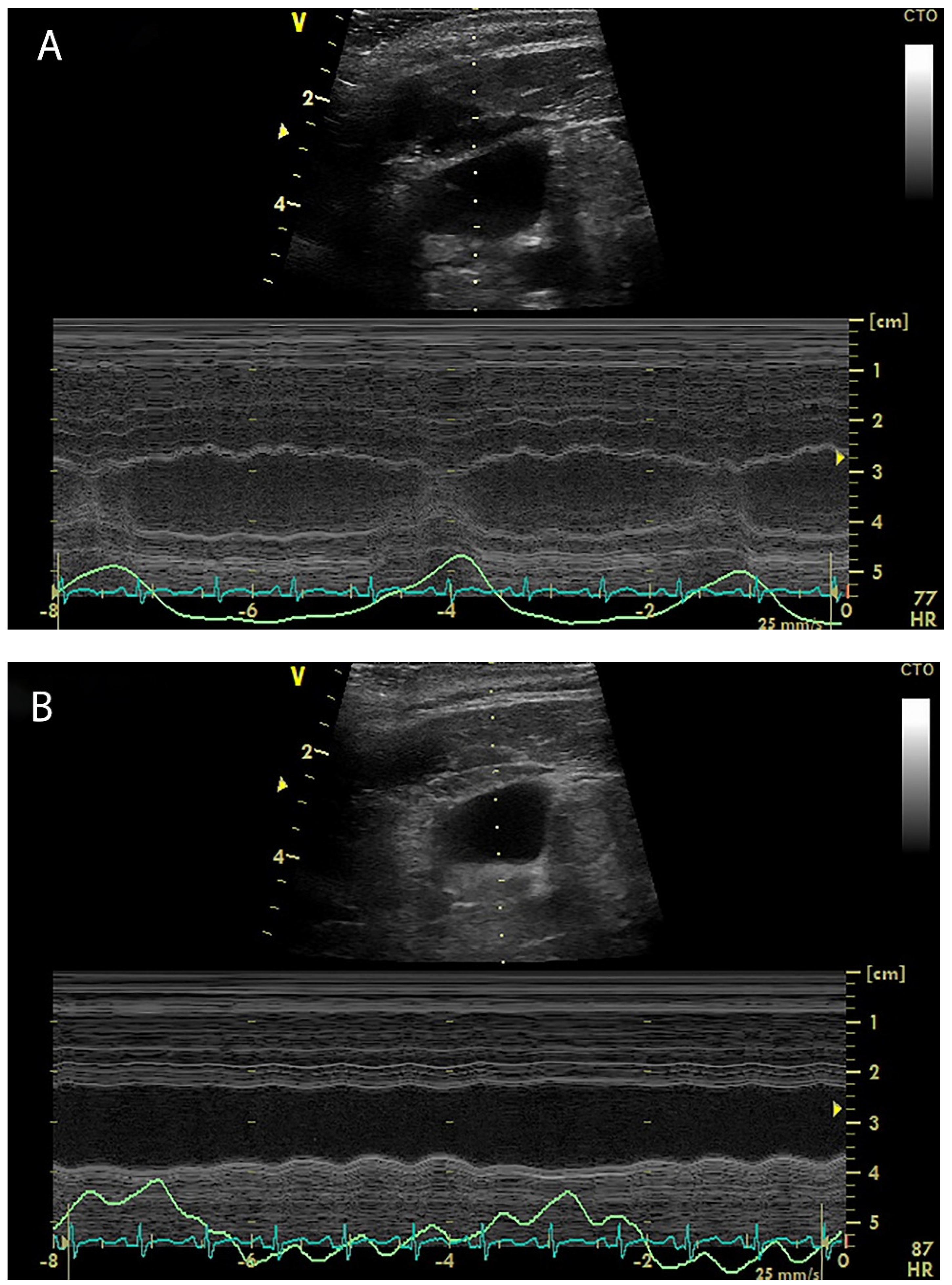
4. Internal Jugular Vein Distensibility
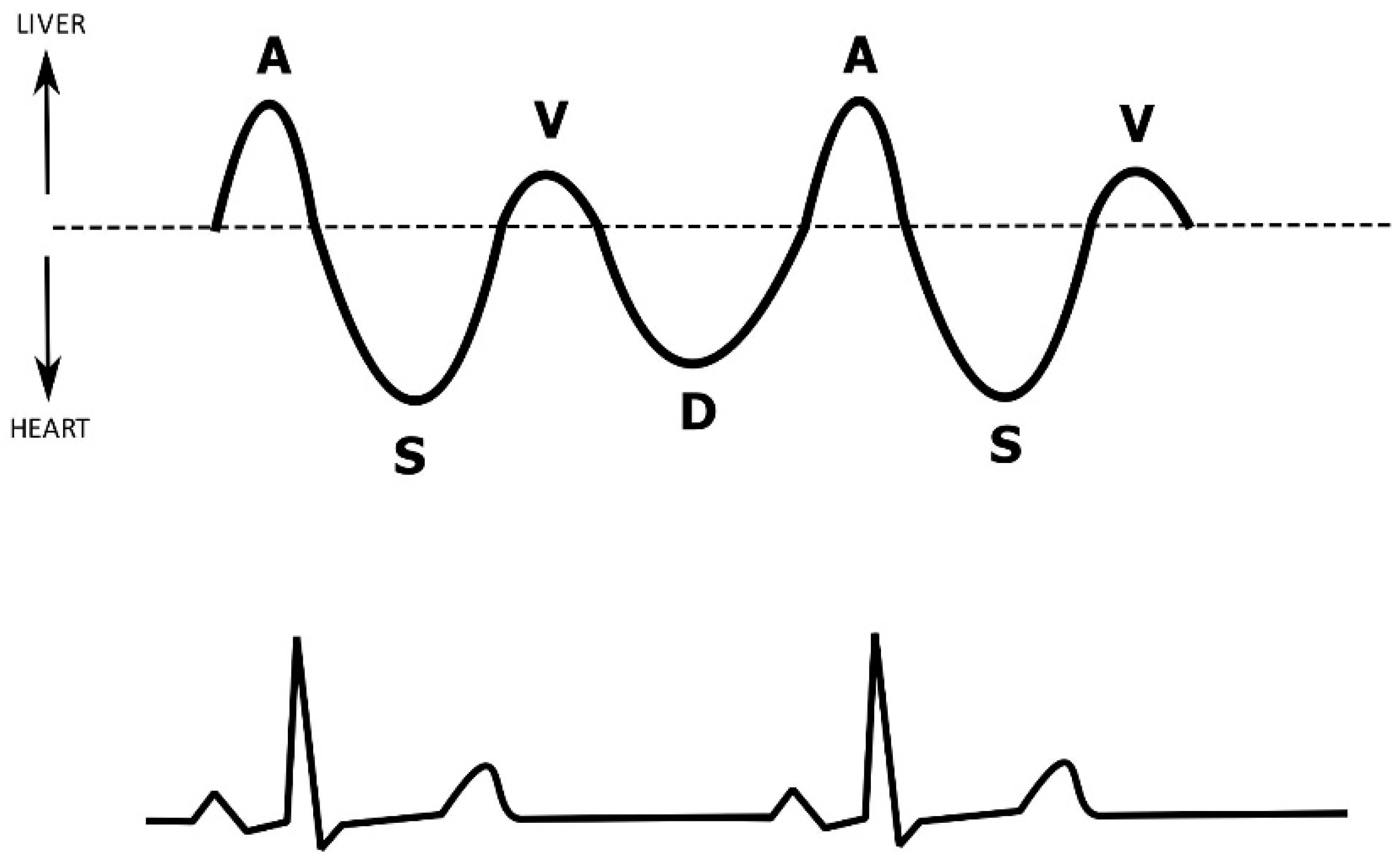
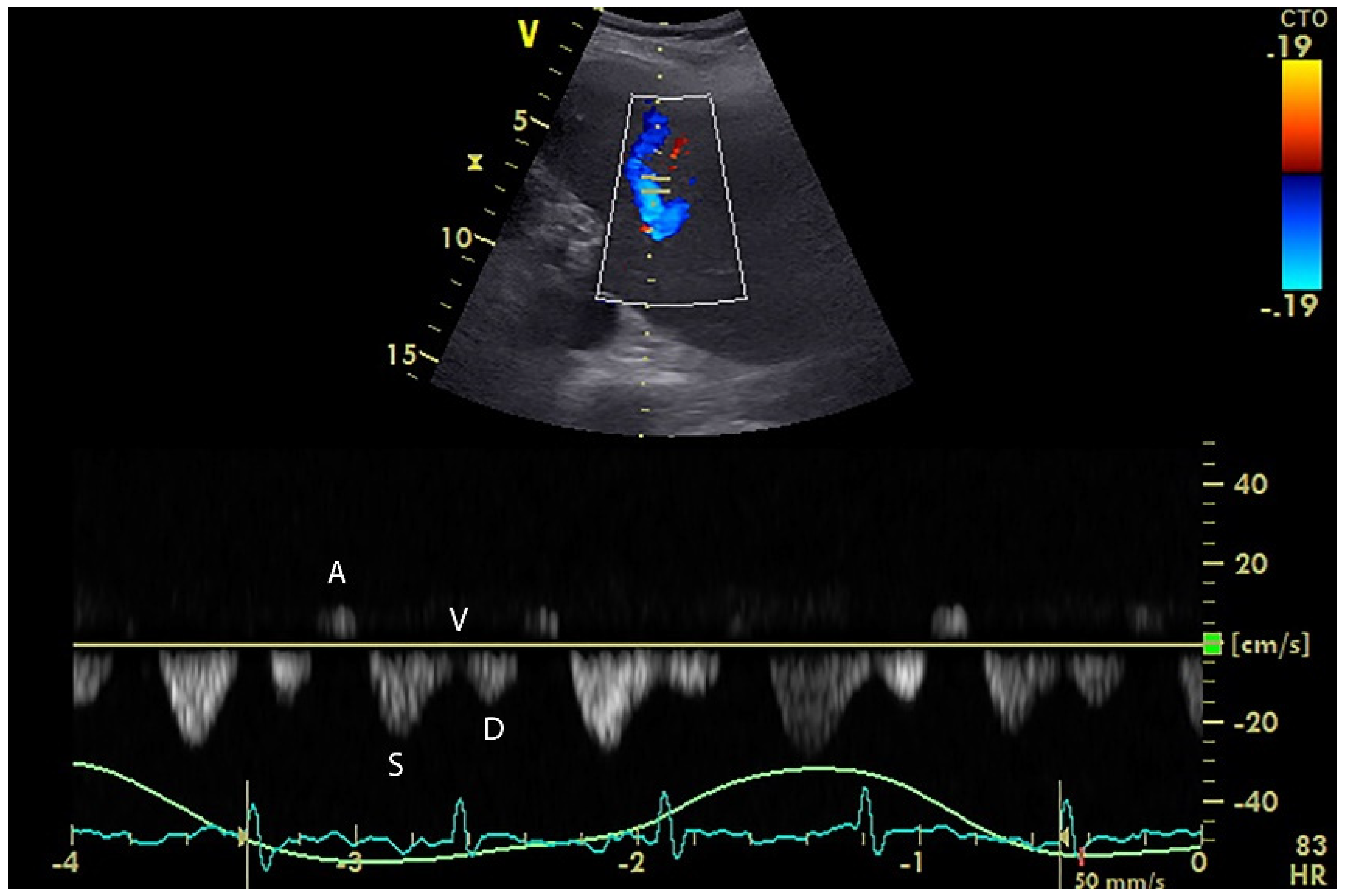
5. Hepatic Venous Flow
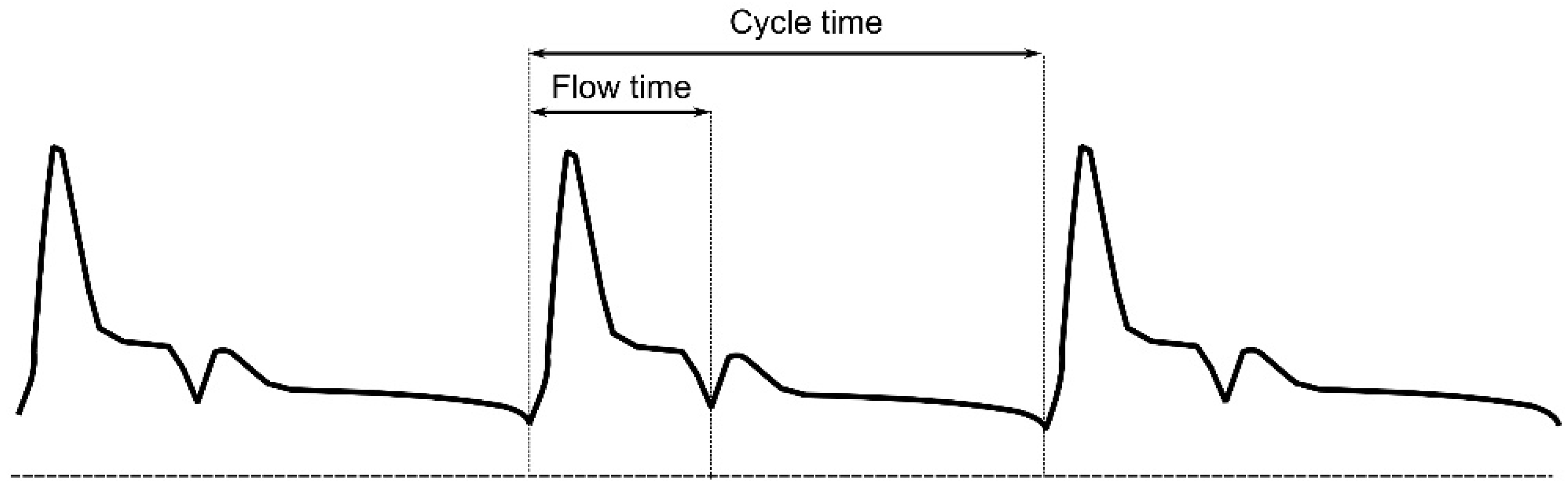
6. Arterial Doppler Monitoring
6.1. Carotid Artery
6.2. Brachial Artery
6.3. Femoral Artery
6.4. Splenic Artery
7. Extrasystoles
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marik, P.E.; Weinmann, M. Optimizing fluid therapy in shock. Curr. Opin. Crit. Care 2019, 25, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Teboul, J.-L. Assessment of volume responsiveness during mechanical ventilation: Recent advances. Crit. Care 2013, 17, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.E.; Levitov, A.; Young, A.; Andrews, L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 2013, 143, 364–370. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Weil, M.H. Fluid challenge revisited. Crit. Care Med. 2006, 34, 1333–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michard, F.; Teboul, J.-L. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest 2002, 121, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, M.; Teboul, J.-L.; Pettila, V.; Wilkman, E.; Molnar, Z.; Della Rocca, G.; Aldecoa, C.; Artigas, A.; Jog, S.; Sander, M.; et al. Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 2015, 41, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, M.E.; Prowle, J.R. Fluid Overload. Crit. Care Clin. 2015, 31, 803–821. [Google Scholar] [CrossRef]
- Wiedemann, H.P.; Wheeler, A.P.; Bernard, G.R. Comparison of two fluid–management strategies in acute lung injury. N. Engl. J. Med. 2006, 354, 2564–2575. [Google Scholar] [CrossRef] [Green Version]
- Acheampong, A.; Vincent, J.L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care 2015, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Boyd, J.; Forbes, J.; Nakada, T.A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Neyra, J.A.; Li, X.; Canepa-Escaro, F.; Adams-Huet, B.; Toto, R.D.; Yee, J.; Hedayati, S.S. Cumulative Fluid Balance and Mortality in Septic Patients With or Without Acute Kidney Injury and Chronic Kidney Disease. Crit. Care Med. 2016, 44, 1891–1900. [Google Scholar] [CrossRef]
- Li, D.K.; Wang, X.T.; Liu, D.W. Association between elevated central venous pressure and outcomes in critically ill patients. Ann. Intensive Care 2017, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Levy, M.M.; Dellinger, R.P.; Townsend, S.R.; Linde-Zwirble, W.T.; Marshall, J.C.; Bion, J.; Schorr, C.; Artigas, A.; Ramsay, G.; Beale, R.; et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit. Care Med. 2010, 38, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Teboul, J.-L. Volume responsiveness. Curr. Opin. Crit. Care 2007, 13, 549–553. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta–analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef]
- Eskesen, T.G.; Wetterslev, M.; Perner, A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016, 42, 324–332. [Google Scholar] [CrossRef]
- Ait-Hamou, Z.A.-O.; Teboul, J.L.; Anguel, N.; Monnet, X. How to detect a positive response to a fluid bolus when cardiac output is not measured? Ann. Intensive Care 2019, 9, 138. [Google Scholar] [CrossRef]
- Pinsky, M.R.; Payen, D. Functional hemodynamic monitoring. Crit. Care 2005, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Michard, F.; Boussat, S.; Chemla, D.; Anguel, N.; Mercat, A.; Lecarpentier, Y.; Richard, C.; Pinsky, M.R.; Teboul, J.L. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am. J. Respir. Crit. Care Med. 2000, 162, 134–138. [Google Scholar] [CrossRef]
- Monnet, X.; Rienzo, M.; Osman, D.; Anguel, N.; Richard, C.; Pinsky, M.R.; Teboul, J.L. Passive leg raising predicts fluid responsiveness in the critically ill. Crit. Care Med. 2006, 34, 1402–1407. [Google Scholar] [CrossRef]
- Mallat, J.; Meddour, M.; Durville, E.; Lemyze, M.; Pepy, F.; Temime, J.; Vangrunderbeeck, N.; Tronchon, L.; Thevenin, D.; Tavernier, B. Decrease in pulse pressure and stroke volume variations after mini–fluid challenge accurately predicts fluid responsiveness. Br. J. Anaesth. 2015, 115, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Michard, F. Volume Management Using Dynamic Parameters. Chest 2005, 128, 1902–1904. [Google Scholar] [CrossRef]
- Mahjoub, Y.; Lejeune, V.; Muller, L.; Perbet, S.; Zieleskiewicz, L.; Bart, F.; Veber, B.; Paugam-Burtz, C.; Jaber, S.; Ayham, A.; et al. Evaluation of pulse pressure variation validity criteria in critically ill patients: A prospective observational multicentre point–prevalence study. Br. J. Anaesth. 2014, 112, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Zogheib, E.; Defouilloy, C.; Mahjoub, Y.; Cherradi, N.; Moubarak, M.; Beloucif, S.; Dupont, H. Modification of hemodynamic effect after passive leg raising test by the use of elastic compression stocking. Intensive Care Med. 2007, 33, S72. [Google Scholar]
- Monnet, X.; Teboul, J.-L. Passive leg raising: Five rules, not a drop of fluid! Crit. Care 2015, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Cherpanath, T.G.; Hirsch, A.; Geerts, B.F.; Lagrand, W.K.; Leeflang, M.M.; Schultz, M.J.; Groeneveld, A.B. Predicting Fluid Responsiveness by Passive Leg Raising: A Systematic Review and Meta–Analysis of 23 Clinical Trials. Crit. Care Med. 2016, 44, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Osman, D.; Ridel, C.; Lamia, B.; Richard, C.; Teboul, J.L. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit. Care Med. 2009, 37, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, M.; Depret, F.; Teboul, J.L.; Alphonsine, J.E.; Lai, C.; Richard, C.; Monnet, X. Predicting Fluid Responsiveness in Critically Ill Patients by Using Combined End-Expiratory and End-Inspiratory Occlusions With Echocardiography. Crit. Care Med. 2017, 45, e1131–e1138. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Bleibtreu, A.; Ferre, A.; Dres, M.; Gharbi, R.; Richard, C.; Teboul, J.L. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit. Care Med. 2012, 40, 152–157. [Google Scholar] [CrossRef]
- Silva, S.; Jozwiak, M.; Teboul, J.L.; Persichini, R.; Richard, C.; Monnet, X. End-expiratory occlusion test predicts preload responsiveness independently of positive end-expiratory pressure during acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 1692–1701. [Google Scholar] [CrossRef]
- Biais, M.; Larghi, M.; Henriot, J.; de Courson, H.; Sesay, M.; Nouette-Gaulain, K. End-Expiratory Occlusion Test Predicts Fluid Responsiveness in Patients With Protective Ventilation in the Operating Room. Anesth. Analg. 2017, 125, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; Tu, G.W.; Cang, J.; Hou, J.Y.; Yu, Y.; Luo, Z.; Guo, K.F. End-expiratory occlusion test predicts fluid responsiveness in cardiac surgical patients in the operating theatre. Ann. Transl. Med. 2019, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Myatra, S.N.; Prabu, N.R.; Divatia, J.V.; Monnet, X.; Kulkarni, A.P.; Teboul, J.L. The Changes in Pulse Pressure Variation or Stroke Volume Variation After a “Tidal Volume Challenge” Reliably Predict Fluid Responsiveness During Low Tidal Volume Ventilation. Crit. Care Med. 2017, 45, 415–421. [Google Scholar] [CrossRef]
- Beurton, A.; Gavelli, F.; Teboul, J.L.; De Vita, N.; Monnet, X. Changes in the Plethysmographic Perfusion Index During an End-Expiratory Occlusion Detect a Positive Passive Leg Raising Test. Crit. Care Med. 2021, 49, e151–e160. [Google Scholar] [CrossRef]
- Depret, F.; Jozwiak, M.; Teboul, J.L.; Alphonsine, J.E.; Richard, C.; Monnet, X. Esophageal Doppler Can Predict Fluid Responsiveness Through End-Expiratory and End–Inspiratory Occlusion Tests. Crit. Care Med. 2019, 47, e96–e102. [Google Scholar] [CrossRef]
- Monnet, X.; Dres, M.; Ferre, A.; Le Teuff, G.; Jozwiak, M.; Bleibtreu, A.; Le Deley, M.C.; Chemla, D.; Richard, C.; Teboul, J.L. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: Comparison with four other dynamic indices. Br. J. Anaesth. 2012, 109, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Georges, D.; de Courson, H.; Lanchon, R.; Sesay, M.; Nouette-Gaulain, K.; Biais, M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: An echocardiographic study. Crit. Care 2018, 22, 32. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Dell’Anna, A.; Baggiani, M.; Torrini, F.; Maresca, G.M.; Bennett, V.; Saderi, L.; Sotgiu, G.; Antonelli, M.; Cecconi, M. Functional hemodynamic tests: A systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit. Care 2019, 23, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavelli, F.; Shi, R.; Teboul, J.-L.; Azzolina, D.; Monnet, X. The end-expiratory occlusion test for detecting preload responsiveness: A systematic review and meta-analysis. Ann. Intensive Care 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Song, X.; Lin, Q.; Nie, Y.; Zhang, G.; Xu, H.; Chen, M.; Wu, J.; Guan, X. Does End-Expiratory Occlusion Test Predict Fluid Responsiveness in Mechanically Ventilated Patients? A Systematic Review and Meta–Analysis. Shock 2020, 54, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Yonis, H.; Bitker, L.; Aublanc, M.; Perinel Ragey, S.; Riad, Z.; Lissonde, F.; Louf-Durier, A.; Debord, S.; Gobert, F.; Tapponnier, R.; et al. Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit. Care 2017, 21, 295. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Montagnini, C.; Cammarota, G.; De Rosa, S.; Giuliani, F.; Muratore, L.; Della Corte, F.; Navalesi, P.; Cecconi, M. Tidal volume challenge to predict fluid responsiveness in the operating room: An observational study. Eur. J. Anaesthesiol. 2019, 36, 583–591. [Google Scholar] [CrossRef]
- Guinot, P.G.; Godart, J.; de Broca, B.; Bernard, E.; Lorne, E.; Dupont, H. End-expiratory occlusion manoeuvre does not accurately predict fluid responsiveness in the operating theatre. Br. J. Anaesth. 2014, 112, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Teboul, J.L. End-expiratory occlusion test: Please use the appropriate tools! Br. J. Anaesth. 2015, 114, 166–167. [Google Scholar] [CrossRef] [Green Version]
- Jozwiak, M.; Mercado, P.; Teboul, J.-L.; Benmalek, A.; Gimenez, J.; Dépret, F.; Richard, C.; Monnet, X. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit. Care 2019, 23, 116. [Google Scholar] [CrossRef] [Green Version]
- Guarracino, F.; Ferro, B.; Forfori, F.; Bertini, P.; Magliacano, L.; Pinsky, M.R. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit. Care 2014, 18, 647. [Google Scholar] [CrossRef] [Green Version]
- Akilli, N.B.; Cander, B.; Dundar, Z.D.; Koylu, R. A new parameter for the diagnosis of hemorrhagic shock: Jugular index. J. Crit. Care 2012, 27, 530.e13–530.e18. [Google Scholar] [CrossRef] [PubMed]
- Broilo, F.; Meregalli, A.; Friedman, G. Right internal jugular vein distensibility appears to be a surrogate marker for inferior vena cava vein distensibility for evaluating fluid responsiveness. Rev. Bras. Ter. Intensiv. 2015, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.G.; Hao, G.W.; Yang, X.M.; Zhu, D.M.; Liu, L.; Liu, H.; Tu, G.W.; Luo, Z. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann. Intensive Care 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haliloglu, M.; Bilgili, B.; Kararmaz, A.; Cinel, I. The value of internal jugular vein collapsibility index in sepsis. Ulus Travma Acil Cerrahi Derg. 2017, 23, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Thudium, M.; Klaschik, S.; Ellerkmann, R.K.; Putensen, C.; Hilbert, T. Is internal jugular vein extensibility associated with indices of fluid responsiveness in ventilated patients? Acta Anaesthesiol. Scand. 2016, 60, 723–733. [Google Scholar] [CrossRef]
- Iizuka, Y.; Nomura, T.; Sanui, M.; Mochida, Y.; Aomatsu, A.; Lefor, A.K. Collapsibility of the Right Internal Jugular Vein Predicts Responsiveness to Fluid Administration in Patients Receiving Pressure Support Ventilation: A Prospective Cohort Study. J. Clin. Med. Res. 2020, 12, 150–156. [Google Scholar] [CrossRef]
- Nagdev, A.D.; Merchant, R.C.; Tirado-Gonzalez, A.; Sisson, C.A.; Murphy, M.C. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann. Emerg. Med. 2010, 55, 290–295. [Google Scholar] [CrossRef]
- Long, E.; Oakley, E.; Duke, T.; Babl, F.E. Does Respiratory Variation in Inferior Vena Cava Diameter Predict Fluid Responsiveness: A Systematic Review and Meta-Analysis. Shock 2017, 47, 550–559. [Google Scholar] [CrossRef]
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of Ultrasonographic Measurements of Inferior Vena Cava to Determine Fluid Responsiveness: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2020, 35, 354–363. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.T.; Long, Y.; Liu, D.W. Monitoring Changes in Hepatic Venous Velocities Flow after a Fluid Challenge Can Identify Shock Patients Who Lack Fluid Responsiveness. Chin. Med. J. 2017, 130, 1202–1210. [Google Scholar] [CrossRef]
- Scheinfeld, M.H.; Bilali, A.; Koenigsberg, M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics 2009, 29, 2081–2098. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Cohen, Y.; Fosse, J.P.; Karoubi, P.; Hoang, P.; Cupa, M. Haemodynamic measurements (continuous cardiac output and systemic vascular resistance) in critically ill patients: Transoesophageal Doppler versus continuous thermodilution. Anaesth. Intensive Care 1999, 27, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dark, P.M.; Singer, M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med. 2004, 30, 2060–2066. [Google Scholar] [CrossRef]
- Blehar, D.J.; Glazier, S.; Gaspari, R.J. Correlation of corrected flow time in the carotid artery with changes in intravascular volume status. J. Crit Care 2014, 29, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D.C.; Khan, N.A.; Blehar, D.; Glazier, S.; Chang, Y.; Stowell, C.P.; Noble, V.E.; Liteplo, A.S. Carotid Flow Time Changes with Volume Status in Acute Blood Loss. Ann. Emerg. Med. 2015, 66, 277–282.e1. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nejad, H.; Mohammadinejad, P.; Lessan-Pezeshki, M.; Davarani, S.S.; Banaie, M. Carotid artery corrected flow time measurement via bedside ultrasonography in monitoring volume status. J. Crit. Care 2015, 30, 1199–1203. [Google Scholar] [CrossRef]
- Chebl, R.B.; Wuhantu, J.; Kiblawi, S.; Abou Dagher, G.; Zgheib, H.; Bachir, R.; Carnell, J. Corrected carotid flow time and passive leg raise as a measure of volume status. Am. J. Emerg. Med. 2019, 37, 1460–1465. [Google Scholar] [CrossRef]
- Shokoohi, H.; Berry, G.W.; Shahkolahi, M.; King, J.; King, J.; Salimian, M.; Poshtmashad, A.; Pourmand, A. The diagnostic utility of sonographic carotid flow time in determining volume responsiveness. J. Crit. Care 2017, 38, 231–235. [Google Scholar] [CrossRef]
- Barjaktarevic, I.; Toppen, W.E.; Hu, S.; Montoya, E.A.; Ong, S.; Buhr, R.G.; David, I.J.; Wang, T.; Rezayat, T.; Chang, S.Y.; et al. Ultrasound Assessment of the Change in Carotid Corrected Flow Time in Fluid Responsiveness in Undifferentiated Shock. Crit. Care Med. 2018, 46, e1040–e1046. [Google Scholar] [CrossRef]
- Judson, P.I.; Abhilash KP, P.; Pichamuthu, K.; Chandy, G.M. Evaluation of Carotid Flow Time to Assess Fluid Responsiveness in the Emergency Department. J. Med. Ultrasound 2021, 29, 99–104. [Google Scholar] [CrossRef]
- Garcia, M.I.M.; Cano, A.G.; Monrove, J.C.D. Brachial artery peak velocity variation to predict fluid responsiveness in mechanically ventilated patients. Crit. Care 2009, 13, R142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, J.M.; Blair, J.E.; Hampole, C.; Goonewardena, S.; Vasaiwala, S.; Shah, D.; Spencer, K.T.; Schmidt, G.A. Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand-carried ultrasound devices. Chest 2007, 131, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Préau, S.; Saulnier, F.; Dewavrin, F.; Durocher, A.; Chagnon, J.L. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit. Care Med. 2010, 38, 819–825. [Google Scholar] [CrossRef]
- Luzi, A.; Marty, P.; Mari, A.; Conil, J.M.; Geeraerts, T.; Lepage, B.; Fourcade, O.; Silva, S.; Minville, V. Noninvasive assessment of hemodynamic response to a fluid challenge using femoral Doppler in critically ill ventilated patients. J. Crit. Care 2013, 28, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Corradi, F.; Brusasco, C.; Garlaschi, A.; Santori, G.; Vezzani, A.; Moscatelli, P.; Pelosi, P. Splenic Doppler resistive index for early detection of occult hemorrhagic shock after polytrauma in adult patients. Shock 2012, 38, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, C.; Tavazzi, G.; Robba, C.; Santori, G.; Vezzani, A.; Manca, T.; Corradi, F. Splenic Doppler Resistive Index Variation Mirrors Cardiac Responsiveness and Systemic Hemodynamics upon Fluid Challenge Resuscitation in Postoperative Mechanically Ventilated Patients. Biomed. Res. Int. 2018, 2018, 1978968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doctor, M.; Siadecki, S.D.; Cooper Jr, D.; Rose, G.; Drake, A.B.; Ku, M.; Suprun, M.; Saul, T. Reliability, Laterality and the Effect of Respiration on the Measured Corrected Flow Time of the Carotid Arteries. J. Emerg. Med. 2017, 53, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Girotto, V.; Teboul, J.L.; Beurton, A.; Galarza, L.; Guedj, T.; Richard, C.; Monnet, X. Carotid and femoral Doppler do not allow the assessment of passive leg raising effects. Ann. Intensive Care 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Vistisen, S.T.; Andersen, K.K.; Frederiksen, C.A.; Kirkegaard, H. Variations in the pre-ejection period induced by ventricular extra systoles may be feasible to predict fluid responsiveness. J. Clin. Monit. Comput. 2014, 28, 341–349. [Google Scholar] [CrossRef]
- Vistisen, S.T.; Krog, M.B.; Elkmann, T.; Vallentin, M.F.; Scheeren, T.W.; Sølling, C. Extrasystoles for fluid responsiveness prediction in critically ill patients. J. Intensive Care 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistisen, S.T. Using extra systoles to predict fluid responsiveness in cardiothoracic critical care patients. J. Clin. Monit. Comput. 2017, 31, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Vistisen, S.T.; Berg, J.M.; Boekel, M.F.; Modestini, M.; Bergman, R.; Jainandunsing, J.S.; Mariani, M.A.; Scheeren, T.W.L. Using extra systoles and the micro-fluid challenge to predict fluid responsiveness during cardiac surgery. J. Clin. Monit. Comput. 2019, 33, 777–786. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horejsek, J.; Kunstyr, J.; Michalek, P.; Porizka, M. Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients—A Narrative Review. Diagnostics 2022, 12, 513. https://doi.org/10.3390/diagnostics12020513
Horejsek J, Kunstyr J, Michalek P, Porizka M. Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients—A Narrative Review. Diagnostics. 2022; 12(2):513. https://doi.org/10.3390/diagnostics12020513
Chicago/Turabian StyleHorejsek, Jan, Jan Kunstyr, Pavel Michalek, and Michal Porizka. 2022. "Novel Methods for Predicting Fluid Responsiveness in Critically Ill Patients—A Narrative Review" Diagnostics 12, no. 2: 513. https://doi.org/10.3390/diagnostics12020513





