Pelvic Sidewall Anatomy in Gynecologic Oncology—New Insights into a Potential Avascular Space
Abstract
:1. Introduction
2. Materials and Methods
Surgical Procedures
- Superiorly: the peritoneum covering the external iliac vessels and psoas major muscle.
- Inferiorly: the pelvic floor muscles, namely the piriformis, coccygeus, and iliococcygeus muscles.
- Ventrally: inguinal ligament.
- Dorsally: sacrum.
- Laterally: superiorly by the medial aspect of the psoas major muscle and inferiorly by the obturator internus muscle.
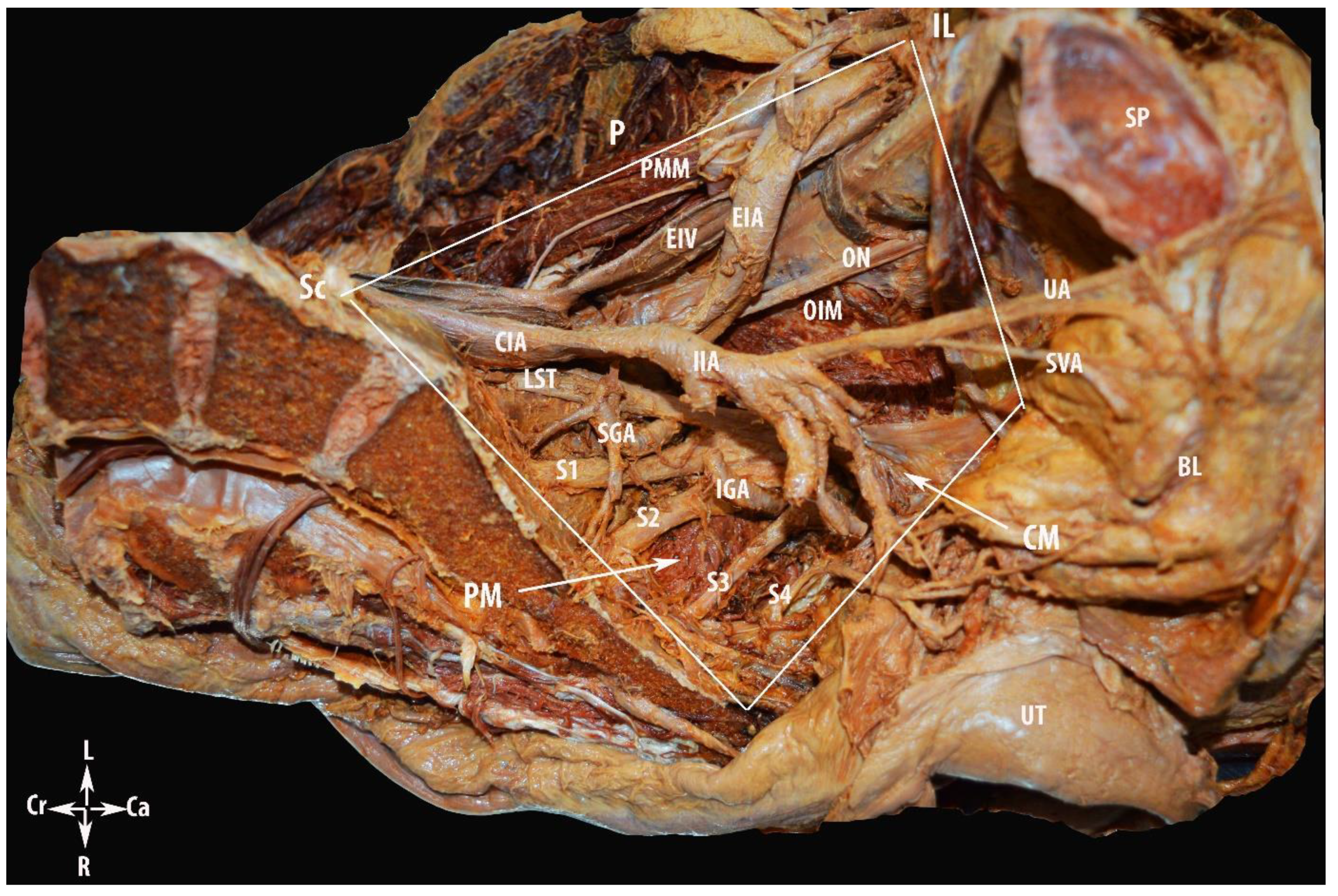
Step 1. Avascular Region
Step 2. Vascular Region
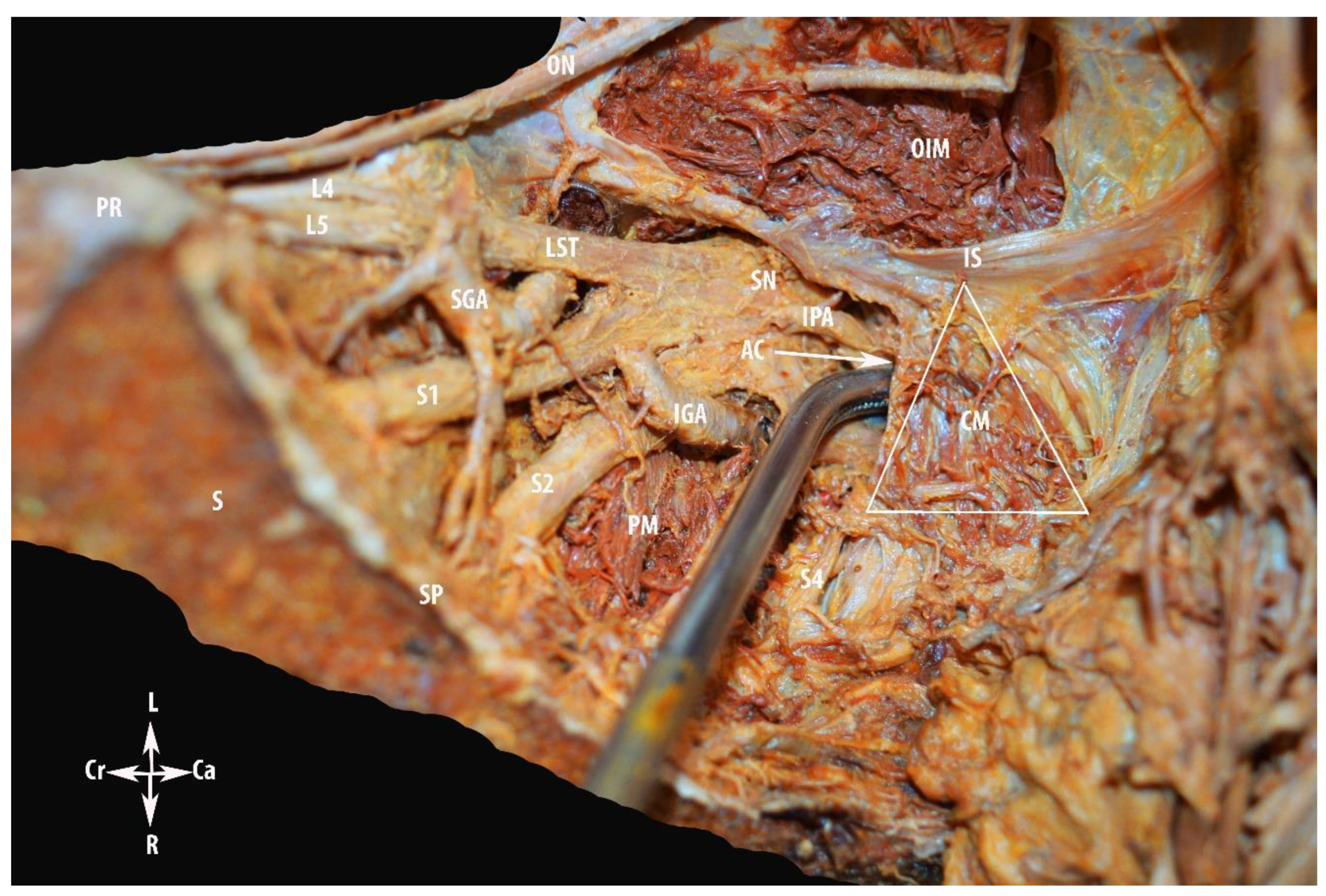
Step 3. Nerve Region
3. Results
4. Discussion
4.1. Terminological Controversies
- Superior: peritoneum covering the psoas major muscle and external iliac vessels.
- Inferior: obturator nerve.
- Ventral: deep circumflex iliac vein.
- Dorsal: common iliac artery bifurcation.
- Lateral: fascia iliacus covering the psoas major muscle, genitofemoral nerve.
- Medial: external iliac artery and vein.
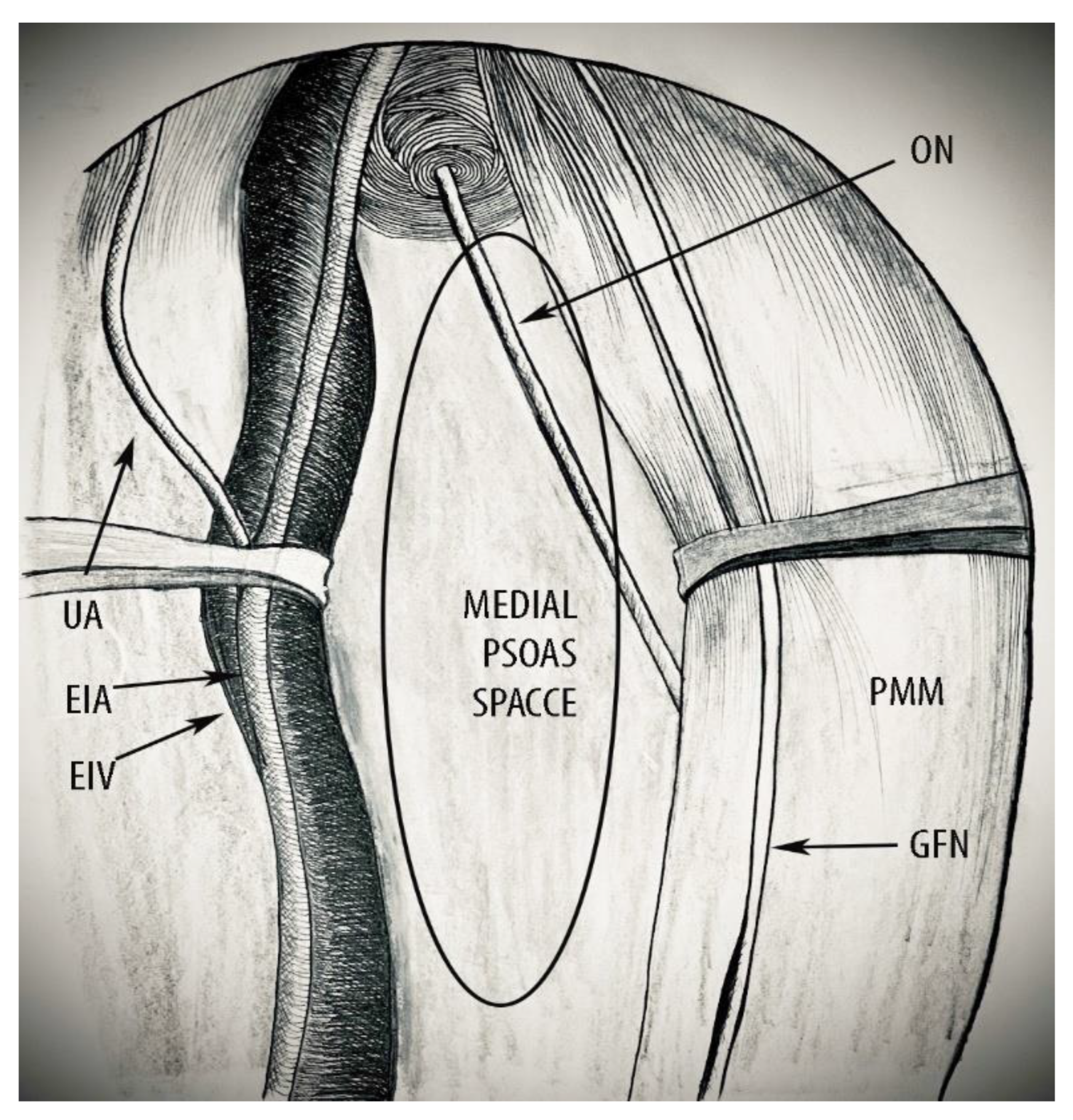

4.2. MPS—Clinical Applications in Gynecologic Oncology
4.2.1. MPS and Avascular Region
Pelvic Lymphadenectomy
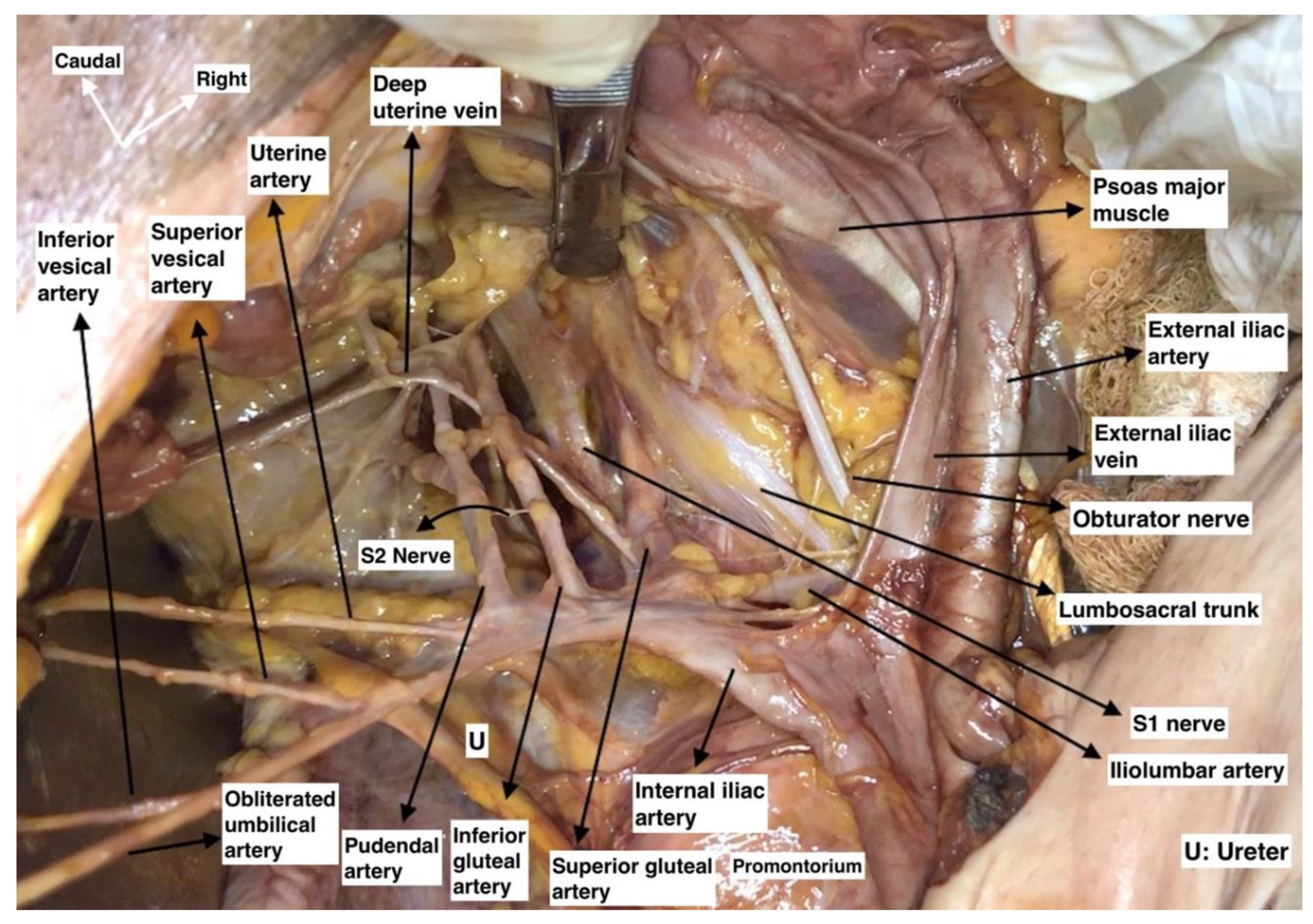
Internal Iliac Artery Ligation
4.2.2. MPS and Vascular Region Dissection
Pelvic Tumors Adhesive to the PSW
Total Mesometrial Resection, Laterally Extended Parametrectomy (LEP) and Laterally Extended Endopelvic Resection (LEER)
Pelvic Sidewall Recurrences
4.2.3. MPS and Nerve Region Dissection
4.3. Anatomical Variations Related to the MPS

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayer, A.; Heinze, T.; Alkatout, I.; Osmonov, D.; Stelzner, S.; Wedel, T. Embryological Development and Topographic Anatomy of Pelvic Compartments-Surgical Relevance for Pelvic Lymphonodectomy. J. Clin. Med. 2021, 10, 708. [Google Scholar] [CrossRef]
- Kostov, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Dimitrov, N.; Yordanov, A. Pelvic Lymphadenectomy in Gynecologic Oncology Significance of Anatomical Variations. Diagnostics 2021, 11, 89. [Google Scholar] [CrossRef]
- Gingold, J.A.; Falcone, T. Retroperitoneal anatomy during excision of pelvic side wall endometriosis. J. Endometr. Pelvic Pain Disord. 2016, 8, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Nishikimi, K.; Tate, S.; Matsuoka, A.; Shozu, M. Removal of the entire internal iliac vessel system is a feasible surgical procedure for locally advanced ovarian carcinoma adhered firmly to the pelvic sidewall. Int. J. Clin. Oncol. 2019, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M. Laterally extended endopelvic resection: Surgical treatment of infrailiac pelvic wall recurrences of gynecologic malignancies. Am. J. Obstet. Gynecol. 1999, 180 Pt 1, 306–312. [Google Scholar] [CrossRef]
- Höckel, M. Laterally extended endopelvic resection (LEER)—Principles and practice. Gynecol. Oncol. 2008, 111 (Suppl. 2), S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Dornhöfer, N. Pelvic exenteration for gynaecological tumours: Achievements and unanswered questions. Lancet Oncol. 2006, 7, 837–847. [Google Scholar] [CrossRef]
- Ungar, L.; Palfalvi, L. Surgical treatment of lymph node metastases in stage IB cervical cancer: The laterally extended parametrectomy (LEP) procedure. Int. J. Gynecol. Cancer 2003, 13, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Căpîlna, M.E.; Ungár, L.; Cozlea, A.L.; Gheorghe, M.; Stanca, M.; Lintner, B.; Moldovan, A.A. Laterally extended parametrectomy. Obstet. Gynecol. Sci. 2021, 64, 470–472. [Google Scholar] [CrossRef]
- Querleu, D. Surgical Spaces, Lymphatic Drainage, Nerves, Topography. Available online: https://eacademy.esgo.org/esgo/2019/esgo-enygo-eso-masterclass/274557/denis.querleu.surgical.spaces.lymphatic.drainage.nerves.topography.html?f=menu%3D16%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D41%2Atopic%3D19404 (accessed on 10 October 2021).
- Cibula, D. Pelvic Side Wall Anatomy. Available online: https://eacademy.esgo.org/esgo/2018/6th-ivw/234207/david.cibula.pelvic.side.wall.anatomy.html?f=menu%3D14%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D10%2Aspeaker%3D48629 (accessed on 2 October 2021).
- Ceccaroni, M.; Clarizia, R.; Roviglione, G.; Ercoli, A. Surgical anatomy of the pelvis. In Minimally Invasive Gynecologic Surgery: Evidenced Based Laparoscopic, Hysteroscopic & Robotic Surgeries, 1st ed.; Einarsson, J.I., Wattiez, A., Eds.; JP Medical: Ashland, OH, USA, 2016; p. 4. [Google Scholar]
- Gray, H.; Standring, S.; Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175. [Google Scholar]
- Kostov, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Dimitrov, N.; Yordanov, A. Lateral Transperitoneal Accesses to the Pelvic Retroperitoneum in Gynecology: Surgical Technique, Anatomical Landmarks and Variations. Indian J. Gynecol. Oncol. 2021, 19, 64. [Google Scholar] [CrossRef]
- Ceccaroni, M.; Clarizia, R.; Alboni, C.; Ruffo, G.; Bruni, F.; Roviglione, G.; Scioscia, M.; Peters, I.; De Placido, G.; Minelli, L. Laparoscopic nerve-sparing transperitoneal approach for endometriosis infiltrating the pelvic wall and somatic nerves: Anatomical considerations and surgical technique. Surg. Radiol. Anat. 2010, 32, 601–604. [Google Scholar] [CrossRef]
- Ercoli, A.; Delmas, V.; Fanfani, F.; Gadonneix, P.; Ceccaroni, M.; Fagotti, A.; Mancuso, S.; Scambia, G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: A dissection-based comparative study. Am. J. Obstet. Gynecol. 2005, 193, 1565–1573. [Google Scholar] [CrossRef]
- Yabuki, Y.; Asamoto, A.; Hoshiba, T.; Nishimoto, H.; Nishikawa, Y.; Nakajima, T. Radical hysterectomy: An anatomic evaluation of parametrial dissection. Gynecol. Oncol. 2000, 77, 155–163. [Google Scholar] [CrossRef]
- Okabayashi, H. Radical abdominal hysterectomy for cancer of the cervix uteri, modification of the Takayama operation. Surg. Gynecol. Obstet. 1921, 33, 335–341. [Google Scholar]
- Possover, M.; Baekelandt, J.; Flaskamp, C.; Li, D.; Chiantera, V. Laparoscopic neurolysis of the sacral plexus and the sciatic nerve for extensive endometriosis of the pelvic wall. Minim. Invasive Neurosurg. 2007, 50, 33–36. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R. Pelvic lymphadenectomy in cervical cancer--surgical anatomy and proposal for a new classification system. Gynecol. Oncol. 2010, 116, 33–37. [Google Scholar] [CrossRef]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Selçuk, İ.; Uzuner, B.; Boduç, E.; Baykuş, Y.; Akar, B.; Güngör, T. Pelvic lymphadenectomy: Step-by-step surgical education video. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 66–69. [Google Scholar] [CrossRef]
- Horn, L.C.; Hentschel, B.; Einenkel, J. Topographic distribution of pelvic lymph node metastases in cervical cancer patients with FIGO stages IB1 to IIB [Cibula D, Abu-Rustum NR. Pelvic lymphadenectomy in cervical cancer--surgical anatomy and proposal for a new classification system. Gynecol Oncol. 2010;116(1):33-7]. Gynecol Oncol. 2010, 118, 93–94. [Google Scholar] [CrossRef]
- Marnitz, S.; Köhler, C.; Bongardt, S.; Braig, U.; Hertel, H.; Schneider, A.; German Association of Gynecologic Oncologists (AGO). Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2006, 103, 35–44. [Google Scholar] [CrossRef]
- Mibayashi, R. Hukushiki-Choukouhannsei-sikyuukeigantekisyutsuzyutsu (Abdominal super-radical hysterectomy). J. Jpn. Obstet. Gynecol. Soc. 1941, 480. (In Japanese) [Google Scholar]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Virchows Arch. Int. J. Pathol. 2018, 472, 919–936. [Google Scholar] [CrossRef] [Green Version]
- Possover, M. Neuropelveology—Lastest Developments in Pelvic Neurofunctional Surgery; The International School of Neuropelveology: Zug, Switzerland, 2015; p. 26. ISBN 97895244533-0-8. [Google Scholar]
- Lolis, E.; Panagouli, E.; Venieratos, D. Study of the ascending lumbar and iliolumbar veins: Surgical anatomy, clinical implications and review of the literature. Ann. Anat. 2011, 193, 516–529. [Google Scholar] [CrossRef]
- Badagabettu Nayak, S.; Padur Aithal, A.; Kumar, N.; Regunathan, D.; Shetty, P.; Alathady Maloor, P. A cadaveric study of variations of external iliac artery and its implication in trauma and radiology. Morphologie 2019, 103, 24–31. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Selçuk, I.; Watrowski, R.; Kornovski, Y.; Yalçın, H.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Yordanov, A. Pelvic Sidewall Anatomy in Gynecologic Oncology—New Insights into a Potential Avascular Space. Diagnostics 2022, 12, 519. https://doi.org/10.3390/diagnostics12020519
Kostov S, Selçuk I, Watrowski R, Kornovski Y, Yalçın H, Slavchev S, Ivanova Y, Dzhenkov D, Yordanov A. Pelvic Sidewall Anatomy in Gynecologic Oncology—New Insights into a Potential Avascular Space. Diagnostics. 2022; 12(2):519. https://doi.org/10.3390/diagnostics12020519
Chicago/Turabian StyleKostov, Stoyan, Ilker Selçuk, Rafał Watrowski, Yavor Kornovski, Hakan Yalçın, Stanislav Slavchev, Yonka Ivanova, Deyan Dzhenkov, and Angel Yordanov. 2022. "Pelvic Sidewall Anatomy in Gynecologic Oncology—New Insights into a Potential Avascular Space" Diagnostics 12, no. 2: 519. https://doi.org/10.3390/diagnostics12020519
APA StyleKostov, S., Selçuk, I., Watrowski, R., Kornovski, Y., Yalçın, H., Slavchev, S., Ivanova, Y., Dzhenkov, D., & Yordanov, A. (2022). Pelvic Sidewall Anatomy in Gynecologic Oncology—New Insights into a Potential Avascular Space. Diagnostics, 12(2), 519. https://doi.org/10.3390/diagnostics12020519








