Radiation Dose and Fluoroscopy Time of Endovascular Coil Embolization in Patients with Carotid Cavernous Fistulas
Abstract
:1. Introduction
2. Materials and Methods
- -
- Age ≥ 18 years
- -
- Presence of a fistula between the ICA and/or ECA and the CS
- -
- Treatment with endovascular embolization using only detachable coils
- -
- CCFs treated with alternative endovascular methods (e.g., detachable balloons, liquid embolic agents, covered stents, flow-diverter stents or combined techniques)
- -
- Recurrent fistulas
- -
- Fistulas located elsewhere but also treated with CS coil embolization
2.1. Procedure
- -
- Low-dose (LD): tube voltage 73 kV, pulse width 50 ms, dose 1820 μGy/pulse
- -
- Normal-dose (ND): tube voltage 73 kV, pulse width 100 ms, dose 3000 μGy/pulse
2.2. Data Collection and Dosimetry Analysis
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrow, D.L.; Spector, R.H.; Braun, I.F.; Landman, J.A.; Tindall, S.C.; Tindall, G.T. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J. Neurosurg. 1985, 62, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ringer, A.J.; Salud, L.; Tomsick, T.A. Carotid cavernous fistulas: Anatomy, classification, and treatment. Neurosurg. Clin. N. Am. 2005, 16, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Korkmazer, B.; Kocak, B.; Tureci, E.; Islak, C.; Kocer, N.; Kizilkilic, O. Endovascular treatment of carotid cavernous sinus fistula: A systematic review. World J. Radiol. 2013, 5, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertl, L.; Brückmann, H.; Patzig, M.; Fesl, G. Endovascular therapy of direct dural carotid cavernous fistulas—A therapy assessment study including long-term follow-up patient interviews. PLoS ONE 2019, 14, e0223488. [Google Scholar] [CrossRef] [Green Version]
- Ertl, L.; Brückmann, H.; Patzig, M.; Dorn, F.; Fesl, G. Patient reported long-term outcome after endovascular therapy of indirect dural carotid cavernous fistulas. PLoS ONE 2020, 15, e0231261. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Melake, M.; Oishi, H.; Yamamoto, M.; Arai, H. Transvenous embolization of dural carotid cavernous fistulas: A series of 44 consecutive patients. AJNR Am. J. Neuroradiol. 2010, 31, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Bink, A.; Goller, K.; Lüchtenberg, M.; Neumann-Haefelin, T.; Dützmann, S.; Zanella, F.; Berkefeld, J.; du Rochemont, R.D.M. Long-term outcome after coil embolization of cavernous sinus arteriovenous fistulas. AJNR Am. J. Neuroradiol. 2010, 31, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.D.; Halbach, V.V.; Hallam, D.K.; Cooke, D.L.; Ghodke, B.V.; Dowd, C.F.; Amans, M.R.; Hetts, S.W.; Higashida, R.T.; Meyers, P.M. Long-term outcomes of endovascular treatment of indirect carotid cavernous fistulae: Superior efficacy, safety, and durability of transvenous coiling over other techniques. Neurosurgery 2019, 85, E94–E100. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.T.; Nguyen, D.; Duc, V.T.; Chau, H.H.; Son, V.T. Direct traumatic carotid cavernous fistula: Angiographic classification and treatment strategies. Study of 172 cases. Interv. Neuroradiol. 2014, 20, 461–475. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, A.M.; Sturiale, C.L.; Bartolo, A.; Romi, A.; Scerrati, A.; Flacco, M.E.; D’Argento, F.; Scarcia, L.; Garignano, G.; Valente, I.; et al. Endovascular treatment of cavernous sinus dural arteriovenous fistulas. Institutional series, systematic review and meta-analysis. Clin. Neuroradiol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Federal Ministry of Justice and Consumer Protection. Regulation on Radiation Protection; Federal Ministry of Justice and Consumer Protection: Berlin, Germany, 2018; Available online: http://www.gesetze-im-internet.de/strlschv_2018 (accessed on 17 January 2022). (In German)
- Bundesamt für Strahlenschutz. Diagnostic Reference Levels. Available online: http://www.bfs.de/DE/themen/ion/anwendung-medizin/diagnostik/referenzwerte/referenzwerte_node.html (accessed on 17 January 2022).
- Forbrig, R.; Ozpeynirci, Y.; Grasser, M.; Dorn, F.; Liebig, T.; Trumm, C.G. Radiation dose and fluoroscopy time of modern endovascular treatment techniques in patients with saccular unruptured intracranial aneurysms. Eur. Radiol. 2020, 30, 4504–4513. [Google Scholar] [CrossRef]
- Forbrig, R.; Stahl, R.; Geyer, L.L.; Ozpeynirci, Y.; Liebig, T.; Trumm, C.G. Radiation dose and fluoroscopy time of endovascular treatment in patients with intracranial lateral dural arteriovenous fistulae. Clin. Neuroradiol. 2021, 31, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Ozpeynirci, Y.; Trumm, C.; Stahl, R.; Fischer, D.; Liebig, T.; Forbrig, R. Radiation dose and fluoroscopy time of diagnostic angiography in patients with spinal dural arteriovenous fistula. Clin. Neuroradiol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Opitz, M.; Alatzides, G.; Zensen, S.; Bos, D.; Wetter, A.; Guberina, N.; Oppong, M.D.; Wrede, K.H.; Hagenacker, T.; Li, Y.; et al. Radiation exposure during diagnostic and therapeutic angiography of carotid-cavernous fistula: A retrospective single center observational study. Clin. Neuroradiol. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Vañó, E.; Miller, D.L.; Martin, C.J.; Rehani, M.M.; Kang, K.; Rosenstein, M.; Ortiz-López, P.; Mattsson, S.; Padovani, R.; Rogers, A. ICRP Publication 135: Diagnostic reference levels in medical imaging. Ann. ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Kwon, J.; Ryu, G.W.; Kim, K.H.; Nam, H.W.; Kim, K.P. Review of national diagnostic reference levels for interventional procedures. Prog. Med. Phys. 2019, 30, 75–88. [Google Scholar] [CrossRef] [Green Version]
- European Commission Radiation Protection N° 180. Diagnostic Reference Levels in Thirty-Six European Countries Part 2/2; European Commission: Luxembourg, 2014. [Google Scholar]
- Australian Radiation Protection and Nuclear Safety Agency. Current Australian National Diagnostic Reference Levels for Image Guided and Interventional Procedures. Available online: https://www.arpansa.gov.au/research-and-expertise/surveys/national-diagnostic-reference-level-service/current-australian-drls/igip (accessed on 17 January 2022).
- Opitz, M.; Zensen, S.; Bos, D.; Li, Y.; Styczen, H.; Wetter, A.; Guberina, N.; Jabbarli, R.; Sure, U.; Forsting, M.; et al. Radiation exposure in the endovascular therapy of cranial and spinal dural arteriovenous fistula in the last decade: A retrospective, single-center observational study. Neuroradiology 2021, 64, 587–595. [Google Scholar] [CrossRef]
- Kien, N.; Rehel, J.-L.; Étard, C.; Aubert, B. Dose patient en neuroradiologie interventionnelle: Bilan d’une enquête multicentrique [article in French]. J. Radiol. 2011, 92, 1101–1112. [Google Scholar] [CrossRef]
- Guenego, A.; Mosimann, P.J.; Pereira, V.M.; Nicholson, P.; Zuber, K.; Lotterie, J.A.; Dobrocky, T.; Marcellus, D.G.; Olivot, J.M.; Piotin, M.; et al. Proposed achievable levels of dose and impact of dose-reduction systems for thrombectomy in acute ischemic stroke: An international, multicentric, retrospective study in 1096 patients. Eur. Radiol. 2019, 29, 3506–3515. [Google Scholar] [CrossRef]
- Söderman, M.; Mauti, M.; Boon, S.; Omar, A.; Marteinsdóttir, M.; Andersson, T.; Holmin, S.; Hoornaert, B. Radiation dose in neuroangiography using image noise reduction technology: A population study based on 614 patients. Neuroradiology 2013, 55, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- van der Marel, K.; Vedantham, S.; van der Bom, I.M.J.; Howk, M.; Narain, T.; Ty, K.; Karellas, A.; Gounis, M.J.; Puri, A.S.; Wakhloo, A.K. Reduced patient radiation exposure during neurodiagnostic and interventional X-ray angiography with a new imaging platform. AJNR Am. J. Neuroradiol. 2017, 38, 442–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, E.N.; Gemmete, J.J.; Chaudhary, N.; Thompson, B.G.; Chen, K.; Christodoulou, E.G.; Pandey, A.S. Radiation dose reduction during neurointerventional procedures by modification of default settings on biplane angiography equipment. J. Neurointerv. Surg. 2016, 8, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Kwon, D.; Bonavia, G.H. Reference levels for patient radiation doses in interventional radiology: Proposed initial values for U.S. practice. Radiology 2009, 253, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
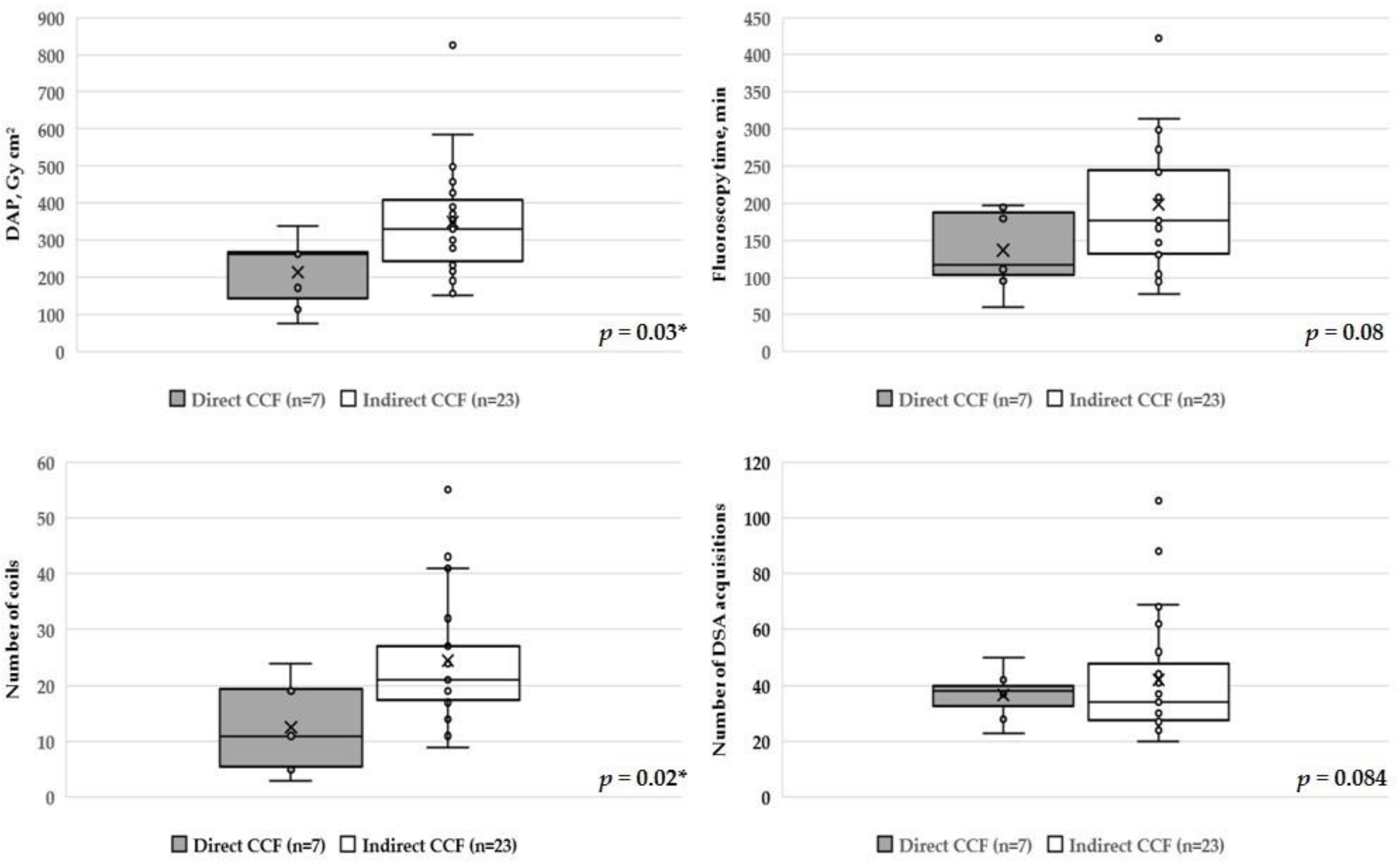

| Mean Age, Years (Range) | 66 (41–85) |
| Female sex | 23/30 (77%) |
| Number of CCFs | |
| -Direct (Type A) | 7/30 (23%) |
-Indirect
| 23/30 (77%) 4/30 (13%) 2/30 (7%) 17/30 (57%) |
| Angiographic outcome | |
| -Total occlusion | 19/30 (63%) |
| -Small remnant | 11/30 (37%) |
| Approach type | |
| -Transarterial | 2/30 (7%) |
| -Transvenous | 27/30 (90%) |
| -Combined | 1/30 (3%) |
| Fistula site | |
| -Right | 9/30 (30%) |
| -Left | 8/30 (27%) |
| -Bilateral | 13/30 (43%) |
| DAP, Gy cm2 | Fluoroscopy Time, Minutes | |||||
|---|---|---|---|---|---|---|
| 25th percentile | Median | 75th percentile | 25th percentile | Median | 75th percentile | |
| All (n = 30) | 230.2 | 291.8 | 376.2 | 115.9 | 174.1 | 241.8 |
| Direct (n = 7) | 113.7 | 264.2 | 271.2 | 95.3 | 117.6 | 194.8 |
| Indirect (n = 23) | 240.2 | 330.5 | 428.8 | 130.9 | 176.2 | 245.8 |
| All CCFs (n = 30) | Direct CCFs (n = 7) | Indirect CCFs (n = 23) | |
|---|---|---|---|
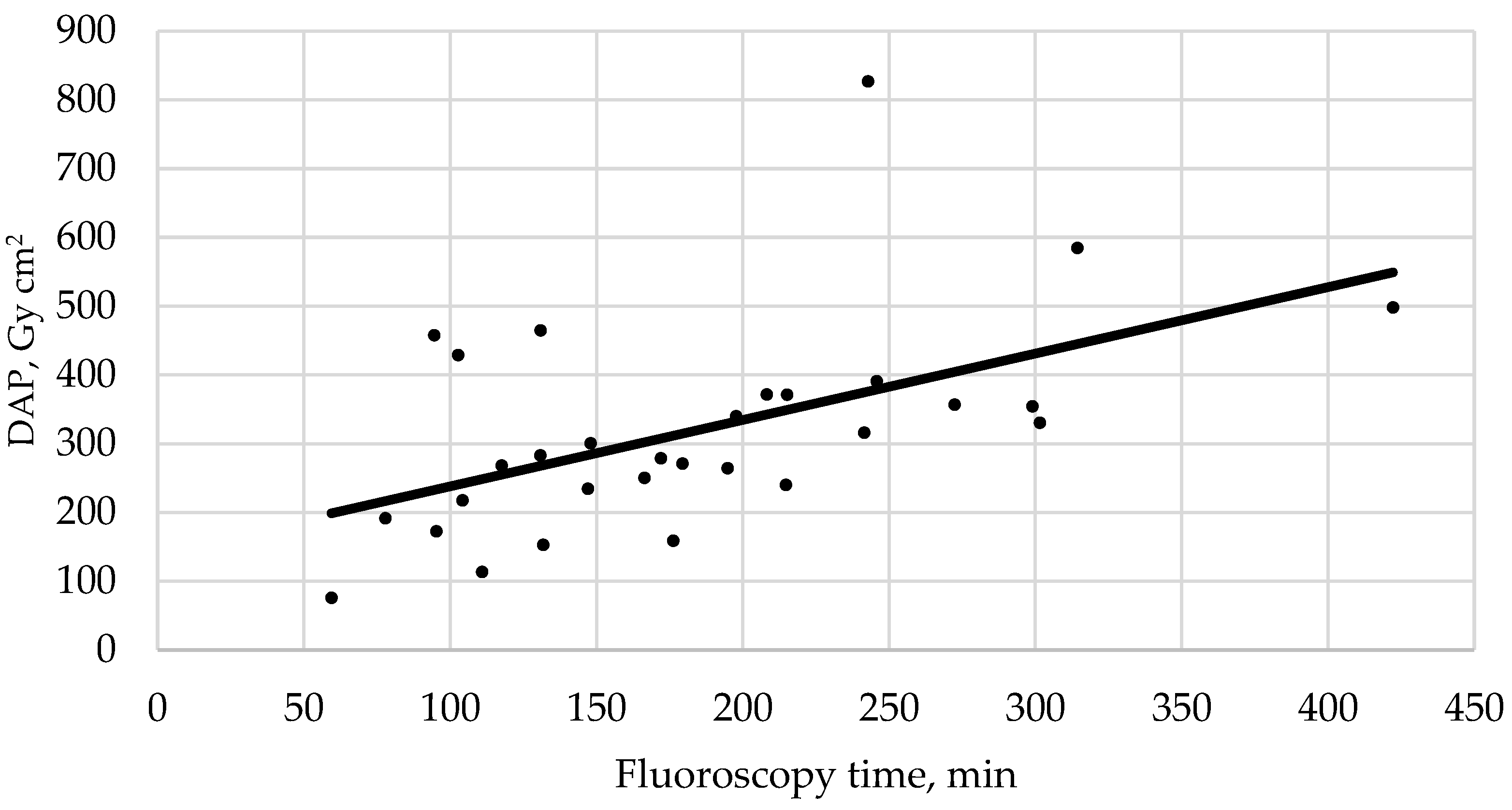
| Mean DAP, Gy cm2 (range) | 318.8 ± 148.1 (75.9–826.6) | 215.1 ± 89 (75.9–339.9) | 350.4 ± 148.1 (153–826.6) |
| Median DAP, Gy cm2 | 291.8 | 264.2 | 330.5 |
| Mean FT, min (range) | 183.8 ± 81.4 (59.4–422.2) | 136.4 ± 50.2 (59.4–197.7) | 198.3 ± 83.6 (77.8–422.2) |
| Median FT, min | 174.1 | 117.6 | 176.2 |
| Mean number of DSA runs (range) | 40.8 ± 19.6 (20–106) | 36.6 ± 8.2 (23–50) | 42 ± 21.8 (20–106) |
| Median number of DSA runs | 35.5 | 38 | 34 |
| Mean number of detached coils (range) | 21.7 ± 11.7 (3–55) | 12.6 ± 7.8 (3–24) | 24.5 ± 11.2 (9–55) |
| Median number of detached coils | 19.5 | 11 | 21 |
| All CCFs (n = 30) | Low-Dose (n = 20) | Normal- and Mixed-Dose (n = 10) | p Value | |
|---|---|---|---|---|
| Mean DAP, Gy cm2 | 318.8 | 274.6 | 407.4 | 0.02 |
| Mean FT, min | 183.8 | 186.1 | 179.5 | 0.84 |
| Mean number of detached coils | 21.7 | 22.4 | 20.3 | 0.65 |
| Mean number of DSA acquisitions | 40.8 | 40.7 | 40.8 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozpeynirci, Y.; Trumm, C.G.; Stahl, R.; Liebig, T.; Forbrig, R. Radiation Dose and Fluoroscopy Time of Endovascular Coil Embolization in Patients with Carotid Cavernous Fistulas. Diagnostics 2022, 12, 531. https://doi.org/10.3390/diagnostics12020531
Ozpeynirci Y, Trumm CG, Stahl R, Liebig T, Forbrig R. Radiation Dose and Fluoroscopy Time of Endovascular Coil Embolization in Patients with Carotid Cavernous Fistulas. Diagnostics. 2022; 12(2):531. https://doi.org/10.3390/diagnostics12020531
Chicago/Turabian StyleOzpeynirci, Yigit, Christoph Gregor Trumm, Robert Stahl, Thomas Liebig, and Robert Forbrig. 2022. "Radiation Dose and Fluoroscopy Time of Endovascular Coil Embolization in Patients with Carotid Cavernous Fistulas" Diagnostics 12, no. 2: 531. https://doi.org/10.3390/diagnostics12020531
APA StyleOzpeynirci, Y., Trumm, C. G., Stahl, R., Liebig, T., & Forbrig, R. (2022). Radiation Dose and Fluoroscopy Time of Endovascular Coil Embolization in Patients with Carotid Cavernous Fistulas. Diagnostics, 12(2), 531. https://doi.org/10.3390/diagnostics12020531







