Abstract
Objective: To compare the diagnostic performance of the automatic breast volume scanner (ABVS) against the handheld ultrasound (HHUS) in the differential diagnosis of benign and malignant breast lesions. Methods: A systematic search and review of studies involving ABVS and HHUS for breast cancer screening were performed. The search involved the data taken from Scopus, PubMed, and science direct databases and was conducted between the year 2011 to 2020. The prospective method was used in determining the inclusion and exclusion criteria while the evidence level was determined using the BI-RADS categories for diagnostic studies. In addition, the parameters of specificity, mean age, sensitivity, tumor number, and diagnostic accuracy of the ABVS and HHUS were summarized. Results: No systematic review or randomized controlled trial were identified in the systematic search while one cross-sectional study, eight retrospective studies, and 10 prospective studies were found. Sufficient follow-up of the subjects with benign and malignant findings were made only in 10 studies, in which only two had used ABVS and HHUS after performing mammographic screening and MRI. Analysis was made of 21 studies, which included 5448 lesions (4074 benign and 1374 malignant) taken from 6009 patients. The range of sensitivity was (0.72–1.0) for ABVS and (0.62–1.0) for HHUS; the specificity range was (0.52–0.98)% for ABVS and (0.49–0.99)% for HHUS. The accuracy range among the 11 studies was (80–99)% and (59–98)% for the HHUS and ABVS, respectively. The identified tumors had a mean size of 2.1 cm, and the detected cancers had a mean percentage of 94% (81–100)% in comparison to the non-cancer in all studies. Conclusions: The evidence available in the literature points to the fact that the diagnostic performance of both ABVS and HHUS are similar with reference to the differentiation of malignant and benign breast lesions.
1. Introduction
Breast disease is common among modern women. It is also one of the leading diseases that threatens the physical health of women. The American Cancer Society predicted that the United States would top the ranking, with 276,480 women with breast cancer in 2020 in the country alone, which accounted for 30% of all cancer patients. With the death of 42,170 of these women, it ranked in second place by accounting for 15% of cancer deaths [1]. In Malaysia, breast cancer is the leading type of cancer, which accounted for 34.1% of all cancer cases in the female population. The diagnosis of a total of 21,634 female breast cancer cases were made between 2012 and 2016, compared to 18,206 cases in the report of 2007–2011. Nonetheless, 19.0% of all new diagnosis of cancer cases between 2012–2016 in comparison to 17.7% in 2007–2011 was attributed to breast cancer, in spite of gender. There has been an increase of 2% for overall cancer among women in a similar comparative period, from 32.1% to 34.1%, for new cases of breast cancer [2].
However, the ideal breast screening system is yet undetermined. Mammography has for some time been the only suggestion for breast screening imaging assessment, and has shown brilliant affectability and particularity. Nonetheless, its utilization in young women under 40 years of age is restricted due to radiation concerns and, often, thick breasts. Presently, there has been a decline in the once-demonstrated power of yearly mammography screening in decreasing the rate of mortality related to breast cancer [3]. The overall clinical limitation of breast screening is similarity in magnetic resonance imaging (MRI) due to inadequate particularity and inadmissible accessibility to non-wealthy regions and individuals [4].
Apart from the obvious benefit of ultrasound in breast cancer diagnosis, it is also beneficial in complementing diagnostic mammograms. It is safe and sensitive at distinguishing the echo of gland tissue and fat, and is good at defining the boundary and morphology of lesions [5,6,7]. Despite the benefits, the duration of the whole-breast examination and operator dependence has rendered the conventional handheld ultrasound (HHUS) with a number of inherent limitations [8]. In contrast, shorter duration of image acquisition, higher reproducibility, and less operator dependence of the automated breast volume scanner (ABVS) has given it a number of advantages over the HHUS [9,10]. Besides, extra information on the diagnosis of the coronal plane that has been reconstructed can also be obtained from the ABVS. Hence, these advantages render the ABVS a promising method for the imaging of breasts in screening as well as diagnostic settings [11,12]. ABUS offers reproducible, high-resolution images and does not depend on the operator, and is achieved using an automated scanner with a larger field of view. Numerous prospective studies have described that adding mammography to ABUS screening resulted in similar positive outcomes to those linked with HHUS screening, such as increased discovery of invasive cancer and reduced rates of interval cancer [5].
In 2012, the approval for the use of ABVS was granted by the U.S. Food and Drug Administration for women with dense breasts and negative mammography findings as an additional whole-breast screening method [13]. The use of ABVS for diagnostic purposes has, over the past decade, been examined by different studies [9,14,15,16,17]. Promising results have been reported in several considerably small patient-population studies [15,18,19,20]. However, the differentiation and characterization of breast lesion discovered through mammography or other screening technologies in present day clinical practice are conducted by a majority of radiologists with the use of conventional HHUS. The controversy regarding the diagnostic performance of ABVS in contrast to HHUS remains. Hence, a systematic review of the differential diagnosis of breast lesions was conducted, comparing HHUS and ABVS diagnostic performance.
2. Materials and Methods
2.1. Search Strategy
A strategy was developed for a systematic search to distinguish significant literature. The search strategy was customized to three databases, namely PubMed, Scopus, and science direct, while the inquiry terms ‘automated breast ultrasound’, ‘handheld ultrasound’, and ‘breast cancer’ were utilized. In particular, all literature was included with reference to automated breast ultrasound (which included its synonyms, for example, automated breast sonography, automated breast scanner, automated whole breast ultrasound, automated breast image, automated whole breast volume scan, 3D automated breast ultrasound, automated breast volume ultrasonography) and handheld (handheld, handheld, handheld, portable, or pocket). The search also spanned the period from the inception of the database to 2020, with the inclusion of journal articles published solely in English.
2.2. Selection Criteria
The criteria for selection depended on the PRISMA Statement [21]. The search mainly focused on the mapping of existing literature on automated breast ultrasound in contrast to handheld ultrasound in medicine, biochemistry, genetics, molecular biology, and the health professions. The search then narrowed down to the medical field. The search span was made between 2011–2020. All articles before 2011 were excluded. The search was not limited to any specific countries, therefore there was no exclusion in this option. The following are the inclusion criteria: (1) both ABVS and HHUS were used in breast lesion diagnosis; (2) the ABVS method was financially accessible; (3) the study population was made up of a minimum of 20 patients; (4) follow-up of histologic analysis (surgery or biopsy), and clinical/imaging for a minimum of 1 year, and unchanged lesions were viewed as pathologically benign. Screening of the relevant literature’s abstracts and titles were conducted, and inspection of the full texts was performed by two researchers independently in determining the inclusion of selected articles in the analysis. A consensus was used in resolving any conflict between the two researchers. At this stage, a total of 311 research articles were excluded while 414 records were extricated.
2.3. Data Extraction
Data collection was made regarding the year of publication, the country in which the examination was performed, the objectives, study design, number of participants, screening methods of assessment, patients’ mean age, and the number of lesions.
2.4. Quality Assessment
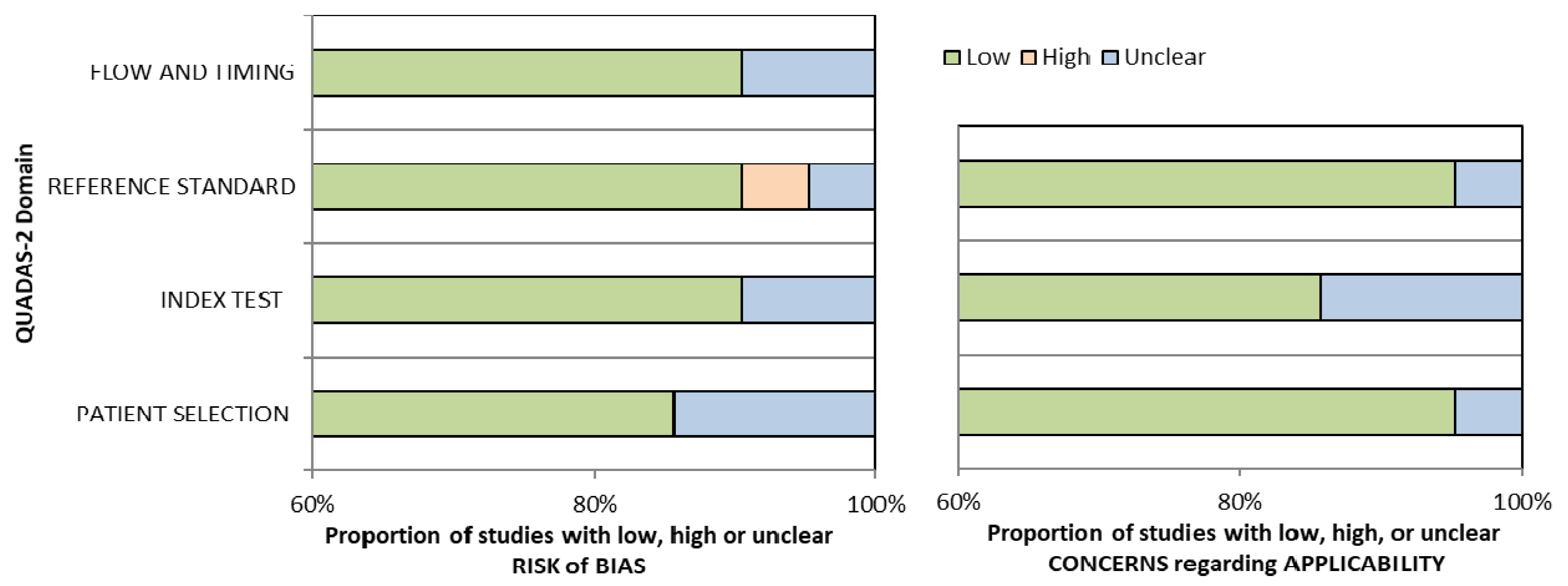
Conference papers, original research articles, and review papers became the basis of this study. A thorough check was made on all duplications in maintaining the nature of the review. In ensuring the relevance and quality of the academic literature included in the review process, detailed examination of the abstracts of articles was conducted in the process of analysis and purification. Next, careful assessment of each research paper was performed. To limit the research only to English-published papers, the subsequent exclusion criterion was therefore used. Therefore, three articles in languages other than English have not been included in the study. In addition to that, the filtration of duplicate records resulted in the removal of 65 more articles. The assessment of each article based on the inclusion and exclusion criteria above resulted in the selection of 21 articles. The exclusion and inclusion of the literature at every stage (PRISMA Statement) is shown in Figure 1. Assessment of the methodological quality was made by two independent reviewers, and to resolve any dispute between the reviewers, mutual suggestion was used. For the inclusion of studies regarding ‘diagnostic accuracy’, the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2) tool was used which involved four domains including ‘index test’, ‘reference standard’, ‘patient selection’, and ‘flow and timing’ [22] (Table 1). Evaluation was made of each domain with regard to its risk of bias (low, high, or unclear), and the initial three domains were identified with regard to their applicability. In general, a study that is viewed as “low” in all domains regarding its applicability or bias is deemed appropriate for an overall judgment of “low concern regarding applicability” or “low risk of bias” for the study. However, judgement of a study is made as having “concerns regarding applicability” or “at risk of bias” if viewed as “unclear” or “high” in one or more domains. Dispute between the two reviewers in assessing the quality of the study was settled through discussion (Figure 2).
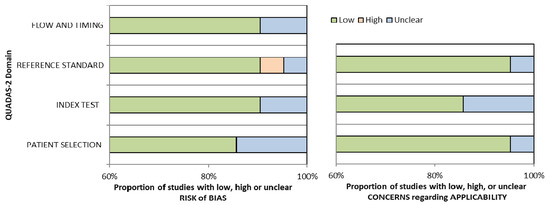
Figure 1.
Bar charts for Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) analysis for 21 studies of diagnostic accuracy.

Table 1.
Quality of studies included in diagnostic accuracy analysis for risk of bias and applicability concerns.
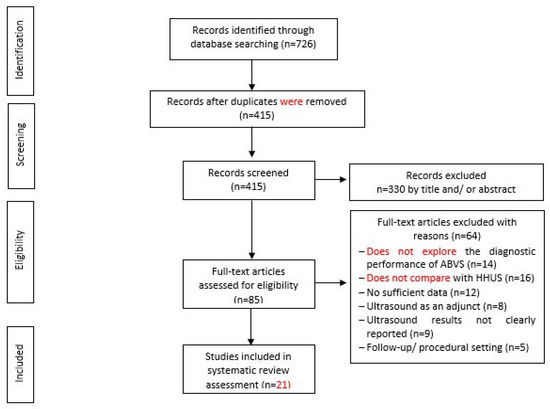
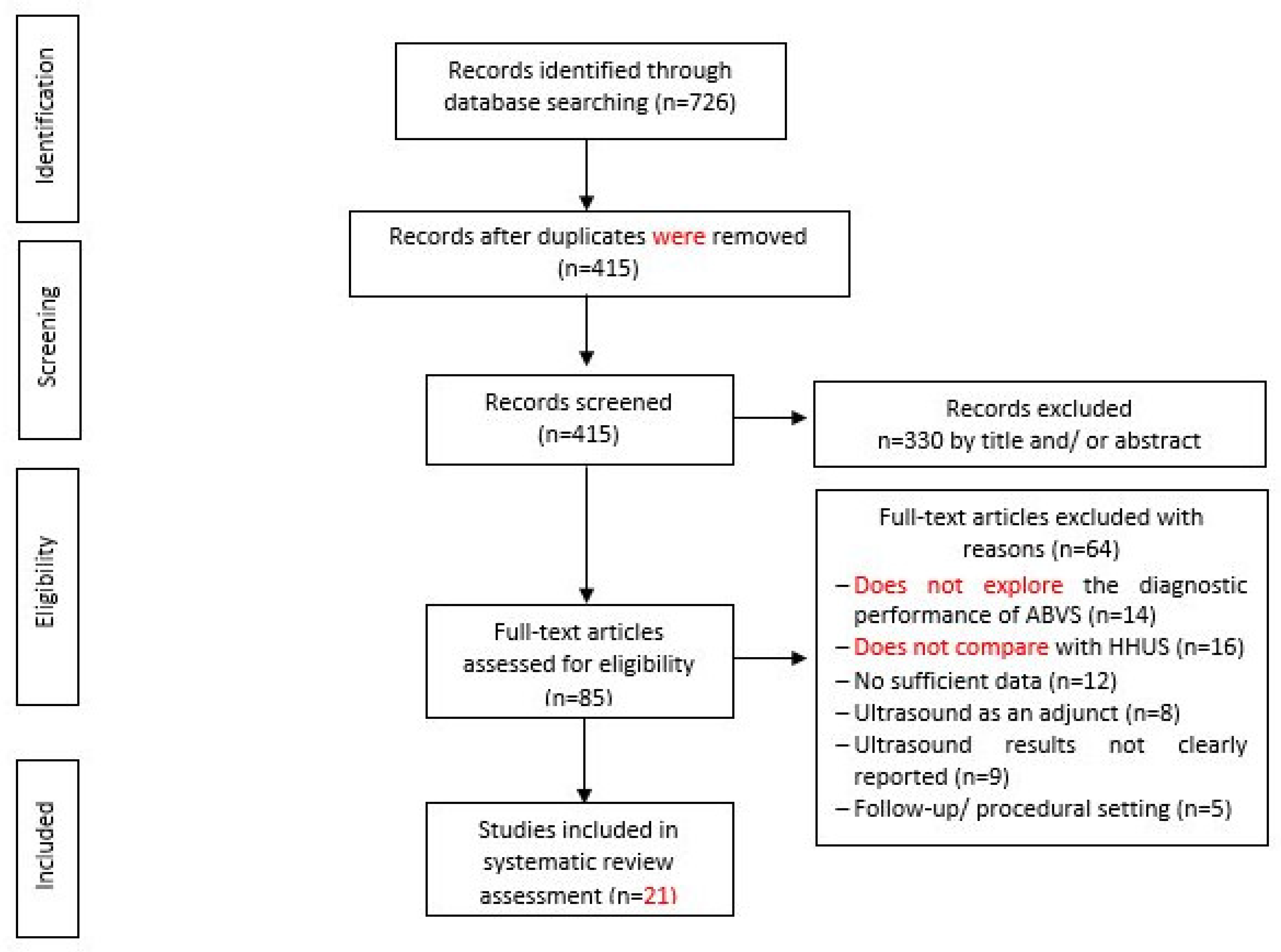
Figure 2.
Flow Chart of Study Selection.
3. Results
3.1. Search Strategy and Study Selection
Based on the search strategy, 726 records were discovered from the electronic databases when, due to duplication issues, 311 items were then discarded. The screening of titles and abstracts based on the inclusion criteria resulted in the exclusion of another 320 items. By reading the full content, 64 more items were excluded based on the examination of the remaining 85 articles. The final selection to be included in the literature review of this study included 21 studies (Depretto et al., 2020; Jia et al., 2020; Tutar et al., 2020; Yun et al., 2019; Zhang et al., 2019; Niu et al., 2019; Choi et al., 2018; Zhang et al., 2018; Schmachtenberg et al., 2017; Hellgren et al., 2016; Kim et al., 2016; Jeh et al., 2015; Choi et al., 2014; Chen et al., 2013; Kim et al., 2013; Lin et al., 2012; Wang et al., 2012; Wojcinski et al., 2011; Chang et al., 2011; Shin et al., 2011). The screening of the included studies’ reference list marks the end of the process of inclusion of studies. A summary of the process involved in the studies selected for the inclusion of this review is shown in the flowchart (see Figure 2).
3.2. Characteristics of the Study and Quality Assessment
From the 21 studies, the inclusion of 5448 lesions in 6009 patients was made, whereby 1374 (23.3%) were malignant while 4074 (69.1%) were benign. Three brands of the ABVS were discovered; ACUSON S2000 (Siemens Healthcare, Erlangen, Germany) as the highest-utilized brand among 13 studies, InveniaTM (GE Healthcare, WI, USA) as the second highest utilized brand among five studies, and SomoVu Scan Station (Usystem, Inc., San Jose, CA, USA), found in three studies although five brands of the HHUS were discovered; ACUSON S2000™; Siemens, Erlangen, Germany as the highest-utilized brand among 8 studies, iU22 Ultrasound System (Philips Medical System, WA, USA) as the second highest utilized brand among 7 studies, Logiq E9 (GE Healthcare; Milwaukee, WI, USA) utilized among three studies, EUB-8500 scanner (Hitachi Medical, Tokyo, Japan) used in two study while Aplio 80 (Toshiba, Tokyo, Japan) just in one study.
Among 14 studies, the sensitivity range was (0.72–1.0) for ABVS and (0.62–1.0) for HHUS, while the specificity range was (0.52–0.98) for ABVS and (0.49–0.99) for HHUS. Among the 11 studies, the accuracy range was (59–98)% and (80–99)% for ABVS and HHUS, respectively. ABVS and HHUS were utilized in 21 studies, thus giving unbiased, extractable data in terms of diagnostic accuracy. For the 21 studies on ‘diagnostic accuracy’, the results of histopathology assessment were the standard of reference. ‘Lesion by lesion’ is the rater approach chosen in all studies. The raters evaluated each lesion detected for malignancy using the BI-RADS. Table 1 provides the summary of the details of the study characteristics. As demonstrated by the QUADAS-2 tool, most of the studies (15 out of 21) had a rather high methodological quality (Table 2). However, due to the use of case-control configuration in this study, one study was judged as high risk [23]. As a result of imprecise review of HHUS and ABVS images, the same radiologist judged five studies as unclear risk, with the use of the blinding method.

Table 2.
Characteristics of the included studies.
3.3. Age Distribution
The report of 18 studies showed an age range between 11 to 82 years old, with the overall age range exceeding 30 years old in every study. Information on the median age was provided in three studies (49, 49, and 52 years), with age range between 32 to 82 years [24,25,26] (Table 2).
3.4. Breast Lesion Diagnosis Using the Categories of BI-RADS
Two studies separated the HHUS and ABVS outcome of breast screening according to the BI-RADS 4 and BI-RADS 5 classification [25,27]. Other studies assessed the results of breast tissue according to BI-RADS 1–BI-RADS 5. Females with BI-RADS 4 and BI-RADS 5 breast categories demonstrated the largest proportion of breast cancer diagnosed through ultrasound screening. Depretto et al. analyzed four carcinomas distinguished by (BI-RADS 4) breast tissue, and 130 cases with breasts in categories (BI-RADS 1 and BI-RADS 2) [28], and 29 malignancies were diagnosed in type 4 and type 5 BI-RADS breast tissue in Jia et al., while two carcinomas were found in three breasts [23].
Using the ABVS, the discovery of 51 circumscribed solid nodules (BI-RADS 3) were made in 42 women. The HHUS exhibited five of these as complicated cysts. The primary HHUS missed five nodules. However, the detection of five BI-RADS 3 solid nodules, one BI-RADS 2 implant rupture, and one BI-RADS 4 distortion was made by HHUS, which were all missed by ABVS. Altogether, 78 lesions were found in 340 women, 71 (91%) of the detected nodules were discovered by ABVS while 68 (87.2%) of the detection was made by the primary HHUS [24]. Niu et al. included 599 masses detected in 398 women (which included solid and cystic masses). The classification of 359 masses by the HHUS and ABUS as category 2 or 3 indicated these as benign masses. The MRI classified two of these masses as category 4 or 5 [29].
It was found at the completion of the study that 496 (83%) of the 599 masses were benign while the remaining 103 (17%) were malignant, with the one-year follow-up information or pathological outcomes as the reference standards. As indicated by the BI-RADS classification for every unit, 258 units (258/320, 80.63%) had BI-RADS classification 1–2, 62 units (19.38%) had BI-RADS classification 3 while 155 lesions had the introductory BI-RADS classifications of 4–5 [30]. Choi et al. confirmed 184 malignant cases of BI-RADS 4 and BI-RADS 5 classes, and 234 lesions were diagnosed as benign (BI-RADS 3) [31]. The remaining 413 lesions were assigned as BI-RADS class 3 (n = 292) or 2 (n = 121). In a study conducted by Zhang et al., 1353 females (68.6%) were characterized as BI-RADS 1, 2, or 3 categories while the other 620 females (31.4%) were classified as BI-RADS category 4 or 5 [32].
Jeh et al. reported the BI-RADS final assessment of 124 classifications as 1, 2, and 3 while 45 malignant classifications were 4 and 5 [33]. Lin et al. identified 15 carcinomas noticed in BI-RADS classification 4–5, and 20 females with breasts in classification 1-3 [34]. Wojcinski et al. identified that 6 out of 14 lesions had been classified as BI-RADS 0, 3 or 4 while 8 out of 14 malignant lesions had been properly classified as BI-RADS 5 [26]. Of masses that were benign, 39 were categorized as BI-RADS 4 while another 7 were characterized as BI-RADS 3. Of masses that were malignant, 16 were accounted for as BI-RADS 5, while 8 masses that were malignant were categorized as BI-RADS 4 [20]. The evaluation of 145 lesions in Shin et al. marked the final evaluation of the BI-RADS classes, where 145 lesions were accounted for by five readers as category 1 or 2 (40%, 240 of 603), 3 (31%, 184 of 603), 4A (11%, 68 of 603), 4B (3%, 19 of 603), 4C (2%, 12 of 603), and 5 (13%, 80 of 603) [35].
3.5. Tumour Stage and Size (Lymph Node and Non-Invasive/Invasive Status)
The detected carcinomas had a mean size of 2.1 cm (mean size ranged between 1.6 cm to 2.6 cm) in seven of the 21 studies [33,34,35,36,37,38,39]. The mean level of malignancy detected was 94% (81–100%) contrasted with non-cancer in all examinations. The status of intramammary lymph node was accounted for in one analysis, with lymph nodes that were negative in all studies [31] (Table 2).
3.6. Assessment Categories for Breast Ultrasound
The positive predictive value concerning the finding of malignancies in biopsies that are prompted/detected by ultrasound was accounted for, or had the option to be derived from the information provided by eleven studies. The percentage of positively-classified findings for carcinoma was then found to range between 4.1–100% for both ABVS and HHUS. The large variation of these positive predictive values is chiefly due to the application of different assessment categories and different sonographic criteria for malignancy. Only three studies reported that all findings were classified as benign post biopsy [35,39,40]. Nonetheless, three studies did not specify the follow-up for patients with positive outcomes [26,33,41] (Table 2).
4. Discussion
ABVS is a novel imaging method for automated breast scanning via ultrasound. The use of this method was first made in the screening setting to enhance the detection of breast cancer [5]. Recently, the evaluation of the use of ABVS in the diagnostic stage has been made by various studies. However, the diagnostic capability of the ABVS continues to be disputable since it is a “novel” method, especially in comparison with the traditional HHUS. It was reported in Meng et al. that ABVS demonstrated decreased specificity compared to HHUS, although both ABVS and HHUS demonstrated equal sensitivity [19]. In a meta-analysis in Zhang et al., the same detection rate (100%) was found in both HHUS and ABVS in detecting breast cancer. However, ABVS demonstrated numerically greater specificity (86% vs. 82%) and sensitivity (93% vs. 90%) in comparison with HHUS [30]. On the other hand, Wang and Qi reported that ABVS and HHUS demonstrated similar specificity and sensitivity in discriminating benign breast lesions from malignant. The pooled values of specificity and sensitivity for ABVS were 82.2% and 90.8%, respectively, while HHUS pooled specificity and sensitivity values were 81.0% and 90.6%, respectively [42].
This systematic review aimed to determine the evidence available on ABVS and HHUS diagnostic accuracy in identifying malignant and benign breast lesions. Based on the data in this study, similar sensitivity and specificity were demonstrated for ABVS and HHUS in discriminating malignant and benign breast lesions. Among 14 studies, the sensitivity range was (0.72–1.0) for ABVS and (0.62–1.0) for HHUS and the specificity range was (0.52–0.98) for ABVS and (0.49–0.99) for HHUS; while among 11 studies, the accuracy range was (59–98)% and (80–99)% for ABVS and HHUS, respectively. On the other hand, as suggested in the studies of Kim et al. and Wang et al., ABVS can be effectively utilized in detecting and characterizing breast lesions, since its sensitivity is not inferior [36,40]. As found in the study of Tutar et al., ABVS succeeded in detecting all malignant lesions, apart from the fact that no interval cancers were detected in the very long follow-up period. A greater number of benign lesions could be fundamentally identified by ABVS in comparison to HHUS. There is a possibility that a proportion of solid nodules detected using ABVS could be focal fat lobules [24].
The article review has shown that a statistically significant difference in results exists between HHUS and ABVS, since HHUS contained a larger amount of BI-RADS 1–2 compared to BI-RADS 0 or 4 discovered in ABVS. This indicates the probability of ABVS resulting in better clinical practice regarding biopsies, follow-ups, and recalls, in comparison to HHUS.
Based on the outcomes of Yun et al. it is confirmed that a relationship exists between ABVS allocation of a lower BI-RADS category and HHUS allocation of US results of irregular shape [25].
Posterior shadowing is a notable limitation of ABVS, identified by the recall or false-negative rate. Notwithstanding the findings for lesion size, no association was revealed in our study group between orientation, margin, or posterior acoustic features and a lower categorization utilizing ABUS. It was shown in the study results of Zhang et al. that the ABVS findings were in greater sync with the pathological outcomes of the BI-RADS 4–5 groups [32]. The 78.6% of females diagnosed with cancer or precancerous lesions, from those classified by ABVS as BI-RADS 4 or 5, was 7.2% greater compared to the female proportion in a similar group of BI-RADS classification based on HHUS. However, the false-negative rates of the BI-RADS 1–2 groups for both ABVS and HHUS were not distinguishable from each other.
On the other hand, emphasis should be made on the fact that HHUS and ABVS diagnostic performance similarity is largely dependent on the interpretation of the Gray-scale ultrasound. No clear statement on the usage of elastography and Doppler ultrasound in HHUS was made in any of the 21 studies included. This suggests that, for breast lesion differential diagnosis, no additional information on vascularity and elasticity was provided, when only the morphological features on Gray-scale images for HHUS and ABVS were used. Breast ultrasound diagnostic accuracy could therefore be significantly improved due to the ability of both elastography and Doppler ultrasound to provide independent diagnostic information apart from Gray-scale imaging. Nevertheless, the investigation of tissue elasticity and lesion vascularity continues currently to be performed by ABVS in the clinical environment. Henceforth, the diagnostic performance of HHUS in the involved studies may be underestimated relative to the clinical reality. Therefore, there is a probability of an underestimation of HHUS diagnostic performance made in the involved studies in relation to clinical reality.
However, a great advantage of ABVS in breast lesion characterization in comparison to HHUS is its capability in obtaining additional data on the reconstructed coronal plane’s morphological features. In the differentiation of breast lesions that are malignant and benign, the ABVS coronal plane retraction phenomenon is perceived as having high probability as a diagnostic feature (Depretto et al., 2020; Jia et al., 2020; Tutar et al., 2020; Yun et al., 2019; Zhang et al., 2019; Niu et al., 2019; Choi et al., 2018; Zhang et al., 2018; Schmachtenberg et al., 2017; Hellgren et al., 2017; Jeh et al., 2015; Choi et al., 2014; Chen et al., 2013; Kim et al., 2013; Lin et al., 2012; Wang et al., 2012; Wojcinski et al., 2011; Chang et al., 2011; Shin et al., 2011). Thus, it can be sensibly concluded that in terms of differential findings assisted by coronal reconstruction, ABVS might be better when compared to HHUS.
Nonetheless, the reviewed article demonstrates that the diagnostic performance of ABVS is similar to the diagnostic performance of HHUS. In terms of benign and malignant breast lesion differential diagnosis, no confirmation was made in our study regarding the added benefit of using ABVS for coronal reconstruction. The results of Zhang et al. are in agreement with those of our study which indicated that, regarding AUC values, ABVS diagnostic performance might not be significantly improved through coronal reconstruction [30]. In the study of Zhang et al., a suggestion was made that ABVS independent value limitation in the differential diagnosis is caused by the low sensitivity (37.0%) of the retraction phenomenon on the coronal plane [32]. The limitations in our study include the substantial dominance of Asian reports, with 15 out of 21.
Variations might have occurred due to the uneven geographic distribution since there are breast cancer differences regarding region and ethnicity between non-Asian and Asian women. Besides, no indication was made in any of the studies included regarding an image quality control statement, which should thus be noted as a variable that is unaccounted for, both for ABVS and HHUS. Third, based on our references, no investigation has made use of Doppler and elastography ultrasound in HHUS, in contrast to the practice in clinical reality. Finally, publication bias might have been prompted since evaluation was made only of articles written in English. Therefore, our reviewed articles may have underestimated the diagnostic performance of HHUS.
5. Conclusions
In relation to malignant and benign breast lesion differentiation, ABVS diagnostic performance based on the evidence available in the literature is similar to that of HHUS. However, ABVS can offer new diagnostic information. ABVS may help to distinguish between real lesions. This technique is feasible for clinical applications and is a promising modality in breast imaging. Nevertheless, since this review of articles was conducted on various studies, most of which were obtained from a single geographical region, further studies are hence required before the generalization of this conclusion can be made. More sound research associating the diagnostic performance of ABVS and mammography/MRI is anticipated and required.
Author Contributions
Conceptualization, S.A.I., R.M., S.M.S. and H.A.H.; methodology, S.A.I. and A.M.D.; software, S.A.I.; validation, S.A.I. and A.S.K.; formal analysis, S.A.I. and A.M.D.; investigation, S.A.I. and A.S.K.; resources, S.A.I.; data curation, S.A.I.; writing—original draft, S.A.I.; writing—review and editing, S.A.I., R.M., S.M.S., H.A.H., A.S.K. and A.M.D.; visualization, S.A.I., R.M., S.M.S., H.A.H., A.S.K. and A.M.D.; supervision, R.M., S.M.S. and H.A.H.; project administration, S.A.I.; funding acquisition, S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for this review.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the studies included in the review that have been cited.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Azizah, A.M.; Hashimah, B.; Nirmal, K.; Siti Zubaidah, A.R.; Puteri, N.A.; Nabihah, A.; Sukumaran, R.; Balqis, B.; Nadia, S.M.R.; Sharifah, S.S.S.; et al. Malaysian National Cancer Registry Report 2012–2016. Malaysia Cancer Statistics, Data and Figure; National Cancer Institute, Ministry of Health: Putrajaya, Malaysia, 2019.
- Van den Ende, C.; Oordt-Speets, A.M.; Vroling, H.; van Agt, H.M. Benefits and harms of breast cancer screening with mammography in women aged 40–49 years: A systematic review. Int. J. Cancer 2017, 141, 1295–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, A.H.; Pagani, O.; Abulkhair, O.; Aebi, S.; Amant, F.; Azim, H.A., Jr.; Harbeck, N. First international consensus guidelines for breast cancer in young women (BCY1). Breast 2014, 23, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Brem, R.F.; Lenihan, M.J.; Lieberman, J.; Torrente, J. Screening breast ultrasound: Past, present, and future. Am. J. Roentgenol. 2015, 204, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Burkett, B.J.; Hanemann, C.W. A review of supplemental screening ultrasound for breast cancer: Certain populations of women with dense breast tissue may benefit. Acad. Radiol. 2016, 23, 1604–1609. [Google Scholar] [CrossRef]
- Rebolj, M.; Assi, V.; Brentnall, A.; Parmar, D.; Duffy, S.W. Addition of ultrasound to mammography in the case of dense breast tissue: Systematic review and meta-analysis. Br. J. Cancer 2018, 118, 1559–1570. [Google Scholar] [CrossRef]
- Kaplan, S.S. Automated whole breast ultrasound. Radiol. Clin. 2014, 52, 539–546. [Google Scholar] [CrossRef]
- Van Zelst, J.C.; Mann, R.M. Automated three-dimensional breast US for screening: Technique, artifacts, and lesion characterization. Radiographics 2018, 38, 663–683. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Chen, Z.H.; Zhou, Q.C.; Wang, Z. The efficacy of automated breast volume scanning over conventional ultrasonography among patients with breast lesions. Int. J. Gynecol. Obstet. 2015, 131, 293–296. [Google Scholar] [CrossRef]
- Kotsianos-Hermle, D.; Wirth, S.; Fischer, T.; Hiltawsky, K.M.; Reiser, M. First clinical use of a standardized three-dimensional ultrasound for breast imaging. Eur. J. Radiol. 2009, 71, 102–108. [Google Scholar] [CrossRef]
- Rella, R.; Belli, P.; Giuliani, M.; Bufi, E.; Carlino, G.; Rinaldi, P.; Manfredi, R. Automated breast ultrasonography (ABUS) in the screening and diagnostic setting: Indications and practical use. Acad. Radiol. 2018, 25, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, H.H.; Cha, J.H. Current status of automated breast ultrasonography. Ultrasonography 2015, 34, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grady, I.; Chanisheva, N.; Vasquez, T. The addition of automated breast ultrasound to mammography in breast cancer screening decreases stage at diagnosis. Acad. Radiol. 2017, 24, 1570–1574. [Google Scholar] [CrossRef]
- Wang, X.L.; Tao, L.; Zhou, X.L.; Wei, H.; Sun, J.W. Initial experience of automated breast volume scanning (ABVS) and ultrasound elastography in predicting breast cancer subtypes and staging. Breast 2016, 30, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, S.; Xie, Y.; Guan, X.; Fu, N.; Huang, P.; Yang, B. Combined use of the automated breast volume scanner and the US elastography for the differentiation of benign from malignant lesions of the breast. BMC Cancer 2014, 14, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.Y.; Yan, L.X.; Huang, B.J.; Xia, H.S.; Wang, X.; Lu, Q.; Wang, W.P. Comparison of retraction phenomenon and BI-RADS-US descriptors in differentiating benign and malignant breast masses using an automated breast volume scanner. Eur. J. Radiol. 2015, 84, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Wojcinski, S.; Gyapong, S.; Farrokh, A.; Soergel, P.; Hillemanns, P.; Degenhardt, F. Diagnostic performance and inter-observer concordance in lesion detection with the automated breast volume scanner (ABVS). BMC Med. Imaging 2013, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Chen, C.; Zhu, Y.; Zhang, S.; Wei, C.; Hu, B.; Shen, E. Diagnostic performance of the automated breast volume scanner: A systematic review of inter-rater reliability/agreement and meta-analysis of diagnostic accuracy for differentiating benign and malignant breast lesions. Eur. Radiol. 2015, 25, 3638–3647. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Park, J.S.; Kim, S.J. Radiologists’ performance in the detection of benign and malignant masses with 3D automated breast ultrasound (ABUS). Eur. J. Radiol. 2011, 78, 99–103. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Tugwell, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (Chinese edition). J. Chin. Integr. Med. 2009, 7, 889–896. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Lin, X.; Zhou, X.; Yan, H.; Chen, Y.; Liu, P.; Sankaranarayanan, R. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res. Treat. 2020, 181, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Tutar, B.; Icten, G.E.; Guldogan, N.; Kara, H.; Arıkan, A.E.; Tutar, O.; Uras, C. Comparison of automated versus hand-held breast US in supplemental screening in asymptomatic women with dense breasts: Is there a difference regarding woman preference, lesion detection and lesion characterization? Arch. Gynecol. Obstet. 2020, 301, 1257–1265. [Google Scholar] [CrossRef]
- Yun, G.; Kim, S.M.; La Yun, B.; Ahn, H.S.; Jang, M. Reliability of automated versus handheld breast ultrasound examinations of suspicious breast masses. Ultrasonography 2019, 38, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcinski, S.; Farrokh, A.; Hille, U.; Wiskirchen, J.; Gyapong, S.; Soliman, A.A.; Hillemanns, P. The automated breast volume scanner (ABVS): Initial experiences in lesion detection compared with conventional handheld B-mode ultrasound: A pilot study of 50 cases. Int. J. Women’s Health 2011, 3, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmachtenberg, C.; Fischer, T.; Hamm, B.; Bick, U. Diagnostic performance of automated breast volume scanning (ABVS) compared to handheld ultrasonography with breast MRI as the gold standard. Acad. Radiol. 2017, 24, 954–961. [Google Scholar] [CrossRef]
- Depretto, C.; Liguori, A.; Primolevo, A.; Di Cosimo, S.; Cartia, F.; Ferranti, C.; Scaperrotta, G.P. Automated breast ultrasound compared to hand-held ultrasound in surveillance after breast-conserving surgery. Tumori J. 2020, 107, 132–138. [Google Scholar] [CrossRef]
- Niu, L.; Bao, L.; Zhu, L.; Tan, Y.; Xu, X.; Shan, Y.; Shen, Y. Diagnostic performance of automated breast ultrasound in differentiating benign and malignant breast masses in asymptomatic women: A comparison study with handheld ultrasound. J. Ultrasound Med. 2019, 38, 2871–2880. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, L.Y.; Tan, Y.J.; Zhu, L.Q.; Xu, X.J.; Zhu, Q.Q.; Liu, J. Diagnostic performance using automated breast ultrasound system for breast cancer in Chinese women aged 40 years or older: A comparative study. Ultrasound Med. Biol. 2019, 45, 3137–3144. [Google Scholar] [CrossRef]
- Choi, E.J.; Choi, H.; Park, E.H.; Song, J.S.; Youk, J.H. Evaluation of an automated breast volume scanner according to the fifth edition of BI-RADS for breast ultrasound compared with hand-held ultrasound. Eur. J. Radiol. 2018, 99, 138–145. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; Tan, Y.; Zhu, Y.; Wang, H.; Feng, R.; Qiao, Y. A multicenter hospital-based diagnosis study of automated breast ultrasound system in detecting breast cancer among Chinese women. Chin. J. Cancer Res. 2018, 30, 231. [Google Scholar] [CrossRef] [PubMed]
- Jeh, S.K.; Kim, S.H.; Choi, J.J.; Jung, S.S.; Choe, B.J.; Park, S.; Park, M.S. Comparison of automated breast ultrasonography to handheld ultrasonography in detecting and diagnosing breast lesions. Acta Radiol. 2016, 57, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, J.; Han, F.; Fu, J.; Li, A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur. J. Radiol. 2012, 81, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, H.H.; Cha, J.H.; Park, J.H.; Lee, K.E.; Kim, J.H. Automated ultrasound of the breast for diagnosis: Interobserver agreement on lesion detection and characterization. Am. J. Roentgenol. 2011, 197, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Jiang, Y.X.; Zhu, Q.L.; Zhang, J.; Dai, Q.; Liu, H.; Sun, Q. Differentiation of benign and malignant breast lesions: A comparison between automatically generated breast volume scans and handheld ultrasound examinations. Eur. J. Radiol. 2012, 81, 3190–3200. [Google Scholar] [CrossRef]
- Choi, W.J.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Kim, H.; Chae, E.Y.; Hong, M.J. Comparison of automated breast volume scanning and hand-held ultrasound in the detection of breast cancer: An analysis of 5,566 patient evaluations. Asian Pac. J. Cancer Prev. 2014, 15, 9101–9105. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, Y.; Diao, X.H.; Fang, L.; Pang, Y.; Cheng, A.Q.; Wang, Y. Comparative study of automated breast 3-D ultrasound and handheld B-mode ultrasound for differentiation of benign and malignant breast masses. Ultrasound Med. Biol. 2013, 39, 1735–1742. [Google Scholar] [CrossRef]
- Hellgren, R.; Dickman, P.; Leifland, K.; Saracco, A.; Hall, P.; Celebioglu, F. Comparison of handheld ultrasound and automated breast ultrasound in women recalled after mammography screening. Acta Radiol. 2017, 58, 515–520. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, B.J.; Choi, B.G.; Choi, J.J.; Lee, J.H.; Song, B.J.; Kim, H. Radiologists’ performance for detecting lesions and the interobserver variability of automated whole breast ultrasound. Korean J. Radiol. 2013, 14, 154. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, B.J.; Kim, S.H.; Lee, E.J. Prospective Study Comparing Two Second-Look Ultrasound Techniques: Handheld Ultrasound and an Automated Breast Volume Scanner. J. Ultrasound Med. 2016, 35, 2103–2112. [Google Scholar] [CrossRef]
- Wang, L.; Qi, Z.H. Automatic breast volume scanner versus handheld ultrasound in differentiation of benign and malignant breast lesions: A systematic review and meta-analysis. Ultrasound Med. Biol. 2019, 45, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).