Strain Imaging for the Early Detection of Cardiac Remodeling and Dysfunction in Primary Aldosteronism
Abstract
:1. Introduction
2. Diagnosis and Screening of Primary Aldosteronism
3. Left Cardiac Evaluation
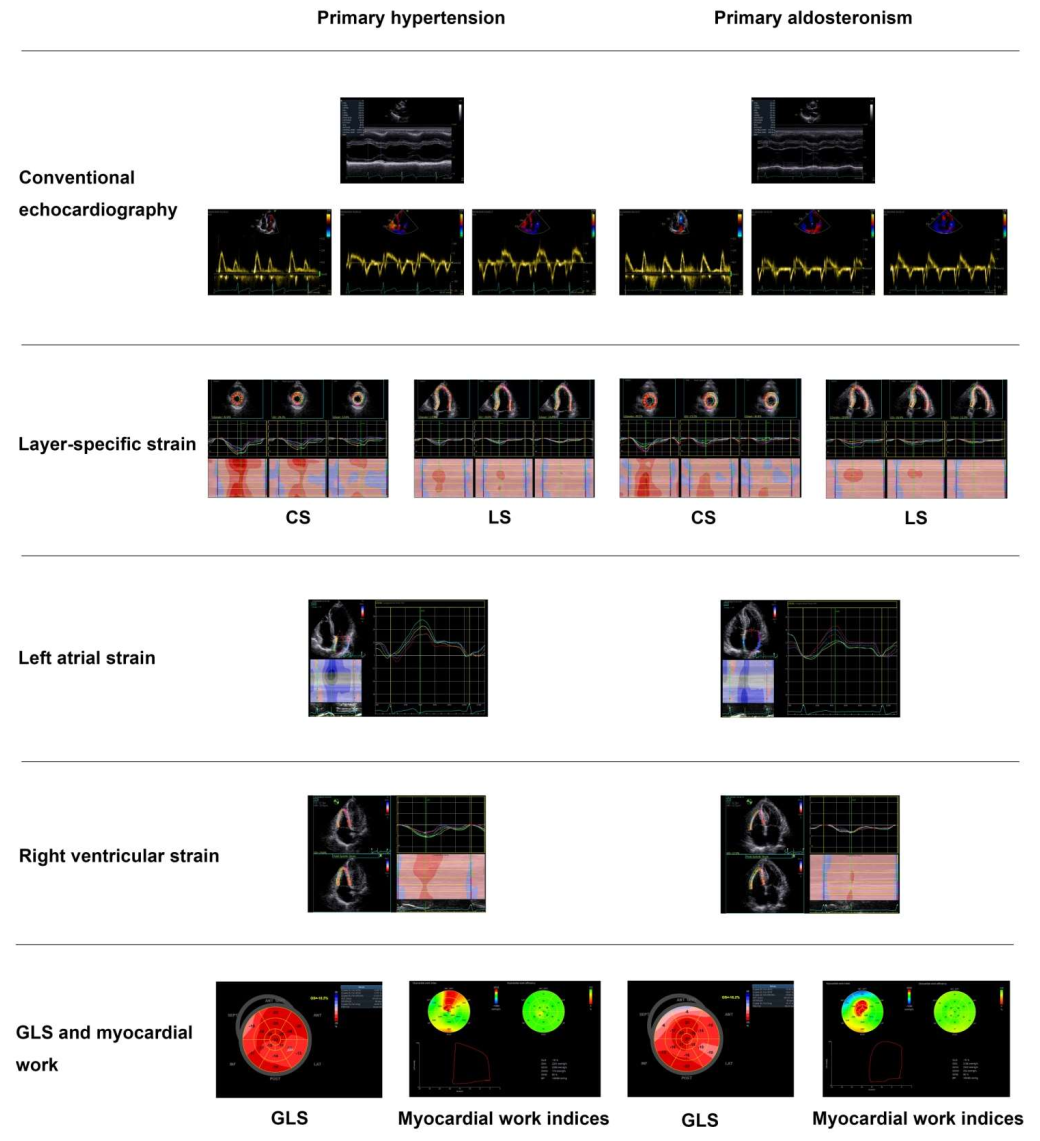
3.1. Left Ventricular Remodeling
3.2. Systolic Dysfunction
3.3. Diastolic Dysfunction
4. Right Cardiac Evaluation
5. Cardiac Myocardial Work Evaluation
6. Myocardial Fibrosis
7. Treatment Effect on Cardiac Structure and Function

| First Author | Year | Study Design | Participants | No. of Participants | Age (y) | Sex (Male) | Outcome Measures | Results | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|
| Rossi GP [9] | 1996 | Cross-sectional | PA vs. PH | 34 vs. 34 | 51 ± 13 vs. 49 ± 12 | 18 vs. 18 | LVMI | 112 ± 5 vs. 98 ± 4 g/m2 | Significantly greater LVMI and higher prevalence of LVH in PA than PH |
| Matsumura K [27] | 2006 | Cross-sectional | PA vs. Renovascular hypertension | 25 vs. 29 | 47 ± 2 vs. 45 ± 4 | 13 vs. 10 | LVMI | 154 ± 7 vs. 135 ± 9 g/m2 | Higher prevalence of LVH in PA than renovascular hypertension |
| Muiesan ML [10] | 2008 | Cross-sectional | PA vs. PH | 125 vs. 125 | 50 ± 11 vs. 51 ± 11 | 71 vs. 71 | LVMI | 50 ± 17 vs. 40 ± 11 g/m2.7 | Significantly higher prevalence of inappropriate LVMI in the absence of traditionally defined LVH in PA than PH |
| Yang Y [12] | 2017 | Cross-sectional | PA vs. PH | 100 vs. 100 | 50 ± 12 vs. 50 ± 12 | 58 vs. 58 | LAVI&E/e’ | LAVI: 23 ± 6 vs. 21 ± 6 mL/m2 E/e’: 13.5 ± 4.3 vs. 11.9 ± 3.3 | Significantly lower e’ and higher E/e’ in PA than PH, in addition to left atrial enlargement |
| Catena C [40] | 2007 | Prospective longitudinal | PA | Surgery 24 vs. Drug treatments 30 | 53 ± 12 in PA patients | 38 inPA patients | LVMI | 53 ± 11 vs. 52 ± 11 g/m2.7 at baseline 45 ± 12 vs. 49 ± 11 g/m2.7 at 1 year 43 ± 11 vs. 44 ± 11 g/m2.7 at the end of study | Earlier response of LVM regression in surgery than drug treatment but later comparable in the two groups during an average of 6.4 years follow-up |
| Lin YH [29] | 2011 | Prospective longitudinal | PA | Surgery 11 | 47 ± 8 | 5 | LVMI | 153 ± 31 at baseline vs. 116 ± 12 g/m2 at 1 year | Significant regression in LVMI at 1 year |
| Ori Y [104] | 2013 | Retrospective | PA | Drug treatment 48 | 61 ± 10 | 28 | LVMI | 142 ± 28 at baseline, 121 ± 21 at 1 year and 112 ± 24 g/m2 at 3 years | Significant decrease in LVMI at 1 year and normalized at 3 years |
| Rossi GP [106] | 2013 | Prospective longitudinal | PA | Surgery 110 vs. Drug treatment 70 | 51 ± 12 in PA patients | 57 in PA patients | LVMI | 53 ± 13 vs. 50 ± 11 g/m2.7 at baseline 49 ± 10 vs. 47 ± 8 g/m2.7 during follow-up | Significant regression in LVMI in surgery but with slight decrease in drug treatment at a median of 36 months follow-up |
| Indra T [111] | 2015 | Prospective longitudinal | PA | Surgery 15 vs. Drug treatment 16 | 49 ± 11 vs. 51 ± 7 | 9 vs. 11 | LVMI&E/e’ | LVMI: 50 ± 12 vs. 53 ± 12 g/m2.7 at baseline 39 ± 9 vs. 49 ± 11 g/m2.7 at the end of study E/e’: 9.6 ± 3.0 vs. 14.4 ± 4.4 at baseline 7.1 ± 1.1 vs. 8.7 ± 1.0 at the end of study | Significant decrease in E/e’ in both surgery and drug treatment groups, with regression of LVMI only in surgery group |
| Freel EM [91] | 2012 | Cross-sectional | PA vs. PH | 27 vs. 53 | 54 ± 11 vs. 55 ± 9 | 21 vs. 42 | LGE | 70% vs. 13% | 4.3 times higher prevalence of non-infarct related replacement fibrosis in PA than PH |
| Su MY [92] | 2012 | Cross-sectional | PA vs. Controls | 25 vs. 12 | 50 ± 13 vs. 49 ± 14 | 6 vs. 7 | EV | 0.43 ± 0.05 vs. 0.36 ± 0.07 | Significantly increased diffuse fibrosis in PA compared with controls |
| First Author | Year | Study Design | Participants | No. of Participants | Age (y) | Sex (Male) | Outcome Measures | Results | Summary of Findings |
|---|---|---|---|---|---|---|---|---|---|
| Chen ZW [34] | 2018 | Cross-sectional | PA vs. PH | 36 vs. 31 | 49 ± 11 vs. 53 ± 12 | 15 vs. 16 | GLS | −17.8 ± 2.4 vs. −20.3 ± 2.3% | Significantly lower GLS in PA than PH |
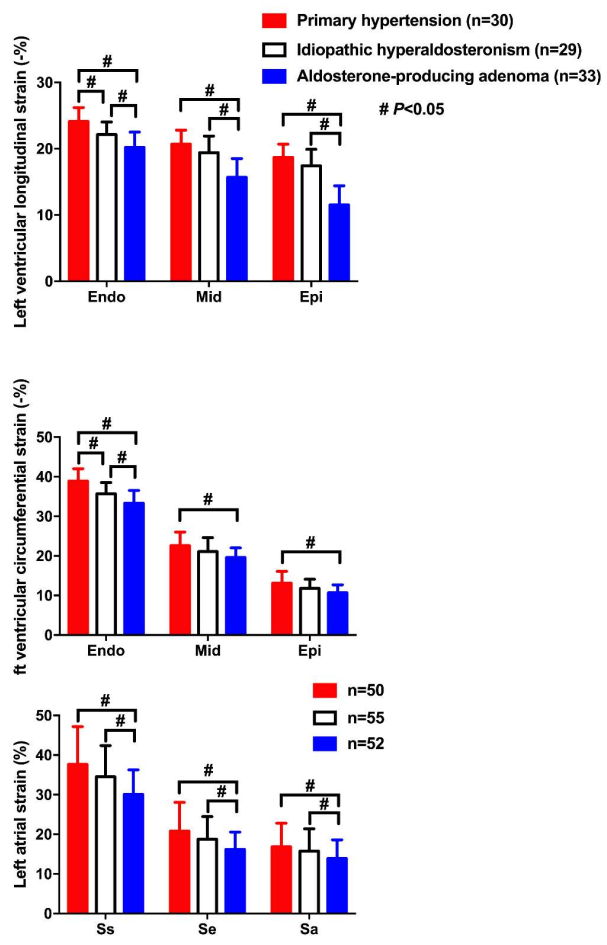
| Wang D [39] | 2019 | Cross-sectional | APA, IHAand PH | 33, 29 and 30 | 49 ± 10, 52 ± 8 and 51 ± 20 | 20, 22 and 18 | LS&CS | LSendo: −20.2 ± 2.3, −22.1 ± 1.9 and −24.1 ± 2.1% LSmid: −15.7 ± 2.8, −19.4 ± 2.5 and −20.7 ± 21% LSepi: −15.8 ± 2.1, −19.6 ± 2.2 and −21.2 ± 1.9% CSendo: −33.3 ± 3.2, −35.7 ± 2.8 and −38.9 ± 3.1% CSmid: −19.6 ± 2.4, −21.1 ± 3.5 and −22.6 ± 3.4% CSepi: −10.7 ± 2.0, −11.8 ± 2.3 and −13.1 ± 3.0% | Lowest CS and LS in endocardium (endo), midmyocardium (mid) and epicardium (epi) in APA, intermediate in IHA, and highest in PH |
| Wang D [50] | 2019 | Cross-sectional | APA, IHA and PH | 52, 55 and 50 | 52 ± 11, 51 ± 10 and 50 ± 17 | 35, 34 and 33 | LAS&LASR | LASs: 30.1 ± 6.2, 34.5 ± 7.9 and 37.7 ± 9.5% LASe: 16.2 ± 4.4, 18.8 ± 5.7 and 20.8 ± 7.3% LASa: 13.9 ± 4.7, 15.8 ± 5.6 and 16.9 ± 6.0% LASRs: 1.8 ± 0.6, 1.9 ± 0.4 and 2.1 ± 0.7/s LASRe: 1.6 ± 0.4, 1.7 ± 0.4 and 1.9 ± 0.6/s LASRa: 1.5 ± 0.5, 1.6 ± 0.6 and 1.8 ± 0.5/s | Significantly lower LAS and LASR during atrial reservoir (s), conduit (e) and contractile (a) phases in APA than IHA and PH |
| Chen YL [66] | 2020 | Cross-sectional | PA vs. PH | 51 vs. 50 | 51 ± 11 vs. 53 ± 11 | 34 vs. 30 | RV4CLS & RVFWLS | RV4CLS: −18.1 ± 2.5 vs.−23.3 ± 3.4% RVFWLS: −21.7 ± 3.7 vs. −27.9 ± 4.5% | Significantdecrease in both RV4CLS and RVFWLS in PA than PH |
| Chen YL [79] | 2021 | Cross-sectional | PA vs. PH | 50 vs. 50 | 51 ± 10 vs. 55 ± 11 | 32 vs. 33 | Strain (GLS) and myocardial work indices (GWI, GCW, GWW, & GWE) | GLS: −18.0 ± 2.1 vs. −19.2 ± 2.0% GWI: 2336 ± 333 vs. 2366 ± 288 mmHg% GCW: 2494 ± 325 vs. 2524 ± 301 mmHg% GWW: 206 ± 75 vs. 142 ± 56 mmHg% GWE: 91.1 ± 2.7 vs. 93.5 ± 2.5% | Significant decrease in GLS and GWE and increase in GWW in PA than PH, with similar GWI and GCW in the two groups |
| Chen YL [116] | 2021 | Prospective longitudinal | PA | Surgery 39 vs. Drug treatment 28 | 49 ± 10 vs. 49 ± 12 | 26 vs. 22 | Strain (GLS) and myocardial work indices (GWI, GCW, GWW, & GWE) | GLS: −18.3 ± 2.7 vs. −18.4 ± 2.3% at baseline 19.2 ± 1.9 vs. 18.4 ± 1.7% at 6 months GWI: 2372 ± 388 vs. 2335 ± 341 mmHg% at baseline 2280 ± 344 vs. 2208 ± 306 mmHg% at 6 months GCW: 2510 ± 360 vs. 2437 ± 293 mmHg% at baseline 2436 ± 335 vs. 2330 ± 311 mmHg% at 6 months GWW: 201 ± 87 vs. 164 ± 56 mmHg% at baseline 142 ± 58 vs. 164 ± 53 mmHg% at 6 months GWE: 91.5 ± 3.1 vs. 92.5 ± 2.2% at baseline 93.6 ± 2.3 vs. 92.4 ± 2.1% at 6 months | Significant improvement in GLS and GWE in surgery but not drug group at 6-month follow-up |
8. Biomarkers in Primary Aldosteronism
9. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Collier, P.; Phelan, D.; Klein, A. A Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Sala, C.; Carugo, S.; Mancia, G.; Grassi, G.; Cuspidi, C. Myocardial strain in hypertension: A meta-analysis of two-dimensional speckle tracking echocardiographic studies. J. Hypertens. 2021, 39, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Bombelli, M.; Grassi, G. Right heart remodeling induced by arterial hypertension: Could strain assessment be helpful? J. Clin. Hypertens. 2018, 20, 400–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzu, H.; Yuda, S.; Muranaka, A.; Doi, T.; Yamamoto, H.; Shimoshige, S.; Hase, M.; Hashimoto, A.; Saitoh, S.; Tsuchihashi, K.; et al. Left Ventricular Hypertrophy Causes Different Changes in Longitudinal, Radial, and Circumferential Mechanics in Patients with Hypertension: A Two-Dimensional Speckle Tracking Study. J. Am. Soc. Echocardiogr. 2011, 24, 192–199. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the use of echocardiography in adult hypertension: A report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef] [Green Version]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Rossi, G.P.; Sacchetto, A.; Visentin, P.; Canali, C.; Graniero, G.R.; Palatini, P.; Pessina, A.C. Changes in Left Ventricular Anatomy and Function in Hypertension and Primary Aldosteronism. Hypertension 1996, 27, 1039–1045. [Google Scholar] [CrossRef]
- Muiesan, M.L.; Salvetti, M.; Paini, A.; Agabiti-Rosei, C.; Monteduro, C.; Galbassini, G.; Belotti, E.; Aggiusti, C.; Rizzoni, D.; Castellano, M.; et al. Inappropriate Left Ventricular Mass in Patients with Primary Aldosteronism. Hypertension 2008, 52, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, A.; Naruse, M.; Naruse, K.; Hase, M.; Yoshimoto, T.; Tanaka, M.; Seki, T.; Demura, R.; Demura, H. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens. Res. 1997, 20, 85–90. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, L.; Xu, J.; Tang, X.; Gao, P. Comparison of left ventricular structure and function in primary aldosteronism and essential hypertension by echocardiography. Hypertens. Res. 2017, 40, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, J.; Hu, J.; Song, Y.; He, W.; Luo, T.; Cheng, Q.; Ma, L.; Luo, R.; Fuller, P.J.; et al. Primary Aldosteronism in Patients in China With Recently Detected Hypertension. J. Am. Coll. Cardiol. 2020, 75, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Douma, S.; Petidis, K.; Doumas, M.; Papaefthimiou, P.; Triantafyllou, A.; Kartali, N.; Papadopoulos, N.; Vogiatzis, K.; Zamboulis, C. Prevalence of primary hyperaldosteronism in resistant hypertension: A retrospective observational study. Lancet 2008, 371, 1921–1926. [Google Scholar] [CrossRef]
- Jansen, P.M.; van den Born, B.-J.H.; Frenkel, W.J.; de Bruijne, E.L.E.; Deinum, J.; Kerstens, M.N.; Smulders, Y.M.; Woittiez, A.J.; Wijbenga, J.A.M.; Zietse, R.; et al. Test characteristics of the aldosterone-to-renin ratio as a screening test for primary aldosteronism. J. Hypertens. 2014, 32, 115–126. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Liu, W.; Su, X. Determining the Prevalence of Primary Aldosteronism in Patients With New-Onset Type 2 Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2020, 105, 1079–1085. [Google Scholar] [CrossRef]
- Chinese Society of Endocrinology. Expert consensus on diagnosis and treatment of primary aldosteronism. Chin. J. Endocrinol. Metab. 2020, 36, 727–736. [Google Scholar]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.-C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928. [Google Scholar] [CrossRef]
- Chen, S.X.; Du, Y.L.; Zhang, J.; Gong, Y.C.; Hu, Y.R.; Chu, S.L.; He, Q.B.; Song, Y.Y.; Zhu, D.L. Aldosterone-to-renin ratio threshold for screening primary aldosteronism in Chinese hypertensive patients. ZhongHua Xin Xue Guan Bing Za Zhi 2006, 34, 868–872. [Google Scholar] [PubMed]
- Mulatero, P.; Sechi, L.A.; Williams, T.A.; Lenders, J.W.M.; Reincke, M.; Satoh, F.; Januszewicz, A.; Naruse, M.; Doumas, M.; Veglio, F.; et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sacchetto, A.; Pavan, E.; Palatini, P.; Graniero, G.R.; Canali, C.; Pessina, A.C. Remodeling of the Left Ventricle in Primary Aldosteronism Due to Conn’s Adenoma. Circulation 1997, 95, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, E.; Gordon, R.D.; Ahmed, A.H.; Cowley, D.; Leano, R.; Marwick, T.H.; Stowasser, M. Cardiac Dimensions Are Largely Determined by Dietary Salt in Patients with Primary Aldosteronism: Results of a Case-Control Study. J. Clin. Endocrinol. Metab. 2011, 96, 2813–2820. [Google Scholar] [CrossRef]
- Palmieri, V.; Wachtell, K.; Gerdts, E.; Bella, J.N.; Papademetriou, V.; Tuxen, C.; Nieminen, M.S.; Dahlöf, B.; de Simone, G.; Devereux, R.B. Left ventricular function and hemodynamic features of inappropriate left ventricular hypertrophy in patients with systemic hypertension: The LIFE Study. Am. Heart J. 2001, 141, 784–791. [Google Scholar] [CrossRef]
- Matsumura, K.; Fujii, K.; Oniki, H.; Oka, M.; Iida, M. Role of Aldosterone in Left Ventricular Hypertrophy in Hypertension. Am. J. Hypertens. 2006, 19, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-H.; Pan, C.-T.; Chang, Y.-Y.; Chen, Z.-W.; Wu, V.-C.; Hung, C.-S.; Lin, Y.-H. Left ventricular remodeling and dysfunction in primary aldosteronism. J. Hum. Hypertens. 2021, 35, 131–147. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lee, H.-H.; Liu, K.-L.; Lee, J.-K.; Shih, S.-R.; Chueh, S.-C.; Lin, W.-C.; Lin, L.-C.; Lin, L.-Y.; Chung, S.-D.; et al. Reversal of myocardial fibrosis in patients with unilateral hyperaldosteronism receiving adrenalectomy. Surgery 2011, 150, 526–533. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, X.-M.; Lee, H.-H.; Lee, J.-K.; Liu, Y.-C.; Chang, H.-W.; Lin, C.-Y.; Wu, V.-C.; Chueh, S.-C.; Lin, L.-C.; et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism: J. Hypertens. 2012, 30, 1606–1613. [Google Scholar] [CrossRef]
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess Aldosterone Is Associated with Alterations of Myocardial Texture in Primary Aldosteronism. Hypertension 2002, 40, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesari, M.; Letizia, C.; Angeli, P.; Sciomer, S.; Rosi, S.; Rossi, G.P. Cardiac remodeling in patients with primary and secondary aldosteronism: A tissue Doppler study. Circ. Cardiovasc. Imaging 2016, 9, e004815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch-Johnston, A.; Gussak, H.; Mobley, J.; Holland, M.; Petrovic, O.; Pérez, J.; Miller, J. Cyclic variation of integrated backscatter: Dependence of time delay on the echocardiographic view used and the myocardial segment analyzed. J. Am. Soc. Echocardiogr. 2000, 13, 9–17. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Huang, K.-C.; Lee, J.-K.; Lin, L.-C.; Chen, C.-W.; Chang, Y.-Y.; Liao, C.-W.; Wu, V.-C.; Hung, C.-S.; Lin, Y.-H. Aldosterone induces left ventricular subclinical systolic dysfunction: A strain imaging study. J. Hypertens. 2018, 36, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Tarascio, M.; Leo, L.A.; Klersy, C.; Murzilli, R.; Moccetti, T.; Faletra, F.F. Speckle-Tracking Layer-Specific Analysis of Myocardial Deformation and Evaluation of Scar Transmurality in Chronic Ischemic Heart Disease. J. Am. Soc. Echocardiogr. 2017, 30, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, S.; Mirea, O.; Duchenne, J.; Pagourelias, E.D.; Bézy, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.-U. Comparison of Feasibility, Accuracy, and Reproducibility of Layer-Specific Global Longitudinal Strain Measurements Among Five Different Vendors: A Report from the EACVI-ASE Strain Standardization Task Force. J. Am. Soc. Echocardiogr. 2018, 31, 374–380.e1. [Google Scholar] [CrossRef]
- Kim, D.; Shim, C.Y.; Hong, G.-R.; Park, S.; Cho, I.; Chang, H.-J.; Ha, J.-W.; Chung, N. Differences in left ventricular functional adaptation to arterial stiffness and neurohormonal activation in patients with hypertension: A study with two-dimensional layer-specific speckle tracking echocardiography. Clin. Hypertens. 2017, 23, 21. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-H.; Liu, Y.-W.; Yang, L.-T.; Tsai, W.-C. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. J. Hypertens. 2016, 34, 1195–1200. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.-Z.; Chen, X.; Chen, Y.; Shao, S.; Zhang, W.; Zhu, L.-M.; Xu, T.-Y.; Li, Y.; Wang, J.-G. Speckle-Tracking Echocardiographic Layer-Specific Strain Analysis on Subclinical Left Ventricular Dysfunction in Patients With Primary Aldosteronism. Am. J. Hypertens. 2019, 32, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Catena, C.; Colussi, G.; Lapenna, R.; Nadalini, E.; Chiuch, A.; Gianfagna, P.; Sechi, L.A. Long-Term Cardiac Effects of Adrenalectomy or Mineralocorticoid Antagonists in Patients With Primary Aldosteronism. Hypertension 2007, 50, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-Y.; Lee, H.-H.; Hung, C.-S.; Wu, X.-M.; Lee, J.-K.; Wang, S.-M.; Liao, M.-T.; Chen, Y.-H.; Wu, V.-C.; Wu, K.-D.; et al. Association between urine aldosterone and diastolic function in patients with primary aldosteronism and essential hypertension. Clin. Biochem. 2014, 47, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Kohncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J.—Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Mondillo, S.; Cameli, M.; Caputo, M.L.; Lisi, M.; Palmerini, E.; Padeletti, M.; Ballo, P. Early Detection of Left Atrial Strain Abnormalities by Speckle-Tracking in Hypertensive and Diabetic Patients with Normal Left Atrial Size. J. Am. Soc. Echocardiogr. 2011, 24, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-Y.; Sun, J.P.; Lee, A.P.-W.; Yang, X.S.; Ji, L.; Zhang, Z.; Li, Y.; Yu, C.-M.; Wang, J.-G. Left Atrial Function as Assessed by Speckle-Tracking Echocardiography in Hypertension. Medicine 2015, 94, e526. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J.—Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: Have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 1–28. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.-Z.; Chen, X.; Xu, T.-Y.; Zhang, W.; Li, Y.; Wang, Y.G. Left atrial myocardial dysfunction in patients with primary aldosteronism as assessed by speckle-tracking echocardiography. J. Hypertens. 2019, 37, 2032–2040. [Google Scholar] [CrossRef]
- Burke, M.A.; Katz, D.H.; Beussink, L.; Selvaraj, S.; Gupta, D.K.; Fox, J.; Chakrabarti, S.; Sauer, A.J.; Rich, J.D.; Freed, B.H.; et al. Prognostic Importance of Pathophysiologic Markers in Patients With Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 288–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nochioka, K.; Querejeta Roca, G.; Claggett, B.; Biering-Sørensen, T.; Matsushita, K.; Hung, C.-L.; Solomon, S.D.; Kitzman, D.; Shah, A.M. Right Ventricular Function, Right Ventricular–Pulmonary Artery Coupling, and Heart Failure Risk in 4 US Communities: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2018, 3, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oketona, O.; Balogun, M.; Akintomide, A.; Ajayi, O.; Adebayo, R.; Mene-Afejuku, T.; Oketona, O.; Bamikole, O. Right ventricular systolic function in hypertensive heart failure. Vasc. Health Risk Manag. 2017, 13, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuspidi, C.; Negri, F.; Giudici, V.; Valerio, C.; Meani, S.; Sala, C.; Esposito, A.; Masaidi, M.; Zanchetti, A.; Mancia, G. Prevalence and clinical correlates of right ventricular hypertrophy in essential hypertension. J. Hypertens. 2009, 27, 854–860. [Google Scholar] [CrossRef]
- Tumuklu, M.M.; Erkorkmaz, U.; Ocal, A. The impact of hypertension and hypertension-related left ventricle hypertrophy on right ventricle function. Echocardiography 2007, 24, 374–384. [Google Scholar] [CrossRef]
- Vriz, O.; Argiento, P.; D’Alto, M.; Ferrara, F.; Vanderpool, R.; Naeije, R.; Bossone, E. Increased pulmonary vascular resistance in early stage systemic hypertension: A resting and exercise stress echocardiography study. Can. J. Cardiol. 2015, 31, 537–543. [Google Scholar] [CrossRef]
- Gregori, M.; Giammarioli, B.; Tocci, G.; Befani, A.; Ciavarella, G.M.; Ferrucci, A.; Paneni, F. Synergic effects of renin and aldosterone on right ventricular function in hypertension: A tissue Doppler study. J. Cardiovasc. Med. 2015, 16, 831–838. [Google Scholar] [CrossRef]
- Gregori, M.; Tocci, G.; Giammarioli, B.; Befani, A.; Ciavarella, G.M.; Ferrucci, A.; Paneni, F. Abnormal Regulation of Renin Angiotensin Aldosterone System Is Associated with Right Ventricular Dysfunction in Hypertension. Can. J. Cardiol. 2014, 30, 188–194. [Google Scholar] [CrossRef]
- Guazzi, M.; Borlaug, B.A. Pulmonary hypertension due to left heart disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yasumura, Y.; Yoshida, C.; Okumura, T.; Tateishi, J.; Yoshida, J.; Abe, H.; Tamaki, S.; Yano, M.; Hayashi, T.; et al. Prognostic importance of right ventricular-vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ. Cardiovasc. Imaging 2020, 13, e011430. [Google Scholar] [CrossRef]
- Vriz, O.; Pirisi, M.; Bossone, E.; Fadl ElMula, F.E.M.; Palatini, P.; Naeije, R. Right ventricular–pulmonary arterial uncoupling in mild-to-moderate systemic hypertension. J. Hypertens. 2020, 38, 274–281. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Yoshifuku, S.; Otsuji, Y.; Takasaki, K.; Yuge, K.; Kisanuki, A.; Toyonaga, K.; Lee, S.; Murayama, T.; Nakashima, H.; Kumanohoso, T.; et al. Pseudonormalized doppler totalejection isovolume (Tei) index in patients with right ventricularacute myocardial infarction. Am. J. Cardiol. 2003, 91, 527–531. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Celic, V.; Pencic-Popovic, B.; Mancia, G. Nocturnal hypertension and right heart remodeling. J. Hypertens. 2018, 36, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Pencic, B.; Ivanovic, B.; Scepanovic, R.; Marjanovic, T.; Jozika, L.; Celic, V. Circadian blood pressure pattern and right ventricular and right atrial mechanics: A two- and three-dimensional echocardiographic study. J. Am. Soc. Hypertens. 2014, 8, 45–53. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A speckle tracking echocardiographic study on right ventricular function in primary aldosteronism. J. Hypertens. 2020, 38, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; Kostyukevich, M.; El Mahdiui, M.; Hansen, G.; Samset, E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. A Roadmap to Assess Myocardial Work: From theory to clinical practice. JACC Cardiovasc. Imaging 2019, 12, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: A novel method ready for clinical testing. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Leclercq, C.; Fournet, M.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Value of Myocardial Work Estimation in the Prediction of Response to Cardiac Resynchronization Therapy. J. Am. Soc. Echocardiogr. 2018, 31, 220–230. [Google Scholar] [CrossRef]
- Cauwenberghs, N.; Tabassian, M.; Thijs, L.; Yang, W.-Y.; Wei, F.-F.; Claus, P.; D’hooge, J.; Staessen, J.A.; Kuznetsova, T. Area of the pressure-strain loop during ejection as non-invasive index of left ventricular performance: A population study. Cardiovasc. Ultrasound 2019, 17, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients With Normal Left Ventricular Function and Wall Motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957. [Google Scholar] [CrossRef] [PubMed]
- van der Bijl, P.; Vo, N.M.; Kostyukevich, M.V.; Mertens, B.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Prognostic implications of global, left ventricular myocardial work efficiency before cardiac resynchronization therapy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure–strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaglan, A.; Roemer, S.; Perez Moreno, A.C.; Khandheria, B.K. Myocardial work in Stage 1 and 2 hypertensive patients. Eur. Heart J.—Cardiovasc. Imaging 2021, 22, 744–750. [Google Scholar] [CrossRef]
- Sahiti, F.; Morbach, C.; Cejka, V.; Tiffe, T.; Wagner, M.; Eichner, F.A.; Gelbrich, G.; Heuschmann, P.U.; Störk, S. Impact of cardiovascular risk factors on myocardial work—insights from the STAAB cohort study. J. Hum. Hypertens. 2021. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A non-invasive left ventricular pressure-strain loop study on myocardial work in primary aldosteronism. Hypertens. Res. 2021, 44, 1462–1470. [Google Scholar] [CrossRef]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of Myocardial Fibrosis with Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Lima, J.A.C. Cardiac MRI: A central prognostic tool in myocardial fibrosis. Nat. Rev. Cardiol. 2015, 12, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Lembo, M.; Manzi, M.V.; Mancusi, C.; Morisco, C.; Rao, M.A.E.; Cuocolo, A.; Izzo, R.; Trimarco, B. Advanced imaging tools for evaluating cardiac morphological and functional impairment in hypertensive disease. J. Hypertens. 2022, 40, 4–14. [Google Scholar] [CrossRef]
- Weber, K.T.; Brilla, C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991, 83, 1849–1865. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive Detection of Fibrosis Applying Contrast-Enhanced Cardiac Magnetic Resonance in Different Forms of Left Ventricular Hypertrophy. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, S.; Janardhanan, R.; Antkowiak, P.; Keeley, E.C.; Adenaw, N.; Brooks, J.; Epstein, F.H.; Kramer, C.M.; Salerno, M. Increased Extracellular Volume and Altered Mechanics Are Associated with LVH in Hypertensive Heart Disease, Not Hypertension Alone. JACC Cardiovasc. Imaging 2015, 8, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.A.; Michaëlsson, E.; Shaw, P.W.; Kuruvilla, S.; Kramer, C.M.; Gan, L.-M.; Keeley, E.C.; Salerno, M. Extracellular volume by cardiac magnetic resonance is associated with biomarkers of inflammation in hypertensive heart disease. J. Hypertens. 2019, 37, 65–72. [Google Scholar] [CrossRef]
- Freel, E.M.; Mark, P.B.; Weir, R.A.P.; McQuarrie, E.P.; Allan, K.; Dargie, H.J.; McClure, J.D.; Jardine, A.G.; Davies, E.; Connell, J.M.C. Demonstration of Blood Pressure-Independent Noninfarct Myocardial Fibrosis in Primary Aldosteronism: A Cardiac Magnetic Resonance Imaging Study. Circ. Cardiovasc. Imaging 2012, 5, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.-Y.M.; Wu, V.-C.; Yu, H.-Y.; Lin, Y.-H.; Kuo, C.-C.; Liu, K.-L.; Wang, S.-M.; Chueh, S.-C.; Lin, L.-Y.; Wu, K.-D.; et al. Contrast-enhanced MRI index of diffuse myocardial fibrosis is increased in primary aldosteronism. J. Magn. Reson. Imaging 2012, 35, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Redheuil, A.; Blanchard, A.; Pereira, H.; Raissouni, Z.; Lorthioir, A.; Soulat, G.; Vargas-Poussou, R.; Amar, L.; Paul, J.-L.; Helley, D.; et al. Aldosterone-Related Myocardial Extracellular Matrix Expansion in Hypertension in Humans. JACC Cardiovasc. Imaging 2020, 13, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Grytaas, M.A.; Sellevåg, K.; Thordarson, H.B.; Husebye, E.S.; Løvås, K.; Larsen, T.H. Cardiac magnetic resonance imaging of myocardial mass and fibrosis in primary aldosteronism. Endocr. Connect. 2018, 7, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimino, S.; Canali, E.; Petronilli, V.; Cicogna, F.; De Luca, L.; Francone, M.; Sardella, G.; Iacoboni, C.; Agati, L. Global and regional longitudinal strain assessed by two-dimensional speckle tracking echocardiography identifies early myocardial dysfunction and transmural extent of myocardial scar in patients with acute ST elevation myocardial infarction and relatively preserved LV function. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lee, A.P.-W.; Li, Z.; Qiao, Z.; Fan, Y.; An, D.; Xu, J.; Pu, J.; Shen, X.; Ge, H.; et al. Impact of Intramyocardial Hemorrhage and Microvascular Obstruction on Cardiac Mechanics in Reperfusion Injury: A Speckle-Tracking Echocardiographic Study. J. Am. Soc. Echocardiogr. 2016, 29, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Zhang, L.; Tian, F.; Wang, B.; Xie, Y.; Sun, W.; Sun, Z.; Yang, Y.; Lv, Q.; et al. Assessment of myocardial fibrosis using two-dimensional and three-dimensional speckle tracking echocardiography in dilated cardiomyopathy with advanced heart failure. J. Card. Fail. 2021, 27, 651–661. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Unlu, S.; Van Cleemput, J.; Papadopoulos, C.E.; Bogaert, J.; Vassilikos, V.P.; Voigt, J.-U. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: A comparison between 2D and 3D echo modalities. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1262–1272. [Google Scholar] [CrossRef]
- Mandoli, G.E.; D’Ascenzi, F.; Vinco, G.; Benfari, G.; Ricci, F.; Focardi, M.; Cavigli, L.; Pastore, M.C.; Sisti, N.; De Vivo, O.; et al. Novel approaches in cardiac imaging for non-invasive assessment of left heart myocardial fibrosis. Front. Cardiovasc. Med. 2021, 8, 614235. [Google Scholar] [CrossRef]
- Galli, E.; Vitel, E.; Schnell, F.; Le Rolle, V.; Hubert, A.; Lederlin, M.; Donal, E. Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography 2019, 36, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Xu, T.-Y.; Chen, C.-H.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-W. An Imaging Study on Cardiac Fibrosis in Primary Aldosteronism. Ruijin Hospital, Shanghai, China. 2021. manuscript in preparation. [Google Scholar]
- Young, W.F., Jr. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2019, 285, 126–148. [Google Scholar] [CrossRef] [Green Version]
- Marzano, L.; Colussi, G.; Sechi, L.A.; Catena, C. Adrenalectomy Is Comparable with Medical Treatment for Reduction of Left Ventricular Mass in Primary Aldosteronism: Meta-Analysis of Long-Term Studies. Am. J. Hypertens. 2015, 28, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ori, Y.; Chagnac, A.; Korzets, A.; Zingerman, B.; Herman-Edelstein, M.; Bergman, M.; Gafter, U.; Salman, H. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol. Dial. Transplant. 2013, 28, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, K.; Corros, C.; Pimenta, E.; Ahmed, M.; Denney, T.; Aban, I.; Inusah, S.; Gupta, H.; Lloyd, S.G.; Oparil, S.; et al. Rapid Reversal of Left Ventricular Hypertrophy and Intracardiac Volume Overload in Patients with Resistant Hypertension and Hyperaldosteronism: A Prospective Clinical Study. Hypertension 2010, 55, 1137–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G.P.; Cesari, M.; Cuspidi, C.; Maiolino, G.; Cicala, M.V.; Bisogni, V.; Mantero, F.; Pessina, A.C. Long-Term Control of Arterial Hypertension and Regression of Left Ventricular Hypertrophy With Treatment of Primary Aldosteronism. Hypertension 2013, 62, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernini, G.; Bacca, A.; Carli, V.; Carrara, D.; Materazzi, G.; Berti, P.; Miccoli, P.; Pisano, R.; Tantardini, V.; Bernini, M.; et al. Cardiovascular changes in patients with primary aldosteronism after surgical or medical treatment. J. Endocrinol. Investig. 2012, 35, 274–280. [Google Scholar] [CrossRef]
- Giacchetti, G.; Ronconi, V.; Turchi, F.; Agostinelli, L.; Mantero, F.; Rilli, S.; Boscaro, M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: An observational study. J. Hypertens. 2007, 25, 177–186. [Google Scholar] [CrossRef]
- Indra, T.; Holaj, R.; Štrauch, B.; Rosa, J.; Petrák, O.; Šomlóová, Z.; Widimský, J. Long-term effects of adrenalectomy or spironolactone on blood pressure control and regression of left ventricle hypertrophy in patients with primary aldosteronism. J. Renin. Angiotensin. Aldosterone Syst. 2015, 16, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Liao, C.; Tsai, C.; Chen, C.; Pan, C.; Chen, Z.; Chen, Y.; Lin, L.; Chang, Y.; Wu, V.; et al. Left Ventricular Dysfunction in Patients With Primary Aldosteronism: A Propensity Score–Matching Follow-Up Study With Tissue Doppler Imaging. J. Am. Heart Assoc. 2019, 8, e013263. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Tsai, C.-H.; Peng, S.-Y.; Chen, Z.-W.; Chang, C.-C.; Lee, B.-C.; Liao, C.-W.; Pan, C.-T.; Chen, Y.-L.; Lin, L.-C.; et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated With a Worse Baseline Status and Better Recovery of Left Ventricular Remodeling and Diastolic Function. Hypertension 2021, 77, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Dal Ferro, M.; De Paris, V.; Collia, D.; Stolfo, D.; Caiffa, T.; Barbati, G.; Korcova, R.; Pinamonti, B.; Zovatto, L.; Zecchin, M.; et al. Left Ventricular Response to Cardiac Resynchronization Therapy: Insights From Hemodynamic Forces Computed by Speckle Tracking. Front. Cardiovasc. Med. 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Sotomi, Y.; Okamura, A.; Iwakura, K.; Date, M.; Nagai, H.; Yamasaki, T.; Koyama, Y.; Inoue, K.; Sakata, Y.; Fujii, K. Impact of revascularization of coronary chronic total occlusion on left ventricular function and electrical stability: Analysis by speckle tracking echocardiography and signal-averaged electrocardiogram. Int. J. Cardiovasc. Imaging 2017, 33, 815–823. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C. Left ventricular strain and arterial hypertension: Is longitudinal strain ready for primetime? J. Clin. Hypertens. 2020, 22, 683–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Xu, T.-Y.; Xu, J.-Z.; Zhu, L.-M.; Li, Y.; Wang, J.-G. A Prospective Comparative Study on Cardiac Alterations After Surgery and Drug Treatment of Primary Aldosteronism. Front. Endocrinol. 2021, 12, 770711. [Google Scholar] [CrossRef]
- Wu, Q.; Hong, M.; Xu, J.; Tang, X.; Zhu, L.; Gao, P.; Wang, J. Diurnal blood pressure pattern and cardiac damage in hypertensive patients with primary aldosteronism. Endocrine 2021, 72, 835–843. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Marzano, L.; Sechi, L. Predictive factors of left ventricular mass changes after treatment of primary aldosteronism. Horm. Metab. Res. 2012, 44, 188–193. [Google Scholar] [CrossRef]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, Y.B.; Wang, J.G. China nationwide screening and registry of primary aldosteronism in hypertensive patients. J. Hum. Hypertens. 2021, 35, 157–161. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xu, T.; Xu, J.; Zhu, L.; Wang, D.; Li, Y.; Wang, J. Strain Imaging for the Early Detection of Cardiac Remodeling and Dysfunction in Primary Aldosteronism. Diagnostics 2022, 12, 543. https://doi.org/10.3390/diagnostics12020543
Chen Y, Xu T, Xu J, Zhu L, Wang D, Li Y, Wang J. Strain Imaging for the Early Detection of Cardiac Remodeling and Dysfunction in Primary Aldosteronism. Diagnostics. 2022; 12(2):543. https://doi.org/10.3390/diagnostics12020543
Chicago/Turabian StyleChen, Yilin, Tingyan Xu, Jianzhong Xu, Limin Zhu, Dian Wang, Yan Li, and Jiguang Wang. 2022. "Strain Imaging for the Early Detection of Cardiac Remodeling and Dysfunction in Primary Aldosteronism" Diagnostics 12, no. 2: 543. https://doi.org/10.3390/diagnostics12020543
APA StyleChen, Y., Xu, T., Xu, J., Zhu, L., Wang, D., Li, Y., & Wang, J. (2022). Strain Imaging for the Early Detection of Cardiac Remodeling and Dysfunction in Primary Aldosteronism. Diagnostics, 12(2), 543. https://doi.org/10.3390/diagnostics12020543






