Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Prostatic Artery Embolization
2.3. MR Imaging Sequences
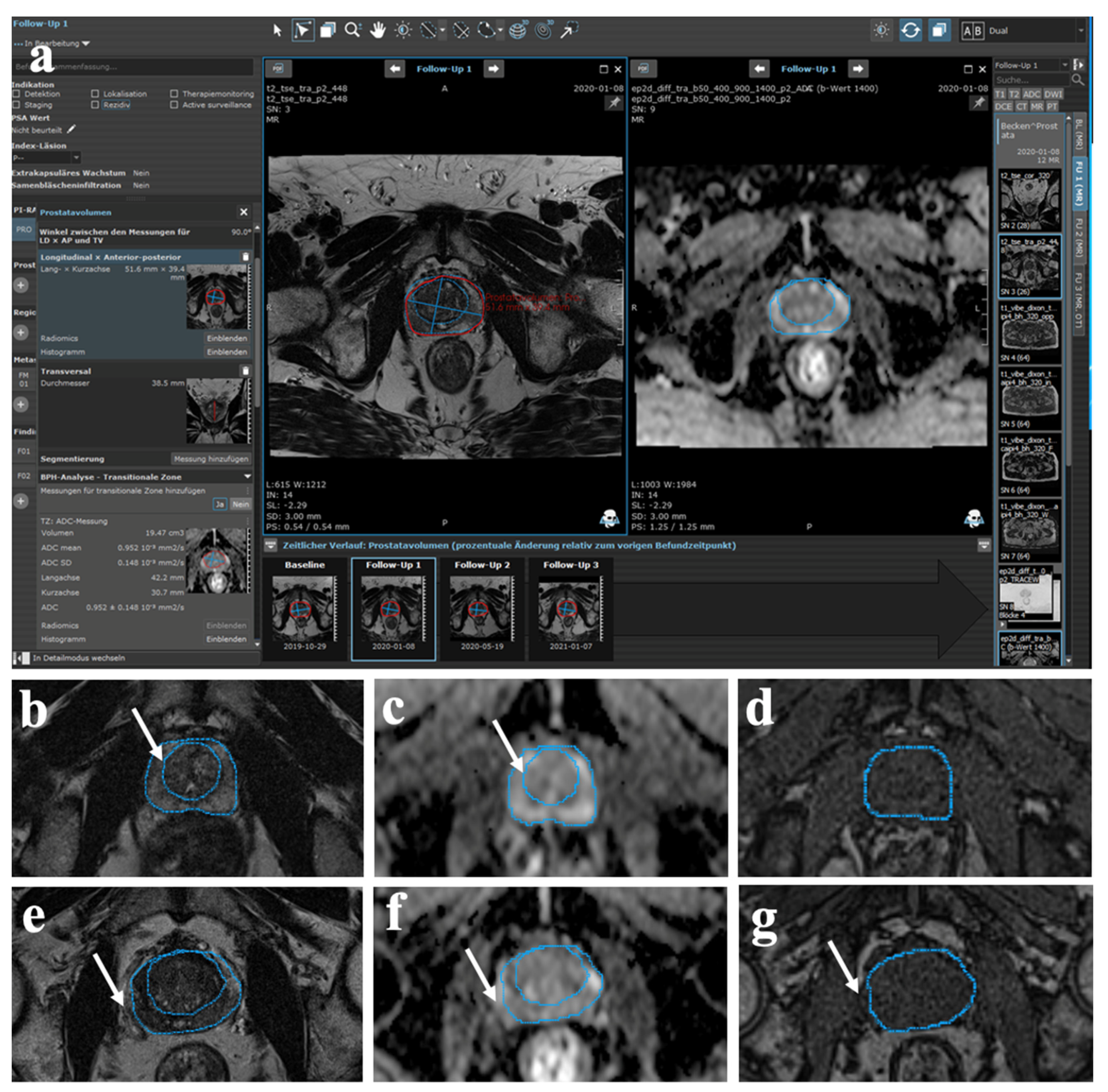
2.4. Semi-Automatic MR Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Volumetric Analyses
3.3. Changes in Signal Intensities
3.4. Clinical Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foo, K.T. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J. Urol. 2019, 37, 1293–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, L.F.; Oto, A.; Allen, B.C.; Akin, O.; Chong, J.; Froemming, A.T.; Fulgham, P.F.; Goldfarb, S.; Maranchie, J.K.; Mody, R.N.; et al. ACR Appropriateness Criteria® Lower Urinary Tract Symptoms-Suspicion of Benign Prostatic Hyperplasia. J. Am. Coll. Radiol. 2019, 16, S378–S383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, M.J.; Fowler, F.J., Jr.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J. Urol. 2017, 197, S189–S197. [Google Scholar] [CrossRef]
- Ng, M.; Baradhi, K.M. Benign Prostatic Hyperplasia; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Abt, D.; Schmid, H.P.; Speakman, M.J. Reasons to consider prostatic artery embolization. World J. Urol. 2021, 39, 2301–2306. [Google Scholar] [CrossRef]
- Lokeshwar, S.D.; Harper, B.T.; Webb, E.; Jordan, A.; Dykes, T.A.; Neal, D.E., Jr.; Terris, M.K.; Klaassen, Z. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl. Urol. 2019, 8, 529–539. [Google Scholar] [CrossRef]
- Magistro, G.; Chapple, C.R.; Elhilali, M.; Gilling, P.; McVary, K.T.; Roehrborn, C.G.; Stief, C.G.; Woo, H.H.; Gratzke, C. Emerging Minimally Invasive Treatment Options for Male Lower Urinary Tract Symptoms. Eur. Urol. 2017, 72, 986–997. [Google Scholar] [CrossRef]
- Salem, R.; Hairston, J.; Hohlastos, E.; Riaz, A.; Kallini, J.; Gabr, A.; Ali, R.; Jenkins, K.; Karp, J.; Desai, K.; et al. Prostate Artery Embolization for Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: Results From a Prospective FDA-Approved Investigational Device Exemption Study. Urology 2018, 120, 205–210. [Google Scholar] [CrossRef]
- Tapping, C.R.; Little, M.W.; Macdonald, A.; Mackinnon, T.; Kearns, D.; Macpherson, R.; Crew, J.; Boardman, P. The STREAM Trial (Prostatic Artery Embolization for the Treatment of Benign Prostatic Hyperplasia) 24-Month Clinical and Radiological Outcomes. Cardiovasc Interv. Radiol. 2020, 44, 436–442. [Google Scholar] [CrossRef]
- Maclean, D.; Kong, M.; Lim, J.; Modi, S.; Harris, M.; Bryant, T.; Hacking, N. Does Prostate Artery Embolization (PAE) Improve Voiding Symptoms, Storage Symptoms, or Both? Cardiovasc Interv. Radiol. 2020, 43, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Rampoldi, A.; Barbosa, F.; Secco, S.; Migliorisi, C.; Galfano, A.; Prestini, G.; Harward, S.H.; Di Trapani, D.; Brambillasca, P.M.; Ruggero, V.; et al. Prostatic Artery Embolization as an Alternative to Indwelling Bladder Catheterization to Manage Benign Prostatic Hyperplasia in Poor Surgical Candidates. Cardiovasc Interv. Radiol. 2017, 40, 530–536. [Google Scholar] [CrossRef]
- Pisco, J.; Bilhim, T.; Pinheiro, L.C.; Fernandes, L.; Pereira, J.; Costa, N.V.; Duarte, M.; Oliveira, A.G. Prostate Embolization as an Alternative to Open Surgery in Patients with Large Prostate and Moderate to Severe Lower Urinary Tract Symptoms. J. Vasc. Interv. Radiol. 2016, 27, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Uflacker, A.; Haskal, Z.J.; Bilhim, T.; Patrie, J.; Huber, T.; Pisco, J.M. Meta-Analysis of Prostatic Artery Embolization for Benign Prostatic Hyperplasia. J. Vasc. Interv. Radiol. 2016, 27, 1686–1697.e1688. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, J.H.; Zhu, Y.B.; Wu, S.J.; Cai, S.L.; Zhou, Y.F.; Qian, X.; Luo, J.W.; Fang, Z.T. Effect of superselective prostatic artery embolization on benign prostatic hyperplasia. Abdom Radiol. 2021, 46, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The Comparison of Imaging and Clinical Methods to Estimate Prostate Volume: A Single-Centre Retrospective Study. Urol. Int. 2021, 105, 804–810. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Pan, J.; Wang, H.; Zhong, Y.; Wang, Y.; Ye, H. MRI features after prostatic artery embolization for the treatment of medium- and large-volume benign hyperplasia. Radiol. Med. 2018, 123, 727–734. [Google Scholar] [CrossRef]
- Ali, R.; Gabr, A.; Mouli, S.K.; Kallini, J.R.; Riaz, A.; Mora, R.; Lewandowski, R.J.; Hohlastos, E.; Casalino, D.D.; Hofer, M.D.; et al. MR imaging findings of the prostate gland following prostate artery embolization: Results from a prospective phase 2 study. Abdom Radiol. 2019, 44, 713–722. [Google Scholar] [CrossRef]
- Sosna, J.; Rofsky, N.M.; Gaston, S.M.; DeWolf, W.C.; Lenkinski, R.E. Determinations of prostate volume at 3-Tesla using an external phased array coil: Comparison to pathologic specimens. Acad Radiol. 2003, 10, 846–853. [Google Scholar] [CrossRef]
- Abt, D.; Hechelhammer, L.; Müllhaupt, G.; Markart, S.; Güsewell, S.; Kessler, T.M.; Schmid, H.P.; Engeler, D.S.; Mordasini, L. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: Randomised, open label, non-inferiority trial. BMJ 2018, 361, k2338. [Google Scholar] [CrossRef] [Green Version]
- Xiang, P.; Guan, D.; Du, Z.; Hao, Y.; Yan, W.; Wang, Y.; Liu, Y.; Liu, D.; Ping, H. Efficacy and safety of prostatic artery embolization for benign prostatic hyperplasia: A systematic review and meta-analysis of randomized controlled trials. Eur. Radiol. 2021, 31, 4929–4946. [Google Scholar] [CrossRef]
- Bilhim, T.; Pisco, J.M.; Rio Tinto, H.; Fernandes, L.; Pinheiro, L.C.; Furtado, A.; Casal, D.; Duarte, M.; Pereira, J.; Oliveira, A.G.; et al. Prostatic arterial supply: Anatomic and imaging findings relevant for selective arterial embolization. J. Vasc. Interv. Radiol. 2012, 23, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monaco, R.; Garategui, L.; Kizilevsky, N.; Peralta, O.; Rodriguez, P.; Palacios-Jaraquemada, J. Human cadaveric specimen study of the prostatic arterial anatomy: Implications for arterial embolization. J. Vasc. Interv. Radiol. 2014, 25, 315–322. [Google Scholar] [CrossRef]
- Lin, Y.T.; Amouyal, G.; Correas, J.M.; Pereira, H.; Pellerin, O.; Del Giudice, C.; Déan, C.; Thiounn, N.; Sapoval, M. Can prostatic arterial embolisation (PAE) reduce the volume of the peripheral zone? MRI evaluation of zonal anatomy and infarction after PAE. Eur. Radiol. 2016, 26, 3466–3473. [Google Scholar] [CrossRef] [PubMed]
- Frenk, N.E.; Baroni, R.H.; Carnevale, F.C.; Gonçalves, O.M.; Antunes, A.A.; Srougi, M.; Cerri, G.G. MRI findings after prostatic artery embolization for treatment of benign hyperplasia. AJR Am. J. Roentgenol 2014, 203, 813–821. [Google Scholar] [CrossRef]
- Shim, S.R.; Kanhai, K.J.; Ko, Y.M.; Kim, J.H. Efficacy and Safety of Prostatic Arterial Embolization: Systematic Review with Meta-Analysis and Meta-Regression. J. Urol. 2017, 197, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Bilhim, T.; Pisco, J.; Pereira, J.A.; Costa, N.V.; Fernandes, L.; Campos Pinheiro, L.; Duarte, M.; Oliveira, A.G. Predictors of Clinical Outcome after Prostate Artery Embolization with Spherical and Nonspherical Polyvinyl Alcohol Particles in Patients with Benign Prostatic Hyperplasia. Radiology 2016, 281, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.J.; Williford, W.O.; Chang, Y.; Machi, M.; Jones, K.M.; Walker-Corkery, E.; Lepor, H. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995, 154, 1770–1774. [Google Scholar] [CrossRef]
- Blanker, M.H.; Alma, H.J.; Devji, T.S.; Roelofs, M.; Steffens, M.G.; van der Worp, H. Determining the minimal important differences in the International Prostate Symptom Score and Overactive Bladder Questionnaire: Results from an observational cohort study in Dutch primary care. BMJ. Open 2019, 9, e032795. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.S.; Sim, H.G.; Lim, K.B.; Wang, D.; Foo, K.T. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int. J. Urol. 2010, 17, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, J.W.; Kim, J.W.; Oh, M.M.; Moon du, G. Defining the degree of intravesical prostatic protrusion in association with bladder outlet obstruction. Korean J. Urol. 2013, 54, 369–372. [Google Scholar] [CrossRef]
- Chia, S.J.; Heng, C.T.; Chan, S.P.; Foo, K.T. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int. 2003, 91, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Topazio, L.; Perugia, C.; De Nunzio, C.; Gaziev, G.; Iacovelli, V.; Bianchi, D.; Vespasiani, G.; Finazzi Agrò, E. Intravescical prostatic protrusion is a predictor of alpha blockers response: Results from an observational study. BMC Urol. 2018, 18, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keqin, Z.; Zhishun, X.; Jing, Z.; Haixin, W.; Dongqing, Z.; Benkang, S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology 2007, 70, 1096–1099. [Google Scholar] [CrossRef]
- Lin, Y.T.; Amouyal, G.; Thiounn, N.; Pellerin, O.; Pereira, H.; Del Giudice, C.; Déan, C.; Sapoval, M. Intra-vesical Prostatic Protrusion (IPP) Can Be Reduced by Prostatic Artery Embolization. Cardiovasc Interv. Radiol. 2016, 39, 690–695. [Google Scholar] [CrossRef]
- Grossfeld, G.D.; Coakley, F.V. Benign prostatic hyperplasia: Clinical overview and value of diagnostic imaging. Radiol. Clin. N. Am. 2000, 38, 31–47. [Google Scholar] [CrossRef]
- Kisilevzky, N.; Faintuch, S. MRI assessment of prostatic ischaemia: Best predictor of clinical success after prostatic artery embolisation for benign prostatic hyperplasia. Clin. Radiol. 2016, 71, 876–882. [Google Scholar] [CrossRef]
- Jara, H.; Yu, B.C.; Caruthers, S.D.; Melhem, E.R.; Yucel, E.K. Voxel sensitivity function description of flow-induced signal loss in MR imaging: Implications for black-blood MR angiography with turbo spin-echo sequences. Magn. Reson. Med. 1999, 41, 575–590. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Saritas, E.U.; Lee, J.H.; Nishimura, D.G. SNR dependence of optimal parameters for apparent diffusion coefficient measurements. IEEE Trans Med. Imaging 2011, 30, 424–437. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, D.; Xiao, Z.; Wang, L.; Ma, R.; Chen, H.; Luo, L. Ultrahigh b-values MRI in normal human prostate: Initial research on reproducibility and age-related differences. J. Magn. Reson. Imaging 2017, 46, 801–812. [Google Scholar] [CrossRef]
- Durur-Subasi, I.; Durur-Karakaya, A.; Karaman, A.; Seker, M.; Demirci, E.; Alper, F. Is the necrosis/wall ADC ratio useful for the differentiation of benign and malignant breast lesions? Br. J. Radiol. 2017, 90, 20160803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyng, H.; Haraldseth, O.; Rofstad, E.K. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn. Reson. Med. 2000, 43, 828–836. [Google Scholar] [CrossRef]
- Kwon, J.K.; Han, J.H.; Choi, H.C.; Kang, D.H.; Lee, J.Y.; Kim, J.H.; Oh, C.K.; Choi, Y.D.; Cho, K.S. Clinical significance of peripheral zone thickness in men with lower urinary tract symptoms/benign prostatic hyperplasia. BJU Int. 2016, 117, 316–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Protocol | Sequence | TR (ms) | TE (ms) | FA | FOV (cm) | Matrix | Slice Thickness (mm) | NEX | Bandwidth (Hz/Pixel) |
|---|---|---|---|---|---|---|---|---|---|
| Axial T2w | Turbo SE | 8900 | 119 | 159 | 20 × 24 | 448 × 340 | 3 | 2 | 200 |
| Sagittal T2w | Turbo SE | 12,500 | 114 | 144 | 20 × 20 | 320 × 320 | 3 | 2 | 200 |
| Axial T1w opposed phase | VIBE | 12.5 | 1.3 | 9 | 33 × 40 | 320 × 221 | 2 | 1 | 1040 |
| Axial T1w in phase | VIBE | 12.5 | 2.5 | 9 | 33 × 40 | 320 × 221 | 2 | 1 | 1040 |
| Axial T1w fat | VIBE | 12.5 | 1.26 | 9 | 33 × 40 | 320 × 221 | 2 | 1 | 1040 |
| Axial T1w water | VIBE | 12.5 | 1.26 | 9 | 33 × 40 | 320 × 221 | 2 | 1 | 1040 |
| Axial DWI trace-w (b = 50 s/mm2) | EPI SE | 47,917 | 61 | 90 | 19 × 24 | 96 × 57 | 3 | 16 | 1795 |
| Axial DWI ADC (b = 1000 s/mm2) | EPI SE | 47,917 | 61 | 90 | 19 × 24 | 96 × 57 | 3 | 16 | 1795 |
| Axial DWI (b = 1600 s/mm2) | EPI SE | 47,917 | 61 | 90 | 19 × 24 | 96 × 57 | 3 | 16 | 1795 |
| Characteristics | Total Cohort (n = 27) | |
|---|---|---|
| Age (years) | Mean (range) | 67 (43–84) |
| PSA (ng/mL) | Mean (range) | 4.6 (0.4–26.1) |
| Prostate arteries embolized | ||
| Unilateral | 2 (17.4%) | |
| Bilateral | 25 (92.6%) | |
| Baseline TGV (mL) Baseline CGV (mL) Baseline IPSS Baseline ICIQ-UI SF score | Median (range) Median (range) Median (range) Median (range) | 81 (31–163) 59 (15–154) 23 (5–33) 5 (0–14) |
| Cohort | BL TGV by Ellipse Formula (mL) | Post- Treatment | TGV by Ellipse Formula (mL) | p-Value | BL TGV by Semi-Automatic Segmentation (mL) | Post- Treatment | TGV by Semi- Automatic Segmentation (mL) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total cohort (n = 27) | 87.2 ± 10.2 | 1 month | 73.6 ± 8.8 | 0.005 | 80.4 ± 10.1 | 1 month | 69.9 ± 8.5 | 0.025 |
| 6 months | 71.2 ± 9.0 | 0.005 | 6 months | 69.6 ± 8.9 | 0.025 | |||
| 12 months | 73.1 ± 8.9 | 0.005 | 12 months | 70.2 ± 8.8 | 0.050 | |||
| Baseline TV ≤ 60 mL (n = 12) | 42.1 ± 4.0 | 1 month 6 months | 35.2 ± 2.9 35.1 ± 2.9 | 0.050 0.050 | 35.6 ± 3.6 | 1 month 6 months | 32.9 ± 3.4 31.3 ± 2.7 | 0.001 0.001 |
| 12 months | 35.1 ± 2.8 | 0.050 | 12 months | 31.8 ± 2.5 | 0.001 | |||
| Baseline TV > 60 mL (n = 15) | 115.8 ± 8.5 | 1 month 6 months | 97.7 ± 8.0 94.2 ± 9.3 | 0.024 0.024 | 110.2 ± 9.4 | 1 month 6 months | 94.6 ± 8.1 94.2 ± 8.7 | 0.050 0.050 |
| 12 months | 97.2 ± 8.2 | 0.024 | 12 months | 94.8 ± 8.4 | 0.050 |
| Cohort | BL CGV Semi- Automatic Segmentation (mL) | Post- Treatment | CGV Semi-Automatic Segmentation (mL) | p-Value | BL IPP Coronary (mm) | Post- Treatment | IPP Coronary (mm) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total cohort (n = 27) | 58.1 ± 9.2 | 1 month | 47.6 ± 7.3 | 0.038 | 19.4 ± 2.3 | 1 month | 17.1 ± 2.1 | 0.028 |
| 6 months | 47.8 ± 7.5 | 0.191 | 6 months | 16.9 ± 1.9 | 0.010 | |||
| 12 months | 48.4 ± 7.3 | 0.283 | 12 months | 17.0 ± 2.1 | 0.018 | |||
| Baseline TV ≤ 60 mL (n = 12) | 19.6 ± 3.2 | 1 month 6 months | 16.7 ± 2.4 16.8 ± 2.7 | 0.043 0.043 | 14.4 ± 3.2 | 1 month 6 months | 12.1 ± 2.3 12.1 ± 2.1 | 0.210 0.190 |
| 12 months | 17.3 ± 2.4 | 0.446 | 12 months | 13.0 ± 2.8 | 1.000 | |||
| Baseline TV > 60 mL (n = 15) | 83.7 ± 9.2 | 1 month 6 months | 68.3 ± 7.2 68.6 ± 7.7 | 0.050 0.346 | 22.6 ± 2.9 | 1 month 6 months | 19.8 ± 2.7 19.7 ± 2.7 | 0.029 0.005 |
| 12 months | 69.2 ± 7.2 | 0.439 | 12 months | 20.1 ± 2.8 | 0.154 |
| Cohort | Baseline ADC Value of CG (10−3 mm2/s) | Post- Treatment | ADC Value of CG (10−3 mm2/s) | p-Value | Baseline ADC Value of TG (10−3 mm2/s) | Post- Treatment | ADC Value of TG (10−3 mm2/s) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total cohort (n = 27) | 1.20 ± 0.26 | 1 month | 1.13 ± 0.23 | <0.001 | 1.21 ± 0.32 | 1 month | 1.18 ± 0.23 | 1.000 |
| 6 months | 1.19 ± 0.27 | 0.197 | 6 months | 1.21 ± 0.23 | 1.000 | |||
| 12 months | 1.19 ± 0.22 | 0.353 | 12 months | 1.22 ± 0.18 | 1.000 | |||
| Baseline TV ≤ 60 mL (n = 12) | 1.16 ± 0.39 | 1 month 6 months | 1.08 ± 0.29 1.13 ± 0.31 | <0.001 0.521 | 1.20 ± 0.35 | 1 month 6 months | 1.13 ± 0.31 1.16 ± 0.19 | 0.529 1.000 |
| 12 months | 1.14 ± 0.34 | 0.541 | 12 months | 1.18 ± 0.31 | 1.000 | |||
| Baseline TV > 60 mL (n = 15) | 1.25 ± 0.29 | 1 month 6 months | 1.17 ± 0.30 1.23 ± 0.35 | <0.001 0.280 | 1.21 ± 0.47 | 1 month 6 months | 1.20 ± 0.29 1.24 ± 0.32 | 1.000 1.000 |
| 12 months | 1.23 ± 0.22 | 0.517 | 12 months | 1.24 ± 0.21 | 1.000 |
| Cohort | BL IPSS | Post- Treatment | IPSS | p-Value | BL ICIQ-UI SF Score | Post- Treatment | ICIQ-UI SF Score | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total cohort (n = 27) | 21.8 ± 2.0 | 1 month | 14.7 ± 2.1 | 0.002 | 6.4 ± 1.2 | 1 month | 2.4 ± 0.9 | 0.016 |
| 6 months | 11.5 ± 1.8 | <0.001 | 6 months | 2.3 ± 0.8 | 0.020 | |||
| 12 months | 15.9 ± 1.9 | 0.003 | 12 months | 3.5 ± 1.0 | 0.061 | |||
| Baseline TV ≤ 60 mL (n = 12) | 23.3 ± 2.8 | 1 month 6 months | 17.8 ± 3.1 14.5 ± 2.9 | 0.050 0.018 | 6.0 ± 2.2 | 1 month 6 months | 2.0 ± 1.4 1.6 ± 0.9 | 0.012 0.007 |
| 12 months | 17.1 ± 3.0 | 0.049 | 12 months | 1.7 ± 1.1 | 0.009 | |||
| Baseline TV > 60 mL (n = 15) | 20.6 ± 2.9 | 1 month 6 months | 12.2 ± 2.8 9.1 ± 2.1 | 0.022 0.016 | 6.7 ± 1.5 | 1 month 6 months | 2.8 ± 1.3 2.9 ± 1.2 | 0.038 0.042 |
| 12 months | 15.0 ± 2.4 | 0.180 | 12 months | 4.8 ± 1.5 | 0.073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, V.F.; Schirren, M.; Heimer, M.M.; Kazmierczak, P.M.; Cyran, C.C.; Wildgruber, M.; Seidensticker, M.; Ricke, J.; Solyanik, O. Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization. Diagnostics 2022, 12, 585. https://doi.org/10.3390/diagnostics12030585
Schmidt VF, Schirren M, Heimer MM, Kazmierczak PM, Cyran CC, Wildgruber M, Seidensticker M, Ricke J, Solyanik O. Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization. Diagnostics. 2022; 12(3):585. https://doi.org/10.3390/diagnostics12030585
Chicago/Turabian StyleSchmidt, Vanessa F., Mirjam Schirren, Maurice M. Heimer, Philipp M. Kazmierczak, Clemens C. Cyran, Moritz Wildgruber, Max Seidensticker, Jens Ricke, and Olga Solyanik. 2022. "Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization" Diagnostics 12, no. 3: 585. https://doi.org/10.3390/diagnostics12030585
APA StyleSchmidt, V. F., Schirren, M., Heimer, M. M., Kazmierczak, P. M., Cyran, C. C., Wildgruber, M., Seidensticker, M., Ricke, J., & Solyanik, O. (2022). Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization. Diagnostics, 12(3), 585. https://doi.org/10.3390/diagnostics12030585








