Reduced Radiation Exposure Protocol during Computer Tomography of the Left Atrium Prior to Catheter Ablation in Patients with Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
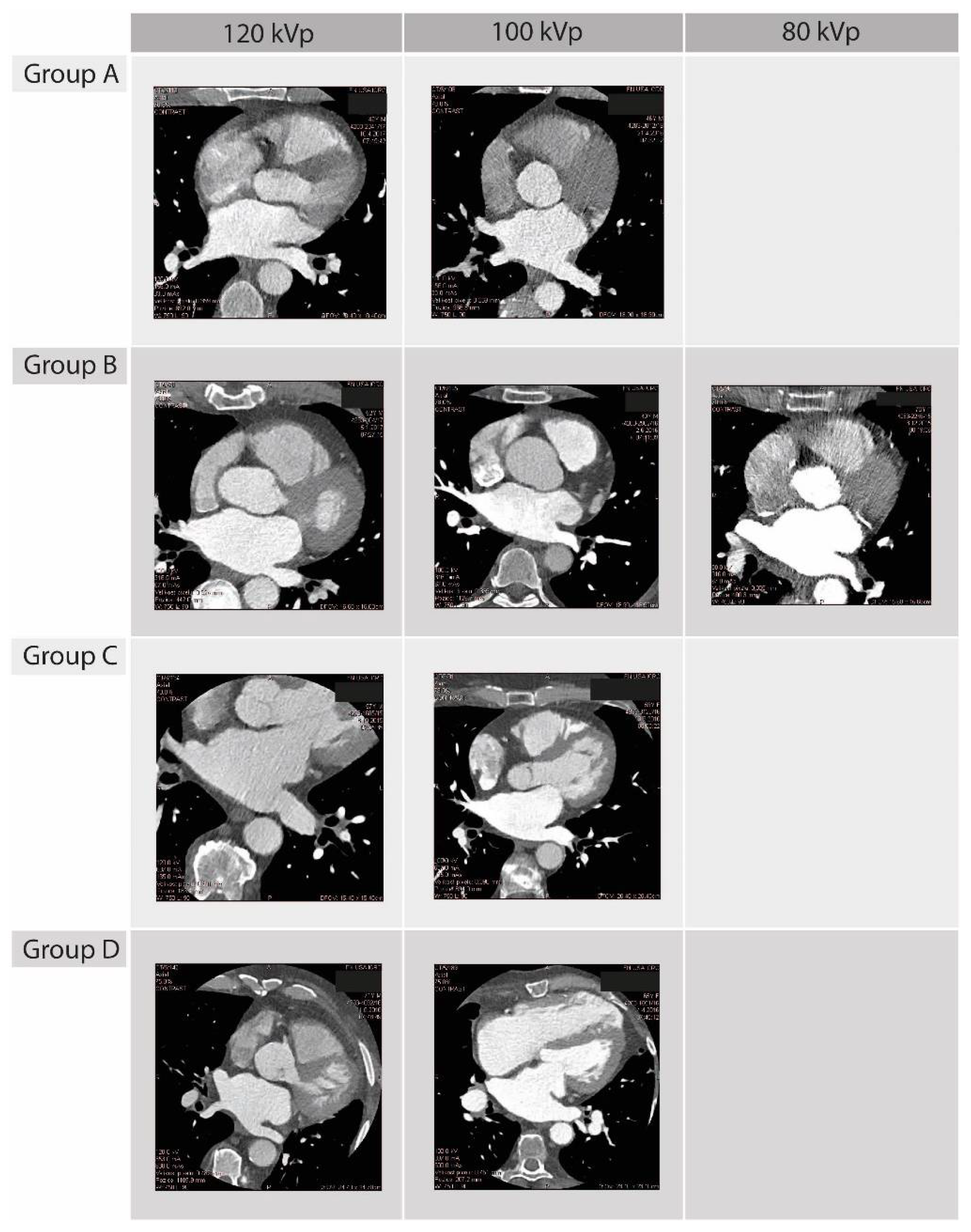
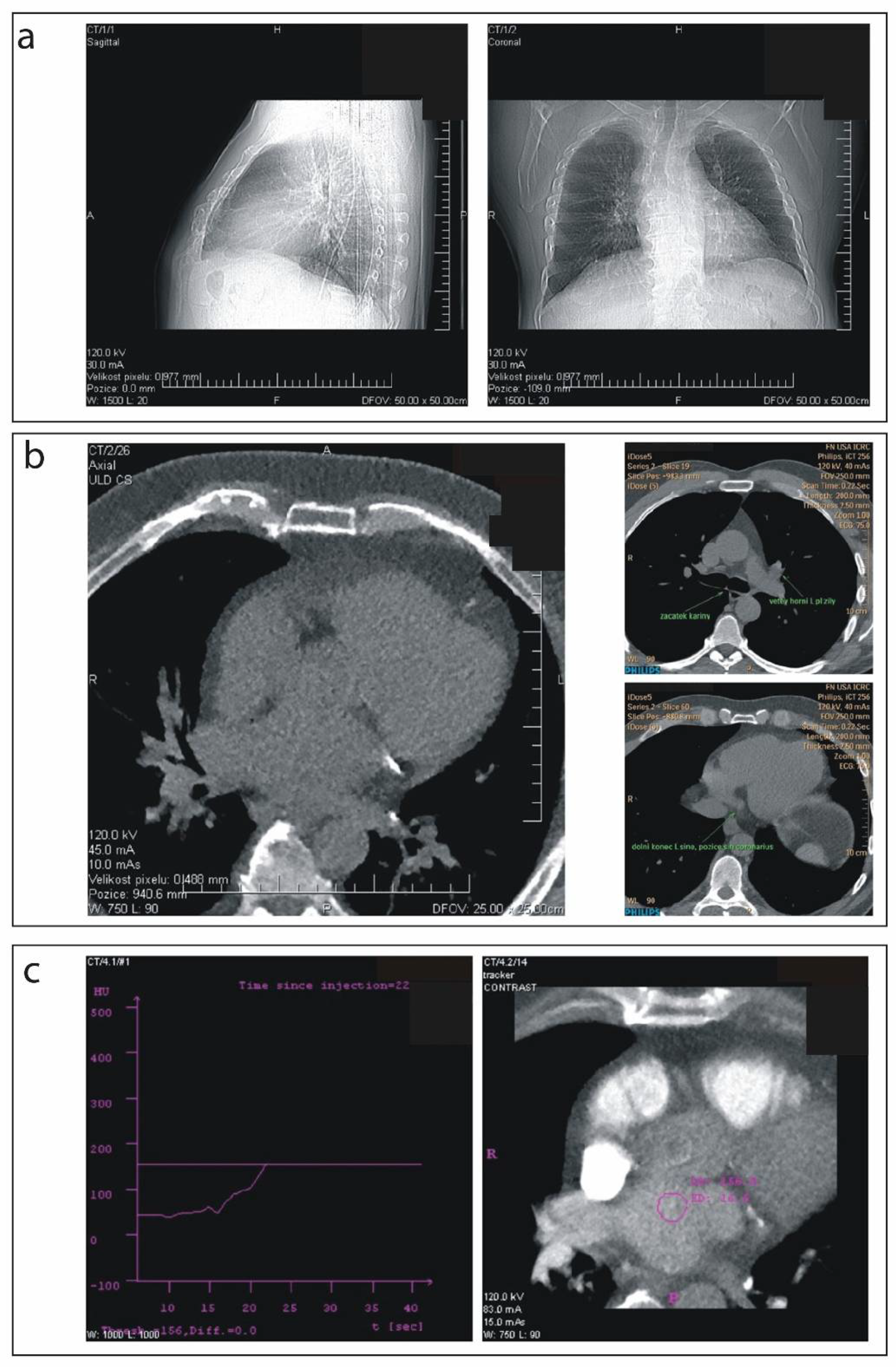
2.2. Scanning Procedure
2.3. Pre-Scanning Settings
2.4. 3D Left Atrium Model Segmentation and Image Quality Assessment
2.5. CT-EAM Integration and Procedural Usefulness
2.6. Statistical Analysis
3. Results
3.1. Dose Results
3.2. Quality of 3D Left Atrium Models, CT-EAM Integration and Procedural Usefulness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kato, R.; Lickfett, L.; Meininger, G.; Dickfeld, T.; Wu, R.; Juang, G.; Angkeow, P.; LaCorte, J.; Bluemke, D.; Berger, R.; et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: Lessons learned by use of magnetic resonance imaging. Circulation 2003, 107, 2004–2010. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.-G.; Xu, H.-Y.; Shi, K.; Long, Q.-H.; Guo, Y.-K. Assessments of pulmonary vein and left atrial anatomical variants in atrial fibrillation patients for catheter ablation with cardiac CT. Eur. Radiol. 2017, 27, 660–670. [Google Scholar] [CrossRef]
- Skowerski, M.; Wozniak-Skowerska, I.; Hoffmann, A.; Nowak, S.; Skowerski, T.; Sosnowski, M.; Wnuk-Wojnar, A.M.; Mizia-Stec, K. Pulmonary vein anatomy variants as a biomarker of atrial fi-brillation—CT angiography evaluation. BMC Cardiovasc. Disord. 2018, 18, 146. [Google Scholar] [CrossRef]
- Anand, R.; Gorev, M.; Poghosyan, H.; Pothier, L.; Matkins, J.; Kotler, G.; Moroz, S.; Armstrong, J.; Nemtsov, S.V.; Orlov, M.V. Prospective randomized comparison of rotational angiography with three-dimensional reconstruction and computed tomography merged with electro-anatomical mapping: A two center atrial fibrillation ablation study. J. Interv. Card. Electrophysiol. 2016, 46, 71–79. [Google Scholar] [CrossRef]
- Dong, J.; Calkins, H.; Solomon, S.B.; Lai, S.; Dalal, D.; Lardo, A.; Brem, E.; Preiss, A.; Berger, R.D.; Halperin, H.; et al. Integrated electroanatomic mapping with three-dimensional computed tomo-graphic images for real-time guided ablations. Circulation 2006, 113, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, P.; Rajappan, K.; Harris, S.; Earley, M.J.; Richmond, L.; Sporton, S.C.; Schilling, R.J. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: A prospective randomized study. Eur. Heart J. 2008, 29, 3029–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, L.; Rajappan, K.; Voth, E.; Rangavajhala, V.; Earley, M.J.; Thomas, G.; Harris, S.; Sporton, S.C.; Schilling, R.J. Validation of Computed Tomography Image Integration into the EnSite NavX Mapping System to Perform Catheter Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2008, 19, 821–827. [Google Scholar] [CrossRef]
- Park, J.J. Computed Tomography for Assessment of Left Atrial Appendage Function. Korean Circ. J. 2019, 49, 181–182. [Google Scholar] [CrossRef]
- Protection, R. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Wolf, J.; Stárek, Z.; Jež, J.; Lehar, F.; Lukasova, M.; Kulik, T.; Novak, M. Rotational angiography of left ventricle to guide ventricular tachycardia ablation. Int. J. Cardiovasc. Imaging 2015, 31, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Fahlenkamp, U.L.; Diaz Ramirez, I.; Wagner, M.; Schwenke, C.; Huppertz, A.; Hamm, B.; Lembcke, A. Image quality of low-radiation dose left atrial CT using filtered back projection and an iterative reconstruction algorithm: Intra-individual comparison in unselected patients undergoing pul-monary vein isolation. Acta Radiol. 2018, 59, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’Alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. EP Eur. 2019, 21, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in col-laboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.J.; Ginks, M.; Ang, R.; Diab, I.; Goromonzi, F.C.; Page, S.; Baker, V.; Richmond, L.; Tayebjee, M.; Sporton, S.; et al. Impact of variant pulmonary vein anatomy and image integration on long-term out-come after catheter ablation for atrial fibrillation. Europace 2010, 12, 1691–1697. [Google Scholar] [CrossRef]
- Malchano, Z.J.; Neuzil, P.; Cury, R.C.; Holmvang, G.; Weichet, J.; Schmidt, E.J.; Ruskin, J.N.; Reddy, V.Y. Integration of Cardiac CT/MR Imaging with Three-Dimensional Electroanatomical Mapping to Guide Catheter Manipulation in the Left Atrium: Implications for Catheter Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2006, 17, 1221–1229. [Google Scholar] [CrossRef]
- Mah, D.Y.; Miyake, C.Y.; Sherwin, E.D.; Walsh, A.; Anderson, M.J.; Western, K.; Abrams, D.; Alexander, M.E.; Cecchin, F.; Walsh, E.P.; et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace 2013, 16, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Stárek, Z.; Lehar, F.; Jež, J.; Wolf, J.; Novak, M. 3D X-ray imaging methods in support catheter ablations of cardiac arrhythmias. Int. J. Cardiovasc. Imaging 2014, 30, 1207–1223. [Google Scholar] [CrossRef]
- Tian, J.; Jeudy, J.; Smith, M.F.; Jimenez, A.; Yin, X.; Bruce, P.A.; Lei, P.; Turgeman, A.; Abbo, A.; Shekhar, R.; et al. Three-Dimensional Contrast-Enhanced Multidetector CT for Anatomic, Dynamic, and Perfusion Characterization of Abnormal Myocardium To Guide Ventricular Tachycardia Ablations. Circ. Arrhythmia Electrophysiol. 2010, 3, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Picano, E.; Vano, E. The Radiation Issue in Cardiology: The time for action is now. Cardiovasc. Ultrasound 2011, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Butler, C.; Rief, M.; Beling, M.; Durmus, T.; Huppertz, A.; Voigt, A.; Baumann, G.; Hamm, B.; Lembcke, A.; et al. Comparison of non-gated vs. electrocardiogram-gated 64-detector-row computed tomography for integrated electroanatomic mapping in patients undergoing pulmonary vein isolation. Europace 2010, 12, 1090–1097. [Google Scholar] [CrossRef]
- Hlaihel, C.; Boussel, L.; Cochet, H.; Roch, J.A.; Coulon, P.; Walker, M.J.; Douek, P.C. Dose and image quality comparison between prospectively gated axial and retro-spectively gated helical coronary CT angiography. Br. J. Radiol. 2011, 84, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Thai, W.-E.; Wai, B.; Lin, K.; Cheng, T.; Heist, E.K.; Hoffmann, U.; Singh, J.P.; Truong, Q.A. Pulmonary Venous Anatomy Imaging with Low-Dose, Prospectively ECG-Triggered, High-Pitch 128-Slice Dual-Source Computed Tomography. Circ. Arrhythmia Electrophysiol. 2012, 5, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwayama, T.; Arimoto, T.; Ishigaki, D.; Hashimoto, N.; Kumagai, Y.; Koyama, Y.; Kiribayashi, N.; Netsu, S.; Nishiyama, S.; Takahashi, H.; et al. The Clinical Value of Nongated Dual-Source Computed Tomography in Atrial Fibrillation Catheter Ablation. J. Cardiovasc. Electrophysiol. 2015, 27, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Annoni, A.D.; Andreini, D.; Pontone, G.; Formenti, A.; Petullà, M.; Consiglio, E.; Nobili, E.; Baggiano, A.; Conte, E.; Mushtaq, S.; et al. Ultra-low-dose CT for left atrium and pulmonary veins imaging using new model-based iterative reconstruction algorithm. Eur. Heart J.—Cardiovasc. Imaging 2015, 16, 1366–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cereda, A.F.; de Luca, F.; Lanzone, A.M.; Cottini, M.; Pastori, L.; Sangiorgi, G. Case report and systematic review of iatrogenic left atrial dissection in different cardiovascular specialties: A common treatment for an uncommon complication? Catheter. Cardiovasc. Interv. 2020, 95, E30–E36. [Google Scholar] [CrossRef]
- Thériault-Lauzier, P.; Spaziano, M.; Vaquerizo, B.; Buithieu, J.; Martucci, G.; Piazza, N. Computed Tomography for Structural Heart Disease and Interventions. Interv. Cardiol. 2015, 10, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Oudkerk, M.; Stillman, A.E.; Halliburton, S.S.; Kalender, W.A.; Möhlenkamp, S.; McCollough, C.H.; Vliegenthart, R.; Shaw, L.J.; Stanford, W.; Taylor, A.J.; et al. Coronary artery calcium screening: Current status and recommendations from the European Society of Cardiac Radiology and North American Society for Cardiovascular Imaging. Int. J. Cardiovasc. Imaging 2008, 24, 645–671. [Google Scholar] [CrossRef] [Green Version]
- Hecht, H.S. Coronary artery calcium scanning: Past, present, and future. JACC Cardiovasc. Imaging 2015, 8, 579–596. [Google Scholar] [CrossRef]

| Group A (n = 20) | Group B (n = 18) | Group C (n = 10) | Group D (n = 20) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 61.0 (53.0–68.0) | 62.0 (56.0–66.0) | 56.0 (49.0–68.0) | 63.0 (58.0–69.0) | 0.333 |
| Male, n (%) | 17 (85) | 16 (89) | 7 (70) | 14 (70) | 0.392 |
| BMI (kg/m2) | 28.0 (26.4–33.8) | 30.6 (26.3–34.0) | 31.1 (28.1–32.0) | 30.0 (27.7–32.2) | 0.947 |
| Diabetes mellitus (yes), n (%) | 6 (30) | 4 (22) | 1 (10) | 2 (10) | 0.354 |
| Hypertension (yes), n (%) | 12 (60) | 12 (67) | 4 (40) | 11 (55) | 0.578 |
| Dyslipidemia (yes), n (%) | 10 (50) | 6 (33) | 6 (60) | 8 (40) | 0.514 |
| Ischemic heart diseases (yes), n (%) | 3 (15) | 4 (22) | 1 (10) | 3 (15) | 0.848 |
| TEE, LA diameter (mm) | 54.0 (46.0–60.0) | 48.5 (45.0–52.5) | 52.2 (47.0–58.0) | 46 (40.0–55.0) | 0.227 |
| Preimaging heart rhythm, SR, n (%) | 8 (40) | 8 (44) | 3 (30) | 13 (65) | 0.241 |
| Group A (n = 20) | Group B (n = 18) | Group C (n = 10) | Group D (n = 20) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | A vs. C | A vs. D | B vs. C | B vs. D | C vs. D | |||||
| DLP (mGy × cm) | 48.6 (44.5–64.8) | 90.9 (79.8–98.1) | 171.2 (131.4–174.2) | 550.2 (470.7–590.7) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Effective radiation dose (mSv) § | 0.83 (0.76–1.10) | 1.55 (1.36–1.67) | 2.91 (2.23–2.96) | 9.35 (8.00–10.04) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Total scanning procedure time (min) # | 8.0 (7.0–11.5) | 7.0 (7.0–9.0) | 8.0 (7.0–11.9) | 10.0 (8.0–11.0) | NS. | NS | <0.0001 | NS | <0.0001 | 0.003 |
| Contrast media volume (ml) # | 66.5 (60.0–78.5) | 70.5 (64.0–75.0) | 76.0 (67.0–80.0) | 100.0 (80.0–100.0) | NS | NS | NS | NS | NS | NS |
| IA ± noise (HU) | 386.5 (317.2–469.6) | 370.1 (280.4–451.3) | 378.0 (332.0–424.0) | 341.2 (306.9–97.9) | NS | NS | NS | NS | NS | NS |
| SNR | 6.5 (5.8–7.3) | 7.1 (5.7–8.2) | 10.8 (10.1–11.3) | 12.2 (9.9–15.7) | NS | <0.001 | <0.0001 | 0.0004 | <0.0001 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadczyk, T.; Wolf, J.; Pesl, M.; Soucek, F.; Lehar, F.; Jez, J.; Kulik, T.; Tyshchenko, B.; Belaskova, S.; Ourednicek, P.; et al. Reduced Radiation Exposure Protocol during Computer Tomography of the Left Atrium Prior to Catheter Ablation in Patients with Atrial Fibrillation. Diagnostics 2022, 12, 612. https://doi.org/10.3390/diagnostics12030612
Jadczyk T, Wolf J, Pesl M, Soucek F, Lehar F, Jez J, Kulik T, Tyshchenko B, Belaskova S, Ourednicek P, et al. Reduced Radiation Exposure Protocol during Computer Tomography of the Left Atrium Prior to Catheter Ablation in Patients with Atrial Fibrillation. Diagnostics. 2022; 12(3):612. https://doi.org/10.3390/diagnostics12030612
Chicago/Turabian StyleJadczyk, Tomasz, Jiri Wolf, Martin Pesl, Filip Soucek, Frantisek Lehar, Jiri Jez, Tomas Kulik, Bohdan Tyshchenko, Silvie Belaskova, Petr Ourednicek, and et al. 2022. "Reduced Radiation Exposure Protocol during Computer Tomography of the Left Atrium Prior to Catheter Ablation in Patients with Atrial Fibrillation" Diagnostics 12, no. 3: 612. https://doi.org/10.3390/diagnostics12030612






