Novel Usefulness of Krebs von den Lungen 6 (KL-6) with Hemoglobin and Lactate Dehydrogenase for Assessing Bone Marrow Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Reticulin-Based Bone Marrow Fibrosis Score System
2.3. Measurement of KL-6 and M2BPGi Levels
2.4. Statistical Analysis
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahr, A.A.; Salama, M.E.; Carreau, N.; Tremblay, D.; Verstovsek, S.; Mesa, R.; Hoffman, R.; Mascarenhas, J. Bone marrow fibrosis in myelofibrosis: Pathogenesis, prognosis and targeted strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, J.T.; McMullin, M.F.; Beer, P.A.; Butt, N.; Conneally, E.; Duncombe, A.; Green, A.R.; Michaeel, N.G.; Gilleece, M.H.; Hall, G.W.; et al. Guideline for the diagnosis and management of myelofibrosis. Br. J. Haematol. 2012, 158, 453–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vener, C.; Fracchiolla, N.S.; Gianelli, U.; Calori, R.; Radaelli, F.; Iurlo, A.; Caberlon, S.; Gerli, G.; Boiocchi, L.; Deliliers, G.L. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood 2008, 111, 1862–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.J.; Bareford, D.; Erber, W.N.; Wilkins, B.S.; Wright, P.; Buck, G.; Wheatley, K.; Harrison, C.N.; Green, A.R. Reticulin accumulation in essential thrombocythemia: Prognostic significance and relationship to therapy. J. Clin. Oncol. 2009, 27, 2991–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvasnicka, H.M.; Thiele, J.; Schmitt-Graeff, A.; Diehl, V.; Zankovich, R.; Niederle, N.; Leder, L.D.; Schaeer, H.E. Bone marrow features improve prognostic efficiency in multivariate risk classification of chronic-phase Ph(1+) chronic myelogenous leukemia: A multicenter trial. J. Clin. Oncol. 2021, 19, 2994–3009. [Google Scholar] [CrossRef]
- Jain, A.G.; Zhang, L.; Bennett, J.M.; Komrokji, R. Myelodysplastic syndromes with bone marrow fibrosis: An update. Ann. Lab. Med. 2021, 42, 299–305. [Google Scholar] [CrossRef]
- Thiele, J.; Kvasnicka, H.M.; Facchetti, F.; Franco, V.; Walt, J.; Orazi, A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005, 90, 1128–1132. [Google Scholar]
- Teman, C.J.; Wilson, A.R.; Perkins, S.L.; Hickman, K.; Prchal, J.T.; Salama, M.E. Quantification of fibrosis and osteosclerosis in myeloproliferative neoplasms: A computer-assisted image study. Leuk. Res. 2010, 34, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and tissue fibrosis: Molecular signals, cellular mechanisms and translational implications. Mol. Aspects. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Mackinnon, A.; Forbes, S. Bone marrow contributions to fibrosis. Biochim. Biophys. Acta 2013, 1832, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Kohno, K.; Awaya, Y.; Oyama, T.; Yamakido, M.; Akiyama, M.; Inoue, Y.; Yokoyama, A.; Hamada, H.; Fujioka, S.; Hiwada, K. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am. Rev. Respir. Dis. 1993, 148, 637–642. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Q.; Han, Q.; Huang, J.; Ou, Y.; Chen, M.; Wen, Y.; Mosha, S.S.; Deng, K.; Chen, R. Sequential changes of serum KL-6 predict the progression of interstitial lung disease. J. Thorac. Dis. 2018, 10, 4705–4714. [Google Scholar] [CrossRef]
- Bekki, Y.; Yoshizumi, T.; Shimoda, S.; Itoh, S.; Harimoto, N.; Ikegami, T.; Kuno, A.; Narimatsu, H.; Shirabe, K.; Maehara, Y. Hepatic stellate cells secreting WFA(+) -M2BP: Its role in biological interactions with Kupffer cells. J. Gastroenterol. Hepatol. 2017, 32, 1387–1393. [Google Scholar] [CrossRef]
- Ye, Y.; Fu, Q.; Wang, R.; Guo, Q.; Bao, C. Serum KL-6 level is a prognostic marker in patients with anti-MDA5 antibody-positive dermatomyositis associated with interstitial lung disease. J. Clin. Lab. Anal. 2019, 33, e22978. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.C.; Choi, K.H.; Jacob, J.; Song, J.W. Prognostic role of blood KL-6 in rheumatoid arthritis-associated interstitial lung disease. PLoS ONE 2020, 15, e0229997. [Google Scholar] [CrossRef]
- Jang, S.Y.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Lee, Y.R.; Kim, G.; Hur, K.; Han, M.H.; Lee, W.K. Diagnostic efficacy of serum mac-2 binding protein glycosylation isomer and other markers for liver fibrosis in non-alcoholic fatty liver diseases. Ann. Lab. Med. 2021, 41, 302–309. [Google Scholar] [CrossRef]
- Tamaki, N.; Kurosaki, M.; Loomba, R.; Izumi, N. Clinical utility of mac-2 binding protein glycosylation isomer in chronic liver diseases. Ann. Lab. Med. 2021, 41, 16–24. [Google Scholar] [CrossRef]
- European Publication Server. Available online: https://data.epo.org/publication-server/document?iDocId=5616136&i%20Format=0 (accessed on 21 December 2021).
- Mudireddy, M.; Shah, S.; Lasho, T.; Barraco, D.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A.; Tefferi, A. Prefibrotic versus overtly fibrotic primary myelofibrosis: Clinical, cytogenetic, molecular and prognostic comparisons. Br. J. Haematol. 2018, 182, 594–597. [Google Scholar] [CrossRef] [Green Version]
- Gogtay, N.J. Statistical evaluation of diagnostic tests—Part 2 [pre-test and post-test probability and odds, likelihood ratios, receiver operating characteristic curve, youden’s index and diagnostic test biases]. J. Assoc. Phys. India 2017, 65, 86–91. [Google Scholar]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Strieter, R.M. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc. Am. Thorac. Soc. 2008, 5, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis-a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Györfi, A.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef]
- Agarwal, A.; Morrone, K.; Bartenstein, M.; Zhao, Z.J.; Verma, A.; Goel, S. Bone marrow fibrosis in primary myelofibrosis: Pathogenic mechanisms and the role of TGF-β. Stem Cell Investig. 2016, 3, 5. [Google Scholar] [PubMed]
- Fernandez, E.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming growth factor-β-induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Naymagon, L.; Mascarenhas, J. Myelofibrosis-related anemia: Current and emerging therapeutic strategies. Hemasphere 2017, 1, e1. [Google Scholar] [CrossRef]
- Shah, S.; Mudireddy, M.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A.; Tefferi, A. Marked elevation of serum lactate dehydrogenase in primary myelofibrosis: Clinical and prognostic correlates. Blood Cancer J. 2017, 7, 657. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kisseleva, T. Bone marrow-derived fibrocytes contribute to liver fibrosis. Exp. Biol. Med. 2015, 240, 691–700. [Google Scholar] [CrossRef]
- Savani, M.; Dulery, R.; Bazarbachi, A.H.; Mohty, R.; Brissot, E.; Malard, F.; Bazarbachi, A.; Nagler, A.; Mohty, M. Allogeneic haematopoietic cell transplantation for myelofibrosis: A real-life perspective. Br. J. Haematol. 2021, 195, 495–506. [Google Scholar] [CrossRef]

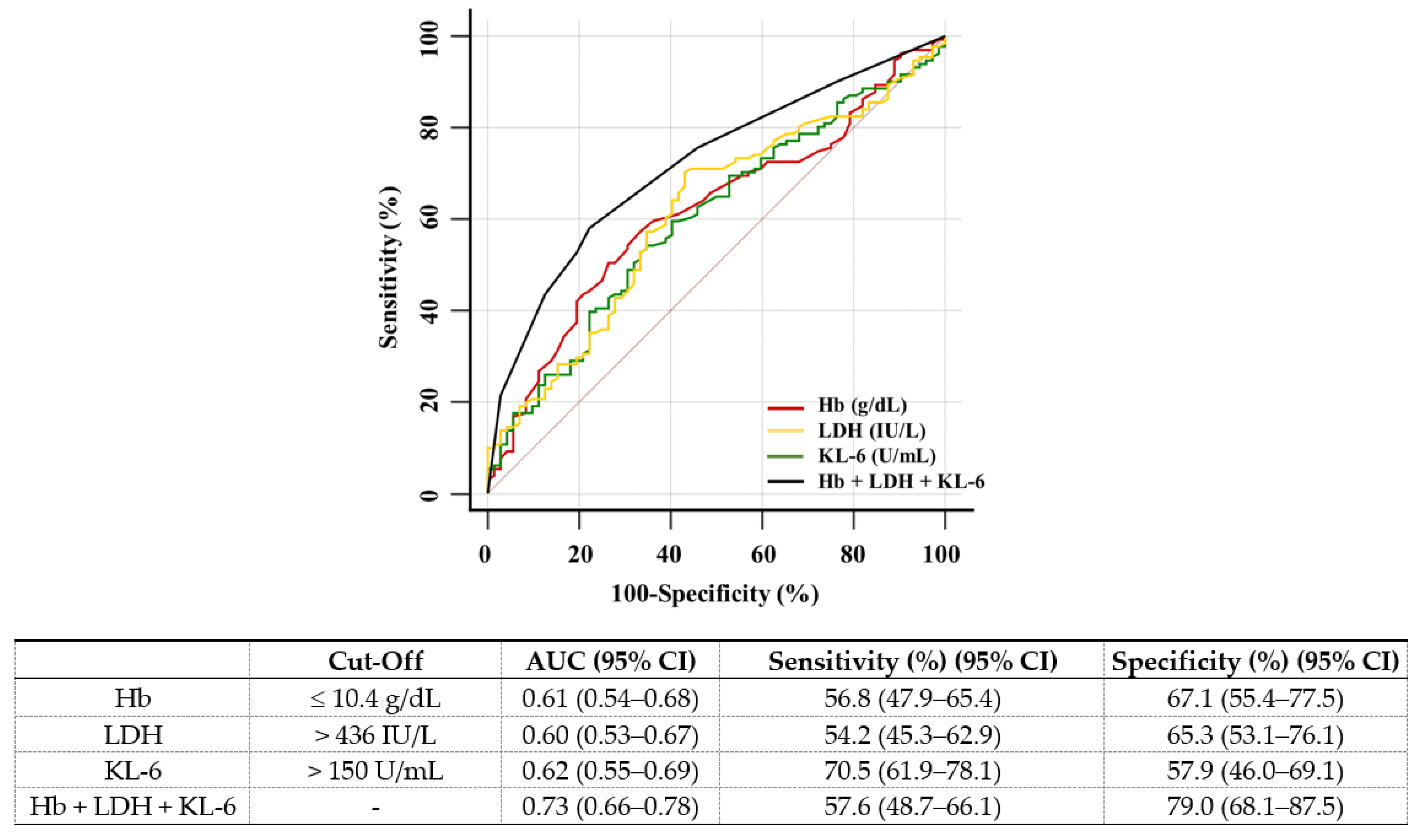

| Characteristics | n = 208 |
|---|---|
| Demographic information | |
| Male | 105 (50.5) |
| Age (years) | 59.0 (43.5–69.3) |
| Clinical information | |
| Diagnosis | |
| Lymphoma | 47 (22.6) |
| AML | 42 (20.2) |
| MDS | 21 (10.1) |
| ALL | 19 (9.1) |
| PCM | 19 (9.1) |
| Cytopenia | 14 (6.7) |
| ITP | 12 (5.8) |
| CML | 10 (4.8) |
| ET | 5 (2.4) |
| PMF | 3 (1.4) |
| Others a | 16 (7.7) |
| Laboratory information | |
| WBC (×109/L) | 5.3 (3.0–7.5) |
| Hb (g/dL) | 10.5 (9.4–12.1) |
| PLT (×109/L) | 134.5 (57–229.3) |
| LDH (IU/L) | 431 (351.5–593.0) |
| BM cellularity (%) | 40 (30–70) |
| Reticulin grade | |
| Grade 0 | 76 (36.5) |
| Grade 1 | 87 (41.8) |
| Grade 2 | 32 (15.4) |
| Grade 3 | 13 (6.3) |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| β | S.E | p | OR (95% CI) | β | S.E | p | OR (95% CI) | |
| WBC (×109/L) | 0.57 | 0.30 | 0.056 | 1.76 (0.99–3.15) | 0.40 | 0.33 | 0.256 | 1.49 (0.75–2.98) |
| Hb (g/dL) | 0.96 | 0.30 | 0.001 | 2.62 (1.46–4.70) | 0.67 | 0.32 | 0.049 | 1.96 (1.00–3.84) |
| PLT (×109/L) | 0.99 | 0.30 | 0.001 | 2.68 (1.50–4.80) | 0.45 | 0.31 | 0.204 | 1.56 (0.79–3.11) |
| LDH (IU/L) | 0.89 | 0.33 | 0.007 | 2.44 (1.27–4.68) | 0.82 | 0.33 | 0.024 | 2.26 (1.11–4.61) |
| KL-6 (U/mL) | 1.19 | 0.30 | <0.001 | 3.28 (1.82–5.91) | 1.07 | 0.33 | 0.001 | 2.91 (1.54–5.51) |
| M2BPGi (C.O.I) | 0.65 | 0.33 | 0.048 | 1.92 (1.01–3.66) | 0.14 | 0.37 | 0.702 | 1.15 (0.56–2.38) |
| Conventional Marker | Combined Marker | AUC (95% CI) | p | IDI | cfNRI | ||
|---|---|---|---|---|---|---|---|
| Estimated Value (95% CI) | p | Estimated Value (95% CI) | p | ||||
| Hb (g/dL) | Hb + KL-6 | 0.70 (0.64–0.76) | <0.001 | 0.034 (0.000–0.069) | 0.056 | 0.331 (0004–0.658) | 0.048 |
| Hb + LDH | 0.68 (0.61–0.75) | <0.001 | 0.026 (0.000–0.051) | 0.043 | 0.294 (−0.033–0.622) | 0.078 | |
| Hb + LDH + KL-6 | 0.73 (0.66–0.78) | <0.001 | 0.044 (0.007–0.081) | 0.020 | 0.434 (0.112–0.756) | 0.008 | |
| LDH (IU/L) | LDH + KL-6 | 0.67 (0.60–0.73) | <0.001 | 0.023 (-0.007–0.054) | 0.131 | 0.364 (0.037–0.692) | 0.029 |
| LDH + Hb | 0.68 (0.61–0.75) | <0.001 | 0.039 (0.022–0.056) | <0.001 | 0.625 (0.344–0.906) | <0.001 | |
| LDH + Hb + KL-6 | 0.73 (0.66–0.78) | <0.001 | 0.057 (0.025–0.090) | 0.001 | 0.625 (0.319–0.931) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, M.; Hur, M.; Park, M.; Kim, H. Novel Usefulness of Krebs von den Lungen 6 (KL-6) with Hemoglobin and Lactate Dehydrogenase for Assessing Bone Marrow Fibrosis. Diagnostics 2022, 12, 628. https://doi.org/10.3390/diagnostics12030628
Nam M, Hur M, Park M, Kim H. Novel Usefulness of Krebs von den Lungen 6 (KL-6) with Hemoglobin and Lactate Dehydrogenase for Assessing Bone Marrow Fibrosis. Diagnostics. 2022; 12(3):628. https://doi.org/10.3390/diagnostics12030628
Chicago/Turabian StyleNam, Minjeong, Mina Hur, Mikyoung Park, and Hanah Kim. 2022. "Novel Usefulness of Krebs von den Lungen 6 (KL-6) with Hemoglobin and Lactate Dehydrogenase for Assessing Bone Marrow Fibrosis" Diagnostics 12, no. 3: 628. https://doi.org/10.3390/diagnostics12030628






